Abstract
A 3-year-old girl suffering from ornithine carbamoyltransferase (OTC) deficiency was poorly equilibrated under conventional diet and scavenger treatment. Following unsuccessful cryopreserved hepatocyte transplantation, she received two infusions of Adult Derived Human Liver Stem/Progenitor Cells (ADHLSCs) expanded in vitro under GMP settings, the quantity being equivalent to 0.75% of her calculated liver mass. Using FISH immunostaining for the Y chromosome, the initial biopsy did not detect any male nuclei in the recipient liver. Two liver biopsies taken 100 days after ADHLSC transplantation showed 3% and 5% of male donor cells in the recipient liver, thus suggesting repopulation by donor cells. Although limited follow-up did not allow us to draw conclusions on long-term improvement, these results provide a promising proof of concept that this therapy is feasible in an OTC patient.
Introduction
The urea cycle is the pathway responsible for the metabolism of wasted nitrogen in humans. This pathway is dependent of the functional integrity of the liver ornithine transcarbamylase (OTC). OTC deficiency is the most common urea cycle enzyme defect. Ammonia incorporation is impaired leading to recurrent high blood values, causing severe neurological damage and long-term cognitive defects (Nassogne et al. 2005; Leonard 2001; Ensenauer et al. 2005). OTC deficiency is an x-chromosomal transmitted disorder. Current treatment encompasses a low natural protein diet, use of ammonium scavengers, and supplements in arginine and citrulline (Enns et al. 2007). Patients can still present episodes of decompensation, and most of them develop secondary anorexia requiring gastric tubing. Their total protein intake remains often below the WHO recommendation. Orthotopic liver transplantation (OLT) applies to the sickest patients, while some other patients are perfectly equilibrated. There is a wide range of clinical presentations, with recurrent episodes of moderate hyperammonemia, and in less severe cases, parents and physicians are balanced between conventional therapy or liver transplantation, the procedure in which access remains highly limited by donor shortage and because of the inherent short- and long-term risks of the procedure. Less invasive, innovative therapies are expected to improve the phenotype and the quality of life of these children and their families.
Liver regenerative medicine aims to repair a deficient liver with cells. In the context of inborn errors of liver metabolism, allogeneic cells are used to correct a liver-based enzyme defect. The proof of concept has been established with hepatocyte transplantation. After infusion in the recipient’s portal system, transplanted hepatocytes have been documented to provide the missing enzyme for urea cycle defects and other metabolic diseases (Sokal et al. 2003; Stéphenne et al. 2005, 2006). However, hepatocyte transplantation faces several limitations, including organ shortage, poor quality of livers offered for cell isolation, poor resistance of mature hepatocytes to cryopreservation and culture conditions, as well as risk of disease transmission with a transplant product (Meyburg and Hoffmann 2010; Jorns et al. 2012; Smets et al., 2008). Therefore, stem/progenitor cells are upcoming as the next generation of cells for regenerative medicine, being developed for their capacity to overcome the limitations of mature hepatocytes, and to be expanded industrially under GMP conditions. This technology brings regenerative medicine of the liver widely available to any patient suffering from this disorder and other inborn metabolic diseases of the liver (Nussler et al. 2006; Sancho-Bru et al. 2009; Khuu et al. 2010; Najimi et al. 2007).
Here we report the first human stem/progenitor cell transplantation, with engraftment and repopulation of recipient liver, using Adult Derived Human Liver Stem Cells (ADHLSCs) intraportal infusion in a 3-year-old girl with OTC deficiency.
Patient and Methods
Patient’s History
A 3-year-old girl suffered severe OTC deficiency with neonatal onset. The diagnosis had been established after a neonatal coma occurring 38 h after birth (NH3 380 μmol/L, TP 10%) and was confirmed by DNA analysis, indicating a de novo deletion of exons 6–8 on the paternal allele of OTC gene. She was under protein restriction diet (1 g/kg/day by amino acid mixture and natural proteins) and received Na-Benzoate (200 mg/kg/day), Na-phenylbutyrate (150 mg/kg/day), citrulline (150 mg/kg/day), and arginine (150 mg/kg/day). Anorexia and impossibility to provide oral feeding led to enteral feeding by gastrostomy tube starting at age 9 months. The child’s growth was normal with good psychomotor development, but despite intensive treatment she displayed recurrent episodes of hyperammonemia (NH3 > 300 μmol/L) and frequent (monthly) hospital admissions. Glutamine levels were persistently elevated (>1,400 μmol/L – normal range: 250–850 μmol/L).
