Abstract
We report a case of false-positive metabolic screening for isovaleric acidemia in a newborn due to treatment of the mother with pivalic acid containing antibiotics before delivery. By using a recently established second-tier test based on the tandem-MS technique, we could identify pivalic acid in a dried blood sample taken during routine neonatal screening. Before this second-tier test was initiated, diverse analytical procedures were performed in the baby to rule out isovaleric acidemia and carnitine supplementation was started. This caused additional psychological burden to the family. The direct use of the second-tier test would have avoided these negative consequences of a false-positive screening result.
Introduction
Electrospray ionization tandem mass spectrometry is used as a standard method for newborn mass screening of inborn errors of metabolism worldwide. Isovaleric acidemia (IVA) is a target disease of the current newborn screening program in Germany with an incidence of 1–1.5 per 100,000 screened newborns (own observation and Ensenauer et al. (2011); Smith and Matern (2010); McHugh et al. (2011)). IVA has a spectrum of biochemical and clinical phenotypes, including an attenuated form that makes clinical diagnosis difficult and may be associated with only mild elevation of isovalerylcarnitine in newborn screening.
A known cause of false-positive testing for IVA is the presence of the isomer pivalic acid in the dried blood spots. The established newborn mass screening method is not suited to separate isomers. Antibiotics given to the mother before delivery are a common source of the C5 isoform pivalic acid (Abdenur et al. 1998). Pivampicillin is hydrolyzed to pivalic acid after absorption (Melegh et al. 1987).
False-positive results have to be avoided whenever possible as they will cause a considerable psychological burden to the family. Furthermore, they give rise to unwarranted follow-up procedures that are invasive and sometimes even require hospital admission (Smith and Matern 2010; Hewlett and Waisbren 2006; Fyrö and Bodegård 1987; Lipstein et al. 2009).
We report a case of false-positive metabolic screening for IVA in a newborn due to treatment of the mother with antibiotics before delivery, which resulted in pivalic acid production. By using a recently established second-tier test based on the tandem-MS technique (Janzen et al. 2013), we could identify pivalic acid in a dried blood sample taken during routine neonatal screening. Second-tier tests are not uncommon in newborn screening to identify false-positive results, for example in tyrosinemia type I (Sander et al. 2006).
Case Report
The patient is the first child of non-consanguineous healthy parents. After an uncomplicated pregnancy, the child was born via caesarean section at 36 weeks of gestation during a family holiday in Denmark. Because of early rupture of membranes, the mother received an antibiotic treatment with six doses of Pondocilline® (à 500 mg pivampicillin) and metronidazole (à 500 mg). The treatment was stopped immediately after delivery.
Delivery was uncomplicated, and cardiorespiratory adaptation of the child was fine. The child was breastfed, supplemented with formula milk. A first newborn screening for inborn errors of metabolism was carried out in Denmark at the age of 3 days with normal results. IVA is not included in the standard Danish neonatal screening program. A second screening test was performed by the local German pediatrician on day 5, as he wanted to offer the family the newborn screening panel available at the local program. This test showed an elevated level of isovalerylcarnitine at 2.6 μmol/l (reference <0.5 μmol/l). The isovalerylcarnitine/acetylcarnitine ratio was increased (0.1; reference <0.03). Values were similar in the recall sample.
Follow-up testing in dried blood spots showed a spontaneous decrease of isovalerylcarnitine to 1.0 μmol/l on day 9, while the isovalerylcarnitine/acetylcarnitine ratio dropped to 0.04 (within reference range). Organic acids in urine were negative for isovaleric acid and isovalerylglycine. As a variant form of IVA was suspected after the initial positive result, carnitine supplementation (50 mg/kg per day) was started. In the meantime, second-tier testing as described below revealed substantial amounts of pivaloylcarnitine in the blood, while isovalerylcarnitine was normal. After obtaining this result, carnitine supplementation was discontinued. Further controls showed a normalization of blood “isovalerylcarnitine,” i.e., pivaloylcarnitine.
Initially, the mother was not aware that she had been given antibiotics. Subsequently she remembered that some medication was given to her before delivery. The clinical file was obtained from the Danish hospital where birth took place. It revealed treatment with Pondocilline® and metronidazole because of early rupture of membranes. Pondocilline® contains pivampicillin which is broken down to ampicillin and pivalic acid in vivo. The initial newborn screening sample from Denmark was sent to us for analysis and showed a high peak of pivaloylcarnitine.
Materials and Methods
A second-tier test for differentiation of C5 acylcarnitines using UPLC-MS/MS in dried blood was carried out using the method recently described (Janzen et al. 2013).
Results
The time course of acylcarnitine levels is summarized in Table 1. A chromatogram from the patient is shown in Fig. 1.
Table 1.