In an attempt to improve her metabolic control, and decrease the risk related to recurrent hyperammoniemia, she was proposed to receive hepatocyte transplantation, according to a protocol used in previously reported patients (Sokal et al. 2003). The project and procedure was approved by the institution ethical committee. Hepatocytes were procured from a ministry of health accredited tissue bank.
She received two infusions of 1.25 and 2.3 billions cryopreserved/thawed female hepatocytes over two days (viability post thawing 72% and 84%, respectively, using trypan blue exclusion assay). Four months later, she received a second series of infusions with 2.45 billions of cryopreserved/thawed male hepatocytes (viability post thawing 91%). The cells were infused through a percutaneous portal catheter, at a concentration of 10–15 × 106cells/mL, at a rate of 1 mL/min. Immunosuppression included Tacrolimus (Prograf, Astellas, Belgium). The tolerance was good without complications, but there was however no evidence of benefit from these hepatocyte transplantation courses.
During the 6-month follow-up treatment, she continued to have monthly episodes of metabolic decompensation with hyperammonemia up to 300 μmol/L and she was proposed to receive a new cell therapy course using ADHLSC. This protocol was approved by the ethical committee of the institution and informed consent was given to and received from the parents by an independent physician.
Large-Scale Production of ADHLSC
ADHLSCs were obtained after primary culture of cryopreserved/thawed liver cells previously isolated from the right lobe of a healthy cadaveric liver donor, an 11-year-old male. Emerging ADHLSCs become predominant after the second passage according to our previous documented observations (Najimi et al. 2007; Scheers et al. 2012). ADHLSCs were expanded in cell-stack 10 chambers to reach 15 cumulative population doublings after 6 passages.
The purity of the ADHLSC culture was investigated at the morphology, the hepato-mesenchymal phenotype (Albumin+, alpha smooth muscle actin (α-SMA+) and the absence of epithelial markers such as cytokeratins (CK) 7, 8, 18, and 19 as well as CD133 using immunocytochemistry, RT-PCR, and flow cytometry.
The hepatogenic differentiation potential was also evaluated as described elsewhere (Khuu et al. 2010; Khuu et al. 2012). The differentiated cells were analyzed at the morphological, genetic, and functional levels (Khuu et al. 2010; Najimi et al. 2007). The quality of cell suspensions recovered was also checked at the safety level. Cell cultures were analyzed at all passages and no bacterial or virus (EBV, CMV, HBV, and HCV) contamination was detected. In addition, no cytogenetic abnormalities were detected in ADHLSC cultures at passage 7 (end of large-scale production). No tumor formation was observed 24 weeks after subcutaneous injection to nude mice (Scheers et al. 2012).
Cryopreservation
Cells were resuspended in solution of 90% fetal bovine serum and 10% DMSO. Cell suspension concentration was adjusted to 2.4 million cells/mL. Cell suspension was cryopreserved using a programmed freezer equipment (Cryoson) using a stepwise decrease in temperature (1°C/min until −40°C and then 2°C/min until −90°C). While reaching −90°C, cells were transferred into liquid nitrogen tank.
Thawing and Formulation Before Infusion
Cells were thawed at 37°C for a few minutes and washed with thawing solution (human albumin 5%, glucose 5 g/100 mL, bicarbonate 84 mg/mL, and heparin 10 UI/mL) at a dilution of 1/10. Cells were washed and centrifuged twice before counting and viability evaluation. Cells were resuspended in infusion solution (thawing solution with N-AcetylCystein 10 mM), at a concentration of 10 millions of cells/mL in 50 mL syringe. Viability of cells after thawing was always above 85% using trypan blue exclusion assay.
Adult Derived Human Liver Stem Cells Infusion
ADHLSCs were obtained and expanded in 2009 from liver cell batches previously isolated and cryopreserved in 2007 (e.g., 11-year-old male donor). ADHLSCs were infused through a percutaneous intraportal catheter for a total of 0.9 billion cells (this corresponds to 60 million ADHLSCs per kg body weight, i.e., 0. 75% of the theoretical liver hepatocyte mass) (14).
In May 2009, two first infusions of respectively 262 millions (viability 89%) and 230 millions (viability 90%) of cryopreserved ADHLSCs were performed under general anesthesia (concentration 10 × 106/mL, infusion rate 1 mL/min). A transcutaneous catheter was placed in the main portal vein under fluoroscopy and ultrasound guidance as previously described (5).
Immunosuppression regimen remained identical, using tacrolimus monotherapy to reach trough levels of 6–8 ng/mL. Prophylactic antibiotherapy with cefazolin (40 mg/kg) was given immediately prior, 8, and 16 h post infusion. The infusion and post-infusion course were unremarkable and the child was discharged from hospital on day three post infusion.
The second infusion occurred 2 weeks later (June 2009) with freshly trypsinized ADHLSC (430 millions of cells, viability 98%) at same concentration and infusion rate as for the first session. Liver Doppler ultrasounds were performed before and after each cell infusion.
Biochemical Monitoring
The ammonia level in plasma was measured by using ammonia reagent by a timed endpoint method (Synchron LX system, Fullerton, CA) (nl < 125 μg/dL).
The glutamine measurement was performed by ion exchange chromatography and ninhydrin detection (Jeol Aminotac analyzer).
Liver Biopsies: Fluorescence In Situ Hybridization
Liver biopsies were performed before ADHLSC infusion and three and half months after the infusions. FISH immunostaining was performed for the Y chromosome on liver-biopsy fingerprints mounted on slides. Transplanted male cells were detected by using a probe specific to the SRY gene labeled with a SpectrumOrange, with a CEP X SpectrumGreen control probe. Hybridized slides were examined using either a DMRB microscope or an axioplan 2 microscope equipped with a single-by-pass filter for excitation of DAPI, FITC, and rhodamine; double-by-pass filters for excitation of FITC/rhodamine and triple-by-pass filters for excitation of DAPI/FITC/rhodamine. Images were captured by using a Photometrics camera and processed by Isis software. For each hybridization, 200 cells in interphase were analyzed.
Statistical Analyses
Three different 6-month treatment phases have been defined:
Baseline (BSL): within 6 months before start of hepatocytes infusion
Post-hepatocytes (Post-Hep): over 6 months after end of hepatocytes infusion
Post-cells: over 6 months after infusion with progenitor cells
Within each of these treatment phases, two different periods of interest have been considered: from 0 to 3 months and from 3 to 6 months.
Only data collected during these treatment phases have been included in the statistical analysis. To account for their lognormal distribution, glutamine, ammonia, and orotic acid concentrations have been log-transformed (Ln) prior statistical analysis. The Ln-transformed levels have been statistically analyzed using a two-way analysis of variance, with the phase, the period, and the interaction between the phase and the period included as fixed factors in the model.
Specific contrasts have been used to derive estimates (with 95% confidence interval) for each treatment period of interest and for the following comparisons:
Post-Hep vs. Baseline
Post-Cells vs. Baseline
Post-Cells vs. Post-Hep (0–6 months)
Post-Cells vs. Post-Hep (0–3 months)
Post-Cells vs. Post-Hep (3–6 months)
Results
ADHLSC Engraftment and Clinical Outcome
Prior to the first infusion of ADHLSC, (9 months after the last hepatocyte infusion), a biopsy was performed to evaluate the engraftment of previously received male hepatocytes using Fluorescent in situ hybridization (FISH) technique for the Y chromosome (male donor and female receiver). No Y-chromosome-positive cells were detected in the recovered biopsy. Three and half months after both infusions of ADHLSCs, two percutaneous biopsies were performed in two separate areas of the right liver lobe to evaluate cell engraftment. Demonstration was made of the presence of 3% of male donor cells in the first biopsy and 5% in the second biopsy, while no Y positive cells were found in the basal biopsy. Taking into account the quantity of infused cells (0.9 × 109), the average 4% chimerism, the theoretical liver cell mass of the patient (15 kg × 5 × 109/kg = 75 × 109) (Fox et al. 1998), and the homogeneous liver distribution as shown in other patients (data not shown), this would mean an absolute quantity of donor cells reaching 3 × 109 cells, i.e., 3.3 times the quantity of cells infused. This theoretical calculation suggests a proliferation and liver repopulation capacity of ADHLSC.
The serum transaminases and bilirubin levels during the immediate post infusion period and in the long-term follow-up stayed within normal laboratory values.
The 8-month follow-up period was characterized by temporary recovery from disease related anorexia and improvement of the child’s overall condition as evaluated by the parents, with less frequent hospital admissions (see below). The psychomotor evolution was however not evaluated by behavioral or intellectual score assessments.