Acylcarnitine concentrations in dried blood spots from our patient. The cutoff for the routine screening method with butylation is 0.5 μmol/L. nd – not detected
| Sample number | Day after birth | C5 carnitines (butylated) [μmol/l] | Pivaloylcarnitine [μmol/l] |
2-Methylbutyrylcarnitine [μmol/l] |
Isovalerylcarnitine [μmol/L] |
Valerylcarnitine [μmol/L] |
|---|---|---|---|---|---|---|
| 1 | 3 | 4.12 | 6.02 | nd | 0.05 | nd |
| 2 | 5 | 2.64 | 3.55 | 0.02 | 0.04 | nd |
| 3 | 5 | - | 3.69 | nd | 0.04 | nd |
| 4 | 5 | - | 3.74 | 0.02 | 0.04 | nd |
| 5 | 9 | 1.00 | 1.20 | 0.02 | 0.05 | nd |
| 6 | 19 | 0.32 | 0.29 | 0.04 | 0.08 | nd |
| 7 | 41 | 0.18 | 0.04 | 0.04 | 0.08 | nd |
| 8 | 55 | 0.13 | 0.02 | 0.05 | 0.08 | nd |
| 9 | 126 | 0.08 | 0.01 | 0.03 | 0.05 | nd |
Fig. 1.
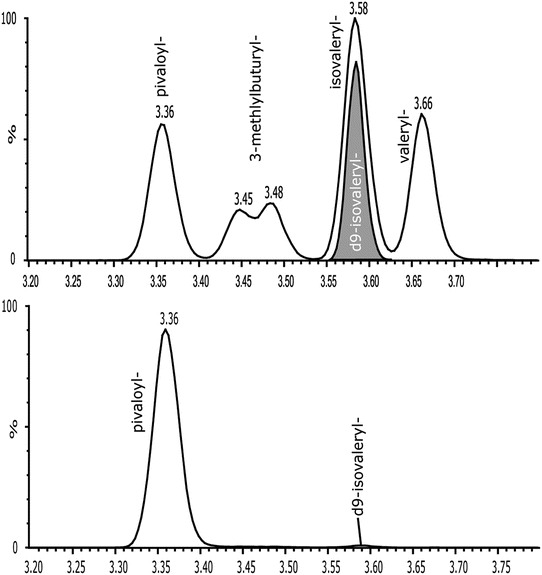
Chromatographic separation of the C5 isoforms (solved in methanol/water). The deuterated d9 isovalerylcarnitine (internal standard) is shown in the bottom chromatogram. Sample of the patient (day 3) eluted from the dried blood spot (bottom chromatogram). The pivaloylcarnitine was the most prominent peak, all other C5 acylcarnitines were only detected in low concentrations
Discussion
IVA is a target disease of the newborn screening program in Germany and other countries. Pivalic acid as a constituent of some antibiotics is known to interfere with IVA-testing leading to false-positive results (Abdenur et al. 1998). This will lead to considerable psychosocial burden for the parents and other relatives and will give rise to further biochemical testing in the baby. We describe the use of a second-tier test that enables screening laboratories to identify pivaloylcarnitine as the cause of elevated C5 giving false-positive results for IVA (Janzen et al. 2013). Over a 2-year period we obtained a total of 350,837 German neonatal samples, 227 cases with elevated C5 were reported as positive (0.064 %). The samples received from abroad (22,084 cases) showed a higher percentage of positive samples (104 cases, 0.47 %). Thus, a significant number of positive samples are found during neonatal screening for IVA. These figures emphasize the need for rapid specification of C5 isomers. The second-tier test described allows rapid separation and quantification of various C5 acylcarnitines which may underlie elevated C5 levels in routine newborn screening in the original blood sample (Janzen et al. 2013).
Maeda et al. (2008, 2007) previously described the chromatographic separation of acylcarnitines from serum and urine. However, their method needs a second sample from the patients (urine or serum) and the sample preparation requires the use of solid phase extraction material (SPE).
Forni et al. (2010) used similar hardware equipment for the separation using plasma or dried blood spot material. Before quantification, they derivatized the acylcarnitines. During this chemical process some acylcarnitines can be fragmented to free acids and carnitine; therefore, we decided to measure the acylcarnitines without any chemical modification.
Ferrer et al. (2007) described a rapid method to separate C4 and C5 acylcarnitine isomers in plasma samples. The validation data agree well with our findings. The separation time was about twice as long, the sample volume was 50 μl. The most important advantage of our method is the option to use blood from the original screening card. By using a UPLC-technique the test is faster, more sensitive, and cheaper compared to the conventional HPLC method. Thus, false-positive results can be rapidly excluded without any burden to the newborn and his family.
The newly established test is able to increase specificity without hampering sensitivity in IVA-testing and does not require additional instrumentation. The small sample volume of a 4.7 mm diameter blood spot equaling 5μl whole blood prolongs the lifetime of the column and the hardware equipment, the method therefore is fast and cost-efficient.
We advocate including this second-tier test in screening in every sample tested “positive” for IVA. It is generally possible to run the method on a conventional HPLC with a less powerful mass spectrometer. This is associated with a longer run time and a lower sensitivity with an increased consumption of flow agents. The advantage of the described method is the use of very little material and rapid separation with high sensitivity.