Metabolic Control After Infusion
The data obtained for each treatment phase and for each period of interest are presented in Figs. 1, 2, 3, and 4 and are summarized in Table 1.
Fig. 1.
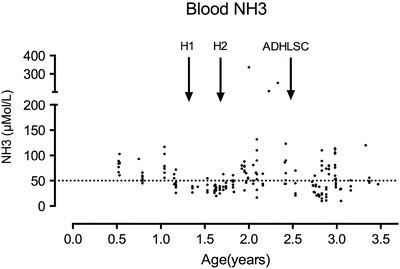
Ammonium blood levels at different points of the evolution. Normal value < 50 μMol/L (dotted horizontal line). H1 and H2 represent the hepatocyte infusions and ADHLSC the ADHLSC infusion
Fig. 2.
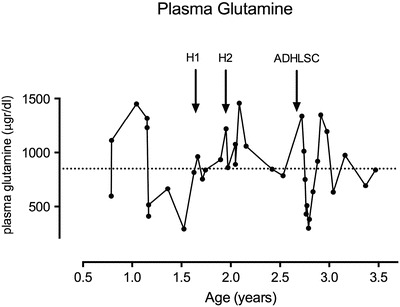
Plasma glutamine levels at different time points of the evolution. Normal values: 250–850 μg/dL (dotted horizontal line = maximal value). H1 and H2 represent the hepatocyte infusions and ADHLSC the ADHLSC infusion
Fig. 3.
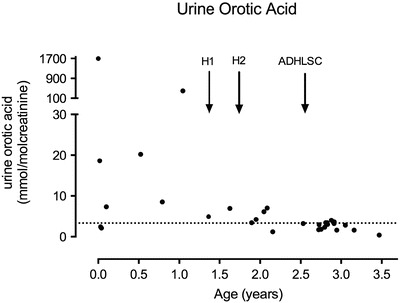
Urine orotic acid excretion at different time points of the evolution. Normal value: <3.3 mmol/mol creatinine (dotted horizontal line). H1 and H2 represent the hepatocyte infusions and ADHLSC the ADHLSC infusion
Fig. 4.
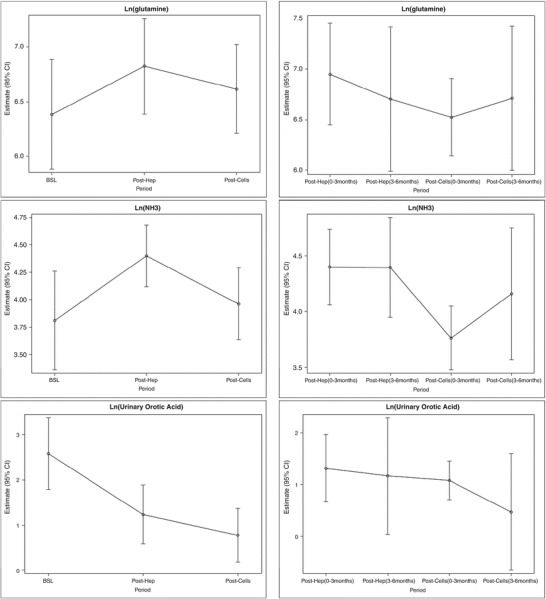
Estimates of Ln(glutamine), Ln(ammoniac), and Ln(orotic acid) levels at each treatment period. Symbols are the mean estimates, errors bars represent the 95% confidence interval Left: 6-month period; Right: over 0–3 and 3–6-month period. Top: Ln(glutamine); Middle: Ln(ammoniac); Bottom: Ln(orotic acid)
Table 1.
Statistical analysis and comparisons of Ln(glutamine), Ln(ammoniac), and Ln(orotic acid) at each treatment period
| Treatment period | Ln(glutamine) | Ln(ammoniac) | Ln(orotic acid) | |||
|---|---|---|---|---|---|---|
| 0–6 months | ||||||
| BSL | 6.38 (5.88–6.89) | 3.81 (3.36–4.26) | 2.59 (1.79–3.38) | |||
| Post-Hep | 6.83 (6.39–7.26) | 4.40 (4.12–4.68) | # | 1.24 (0.59–1.89) | # | |
| Post-Cells | 6.62 (6.21–7.02) | 3.96 (3.63–4.29) | § | 0.77 (0.18–1.37) | ## | |
| 0–3 months | ||||||
| Post-Hep | 6.95 (6.44–7.46) | 4.40 (4.06–4.74) | 1.31 (0.66–1.96) | |||
| Post-Cells | 6.52 (6.14–6.91) | 3.76 (3.48–4.05) | §§ | 1.08 (0.7–1.45) | ||
| 3–6 months | ||||||
| Post-Hep | 6.70 (5.99–7.42) | 4.40 (3.95–4.85) | 1.16 (0.04–2.29) | |||
| Post-Cells | 6.71 (6.00–7.43) | 4.16 (3.57–4.75) | 0.47 (−0.65–1.59) | |||
Values are mean estimates (95% CI) #/##: significantly different from baseline (#: p < 0.05, ##: p < 0.01) §/§§: significantly different from Post-Hep (§: p < 0.05, §§: p < 0.01)
Glutamine levels were not significantly influenced by hepatocytes or ADHLSC infusion.
Ammonia levels were significantly increased after hepatocyte infusion. After ADHLSC infusion, ammonia levels decreased to levels close to baseline, with a significant decrease compared to levels observed after hepatocyte transfusion (Figs. 1 and 4).
Compared to baseline, orotic acid levels were significantly lower after hepatocyte and ADHLSC infusions, without significant differences between both interventional procedures (Figs. 3 and 4).
The number of decompensations was multiple before ADHLSC infusions with four admissions in the 6-month period preceding hepatocyte infusions and three additional severe decompensations between hepatocyte transfusion and ADHLSC infusion (6 months, 12 and 16 months after hepatocytes transfusion, NH3 > 300 μmol/L). After ADHLSC infusions, one mild decompensation occurred at 6 months of the procedure (NH3 100 μmol/L) and a second mild to moderate decompensation at 8 months (NH3 120 μmol/L). The protein content of the diet remained at 1 g/Kg/day (mostly aminoacid mixture). Urea cycle disorder (UCD)-medications were unchanged. In addition, the patient was under tacrolimus therapy at a dosage adapted to reach a level of 5–6 ng/mL.
The follow-up was limited in time, as immunosuppression was stopped 6 months after the ADHLSC infusion, by fear of viral infection during the H1N1 flu epidemic. There was no more evidence of cell effect, and a liver transplantation was proposed 3 months later. The child unfortunately died from the procedure.
Pro-coagulant Effects of ADHLSC
At the end of the second infusion of ADHLSC, sudden increase of portal vein pressure occurred and partial thrombosis of the left intrahepatic portal vein branch was observed by Doppler ultrasound, leading to arrest of infusion. This adverse event was treated by low molecular weight heparin (FraxiparinR, Glaxo Smith Kline, Genval, Belgium) and coumarinic anticoagulant (SintromR, Novartis, Vilvoorde, Belgium), 5 mg/day for 30 days. D-Dimers were markedly elevated after both cell infusion courses, confirming the procoagulant effect of the cell preparation. This adverse event did not lead to any consequence for the patient but encouraged our team to investigate further the procoagulant effect of the progenitor cells and adapt the anticoagulation protocol accordingly.
Discussion
This is the first report demonstrating engraftment of an advanced therapy medicinal product after intraportal transplantation in human. The theoretical calculation tended to show that cells had repopulated the recipient liver, as the percentage of donor cells in two liver biopsies taken 3 months after the infusion, extrapolated to the entire liver, would correspond to a threefold increase of the original transplanted cell number. Such interpretation must be taken cautiously, as sampling error may play a role.
The first attempt to treat the child using cryopreserved hepatocyte has not been clinically successful in improving the child condition, and there was even a trend toward higher ammonium values following hepatocyte infusions.
Following ADHLSC infusion, there was some indication of clinical improvement (parental reporting, decompensation episodes with milder level of NH3). However, we were not able to follow the further metabolic data as immunosuppression was stopped after 6 months; the child was thereafter liver transplanted and died from complications of this procedure.
Thus, we are unable to conclude on the long-term clinical efficacy of the therapy, but overall, the data suggest that cells had engrafted and started to expand and/or to differentiate to a more protective extent. Indeed, patients with neonatal onset OTC are well known to be extremely prone to sudden fatal decompensations, and in the absence of successful liver transplantation, their survival is less than 10% beyond age 5 years (Kido et al. 2012; Uchino et al. 1998). Thus, it is possible that, if immunosuppression had been maintained and in absence of liver transplantation, ADHLSC infusion would have helped to prolong the life of this patient long enough to allow for greater repopulation by the engrafted cells, perhaps as early as during the so-called early “honeymoon” period of the disease (Leonard 2001; Ensenauer et al. 2005).