Further testing in a large newborn cohort is necessary to corroborate our promising results.
Acknowledgments
We are grateful to M. Terhardt, Dr. S. Sander, and the other laboratory staff of the “Screening-Labor Hannover” for technical assistance and the staff from the screening laboratory in Copenhagen for providing the initial screening card from the patient.
Compliance with Ethics Guidelines
Thomas Cloppenborg, Nils Janzen, Hans-Joachim Wagner, Ulrike Steuerwald, Michael Peter, and Anibh Martin Das declare that they have no conflict of interest.
Details of the Contributions of Individual Authors
T. Cloppenborg treated the child at the metabolic clinic and drafted the manuscript, N. Janzen drafted the manuscript and supervised the technical analysis, H.J. Wagner initiated the second neonatal screening and is the community pediatrician of the patient, U. Steuerwald and M. Peter evaluated the results at the screening laboratory, A.M. Das treated the child at the metabolic clinic and finalized the manuscript.
Footnotes
Competing interests: None declared
Authors Cloppenborg T and Janzen N contributed equally
Contributor Information
AM Das, Email: das.anibh@mh-hannover.de1.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Abdenur JE, Chamoles NA, Guinle AE, Schenone AB, Fuertes AN. Diagnosis of isovaleric acidaemia by tandem mass spectrometry: false positive result due to pivaloylcarnitine in a newborn screening programme. J. Inherit. Metab. Dis. 1998;21:624–630. doi: 10.1023/A:1005424331822. [DOI] [PubMed] [Google Scholar]
- Ensenauer R, et al. Newborn screening for isovaleric acidemia using tandem mass spectrometry: data from 1.6 million newborns. Clin. Chem. 2011;57:623–626. doi: 10.1373/clinchem.2010.151134. [DOI] [PubMed] [Google Scholar]
- Ferrer I, et al. Separation and identification of plasma short-chain acylcarnitine isomers by HPLC/MS/MS for the differential diagnosis of fatty acid oxidation defects and organic acidemias. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;860:121–126. doi: 10.1016/j.jchromb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Forni S, Fu X, Palmer SE, Sweetman L. Rapid determination of C4-acylcarnitine and C5-acylcarnitine isomers in plasma and dried blood spots by UPLC-MS/MS as a second tier test following flow-injection MS/MS acylcarnitine profile analysis. Mol. Genet. Metab. 2010;101:25–32. doi: 10.1016/j.ymgme.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Fyrö K, Bodegård G. Four-year follow-up of psychological reactions to false positive screening tests for congenital hypothyroidism. Acta Paediatr Scand. 1987;76:107–114. doi: 10.1111/j.1651-2227.1987.tb10424.x. [DOI] [PubMed] [Google Scholar]
- Hewlett J, Waisbren SE. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J. Inherit. Metab. Dis. 2006;29:677–682. doi: 10.1007/s10545-006-0381-1. [DOI] [PubMed] [Google Scholar]
- Janzen N, et al. UPLC-MS/MS analysis of C5-acylcarnitines in dried blood spots. Clin. Chim. Acta. 2013;421:41–45. doi: 10.1016/j.cca.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Lipstein EA, Perrin JM, Waisbren SE, Prosser LA. Impact of false-positive newborn metabolic screening results on early health care utilization. Genet. Med. 2009;11:716–721. doi: 10.1097/GIM.0b013e3181b3a61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, et al. Simultaneous quantification of acylcarnitine isomers containing dicarboxylic acylcarnitines in human serum and urine by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:799–806. doi: 10.1002/rcm.2905. [DOI] [PubMed] [Google Scholar]
- Maeda Y, et al. Determination of 3-hydroxyisovalerylcarnitine and other acylcarnitine levels using liquid chromatography-tandem mass spectrometry in serum and urine of a patient with multiple carboxylase deficiency. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;870:154–159. doi: 10.1016/j.jchromb.2007.11.037. [DOI] [PubMed] [Google Scholar]
- McHugh DMS, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet. Med. 2011;13:230–254. doi: 10.1097/GIM.0b013e31820d5e67. [DOI] [PubMed] [Google Scholar]
- Melegh B, Kerner J, Bieber LL. Pivampicillin-promoted excretion of pivaloylcarnitine in humans. Biochem. Pharmacol. 1987;36:3405–3409. doi: 10.1016/0006-2952(87)90318-2. [DOI] [PubMed] [Google Scholar]
- Sander J, et al. Newborn screening for hepatorenal tyrosinemia: tandem mass spectrometric quantification of succinylacetone. Clin. Chem. 2006;52:482–487. doi: 10.1373/clinchem.2005.059790. [DOI] [PubMed] [Google Scholar]
- Smith EH, Matern D (2010) Acylcarnitine analysis by tandem mass spectrometry. Curr Protoc Hum Genet, Chapter 17, Unit 17.8.1–20 [DOI] [PubMed]