Markers, such as NH3 and glutamine which represents the gold standard of diagnostic and follow-up studies on UCD disorders, may be unsuitable to detect early limited improvements of potential clinical relevance. We stress the need for longer follow-up and of composite end points to explore more finely any behavioral, dietary, and biochemical effects. The improvement of urea cycle function in vivo could be explored in the future by measurement of C13 incorporated into urea, following C13 Na acetate administration (Yudkoff et al. 2010).
As hepatocyte, islets, and other mesenchymal stromal cells (MSCs) do, the ADHLSCs express tissue factor and induce the coagulation cascade. This was most probably responsible for the partial left portal vein thrombosis observed during the second infusion. This led our group to conduct studies on the procoagulant properties of the ADHLSC and to identify the specific combination of anticoagulant that could prevent thrombosis. In vitro investigation using clotting factor analysis and thromboelastography confirmed the procoagulant effect of both hepatocytes and progenitor cells. However, hepatocyte procoagulant effect can be inhibited by heparin and only partly for the progenitor cells. The mechanisms of procoagulant effect were identified and a specific anticoagulant cocktail containing heparin (10 UI/mL) and bivalirudin (0.75 mg/kg) was developed and submitted for patent application. The use of this anticoagulant combination in successive patients confirmed safety and inhibition of the procoagulant effect of the cells (Stephenne et al. 2012).
In metabolic diseases, cell therapy requires the use of allogeneic cells and hence immunosuppression. ADHLSCs are of mesenchymal phenotype, and like MSCs of other origins, exhibit immunotolerogenic properties (Le Blanc et al. 2008). Trials are being conducted in various autoimmune diseases, with the aim to modulate the patient immune system. We detected similar features in vitro for the liver-derived MSCs, which can block an ongoing mixed lymphocyte reaction (Sana et al. 2013). However, it is not clear whether any such immune suppressive features would persist once the cells engraft and differentiate. For this reason, immunosuppression must be given to the patient, and in our case, we choose to use the same immunosuppression protocol that is widely used in pediatric liver transplantation.
We conclude that liver-based regenerative medicine using AHDLSC is feasible, and that it can lead to a significant engraftment and parenchymal repopulation at medium term after portal infusion. Although the limited follow-up of the patient did not enable us to draw any firm conclusion on the clinical evolution, the histological data provide a very promising proof of concept that ADHLSC therapy is a potential treatment. Urea cycle disease is one of the most desired targets, but composite end points must be further developed to better assess the efficacy and benefits for the patients. Ongoing research is about to bring new tools to improve the level of engraftment.
Our first-in-man study is another example that stem cell–based regenerative medicine is now coming to clinical application, and EMA approved phase I/II clinical trials are currently being conducted using this technology (clinicaltrial.gov identifier NCT01765283), which will allow to evaluate both safety and efficacy of this treatment.
Take-Home Message
Liver stem cells infused in the portal vein engraft and repopulate the liver in an OTC patient.
Compliance with Guidelines
This cell transplantation protocol was approved by the ethical committee of the institution and informed consent was given to and received from the parents by an independent physician.
Conflict of Interest
Etienne Sokal declares to own patent rights on ADHLSC, currently in clinical development plan (clinicaltrial.gov identifier NCT01765283) by Promethera Biosciences, spinoff of the laboratory of pediatric hepatology and cell therapy. ES Sokal is consultant CSO for Promethera Biosciences.
Xavier Stephenne declares that he has no conflict of interest.
Chris Ottolenghi declares no conflict of interest.
Nawal Jazouli declares no conflict of interest.
Florence Lacaille declares no conflict of interest.
Mustapha Najimi declares to own patent rights on ADHLSC, currently in clinical development (clinicaltrial.gov identifier NCT01765283). MN is consultant for Promethera Biosciences.
Pascale de Lonlay declares no conflict of interest.
Françoise Smets declares that she is currently principal investigator of a clinical trial (clinicaltrial.gov identifier NCT01765283).
Animal Rights: All preclinical animal studies done prior to the reported work were approved by the Institution Animal Ethical Committee.
Informed consent of the patient was obtained from her legal representative, and the protocol and informed consent was approved by the Université Catholique de Louvain & Cliniques St Luc Ethical committee. An ethical committee independent delegate explained the study to the patient and her legal representative.
Footnotes
Competing interests: None declared
Contributor Information
Etienne M Sokal, Email: Etienne.sokal@uclouvain.be.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med. 2007;356:2282–2292. doi: 10.1056/NEJMoa066596. [DOI] [PubMed] [Google Scholar]
- Ensenauer R, Tuchman M, El-Youssef M, Kotagal S, Ishitani MB, Matern D, Babovic-Vuksanovic D. Management and outcome of neonatal-onset ornithine transcarbamylase deficiency following liver transplantation at 60 days of life. Mol Genet Metab. 2005;84:363–366. doi: 10.1016/j.ymgme.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201–223. doi: 10.1111/j.1365-2796.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- Khuu DN, Scheers I, Ehnert S, et al. In vitro differentiated adult human liver progenitor cells display mature hepatic metabolic functions: a potential tool for in vitro pharmacotoxicological testing. Cell Transplant. 2010;20(2):287–302. doi: 10.3727/096368910X516655. [DOI] [PubMed] [Google Scholar]
- Khuu DN, Nyabi O, Maerckx C, Sokal E, Najimi M. Adult human liver mesenchymal stem/progenitor cells participate to mouse liver regeneration after hepatectomy. Cell Transplant. 2012;22(8):1369–1380. doi: 10.3727/096368912X659853. [DOI] [PubMed] [Google Scholar]
- Kido J, Nakamura K, Mitsubuchi H, et al. Long-term outcome and intervention of urea cycle disorders in Japan. J Inherit Metab Dis. 2012;35:777–785. doi: 10.1007/s10545-011-9427-0. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Leonard JV. The nutritional management of urea cycle disorders. J Pediatr. 2001;138:S40–S44. doi: 10.1067/mpd.2001.111835. [DOI] [PubMed] [Google Scholar]
- Meyburg J, Hoffmann GF. Liver, liver cell and stem cell transplantation for the treatment of urea cycle defects. Mol Genet Metab. 2010;100(Suppl 1):S77–S83. doi: 10.1016/j.ymgme.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007;16:717–728. doi: 10.3727/000000007783465154. [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Héron B, Touati G, Rabier D, Saudubray JM. Urea cycle defects: management and outcome. J Inherit Metab Dis. 2005;28:407–414. doi: 10.1007/s10545-005-0303-7. [DOI] [PubMed] [Google Scholar]
- Nussler A, Konig S, Ott M, et al. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006;45:144–159. doi: 10.1016/j.jhep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Sana G, Lombard C, Vosters et al (2013) Adult human hepatocytes promote CD4+ T cell hyporesponsiveness via interleukin-10 producing allogeneic dendritic cells. Cell Transplant [DOI] [PubMed]
- Sancho-Bru P, Najimi M, Caruso M, et al. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut. 2009;58:594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]
- Scheers I, Maerckx C, Khuu DN, Marcelle S, Decottignies A, Najimi M, Sokal E. Adult derived human liver progenitor cells in long term culture maintain appropriate gatekeeper mechanisms against transformation. Cell Transplant. 2012;21(10):2241–2255. doi: 10.3727/096368912X639026. [DOI] [PubMed] [Google Scholar]
- Smets F, Najimi M, Sokal EM. Cell transplantation in the treatment of liver diseases. Pediatr Transplant. 2008;12:6–13. doi: 10.1111/j.1399-3046.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- Sokal EM, Smets F, Bourgois A, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- Stéphenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058–2061. doi: 10.1111/j.1600-6143.2005.00935.x. [DOI] [PubMed] [Google Scholar]
- Stéphenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Stephenne X, Nicastro E, Eeckhoudt S, et al. Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS One. 2012;7:e42819. doi: 10.1371/journal.pone.0042819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino T, Endo F, Matsuda I. Neurodevelopmental outcome of long-term therapy of urea cycle disorders in Japan. J Inherit Metab Dis. 1998;21(Suppl 1):151–159. doi: 10.1023/A:1005374027693. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Ah Mew N, Daikhin Y, et al. Measuring in vivo ureagenesis with stable isotopes. Mol Genet Metab. 2010;100(Suppl 1):S37–S41. doi: 10.1016/j.ymgme.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


