Abstract
Background
Essential tremor is regarded to be a disease of the central nervous system. Neuroimaging is a rapidly growing field with potential benefits to both diagnostics and research. The exact role of imaging techniques with respect to essential tremor in research and clinical practice is not clear. A systematic review of the different imaging techniques in essential tremor is lacking in the literature.
Methods
We performed a systematic literature search combining the terms essential tremor and familial tremor with the following keywords: imaging, MRI, VBM, DWI, fMRI, PET and SPECT, both in abbreviated form as well as in full form. We summarize and discuss the quality and the external validity of each study and place the results in the context of existing knowledge regarding the pathophysiology of essential tremor.
Results
A total of 48 neuroimaging studies met our search criteria, roughly divided into 19 structural and 29 functional and metabolic studies. The quality of the studies varied, especially concerning inclusion criteria. Functional imaging studies indicated cerebellar hyperactivity during rest and during tremor. The studies also pointed to the involvement of the thalamus, the inferior olive and the red nucleus. Structural studies showed less consistent results.
Discussion and conclusion
Neuroimaging techniques in essential tremor give insight into the pathophysiology of essential tremor indicating the involvement of the cerebellum as the most consistent finding. GABAergic dysfunction might be a major premise in the pathophysiological hypotheses. Inconsistencies between studies can be partly explained by the inclusion of heterogeneous patient groups. Improvement of scientific research requires more stringent inclusion criteria and application of advanced analysis techniques. Also, the use of multimodal neuroimaging techniques is a promising development in movement disorders research. Currently, the role of imaging techniques in essential tremor in daily clinical practice is limited.
Keywords: Essential tremor, Neuroimaging, Review, MRI, Scintigraphy, Pathophysiology
Abbreviations: MRI, magnetic resonance imaging; VBM, voxel-based morphometry; DWI, diffusion weighted imaging; fMRI, functional magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography
Highlights
-
•
We conducted a systematic review of neuroimaging studies in essential tremor.
-
•
Cerebellar involvement is the most consistent finding.
-
•
GABAergic dysfunction is worthwhile investigating more intensively.
-
•
We encourage multimodal neuroimaging focussing on brain networks.
1. Introduction
Amongst the movement disorders, essential tremor is one of the most prevalent disorders. Up to 5% of individuals above the age of 65 years are coping with essential tremor (Louis and Ferreira, 2010). Clinical diagnostic criteria have been developed over the years. The exact clinical definition of essential tremor is however still under debate. Current clinical diagnosis based on the consensus statement of the Movement Disorder Society typically has an estimated error margin of 37% of false-positives (Jain et al., 2006). Neuroimaging techniques could potentially lower the margin of error in diagnostics by gaining insight into underlying brain pathology and could ultimately be used as a valid diagnostic tool.
The pathophysiology of essential tremor is only partially understood. Thus far, surgical, post-mortem, neurophysiological and animal studies point to the involvement of the inferior olive, the cerebellum, the red nucleus, the thalamus, the cortex and their neurotransmitter systems. These areas make up a network known as the cerebello-thalamo-cortical network or tremor network (Fig. 1) (Hallett, 2014). Olivary afferents travel through the inferior cerebellar peduncle to end in cerebellar nuclei or form synapses with GABAergic inhibitory Purkinje cells in the cerebellar cortex. Purkinje cells send inhibitory projections to the deep cerebellar nuclei including the dentate nucleus. Cerebellar nuclei project via the thalamus to the cerebral cortex, yet other projections end in the red nucleus. The exact mechanisms and possible structural or functional changes within the tremor network are not fully understood. There is an ongoing debate whether essential tremor is primarily (1) a neurodegenerative disorder with actual progressive cell loss, (2) a disorder with localized GABAergic dysfunction, or (3) a disorder caused by abnormal neuronal oscillations within the tremor network (Bonuccelli, 2012; Deuschl and Elble, 2009; Helmich et al., 2013; Louis, 2009; Rajput et al., 2012a). These hypotheses are not mutually exclusive per se. Neuroimaging techniques might give insight into these three and even other concepts concerning the pathophysiology of essential tremor. Neuroimaging is a rapidly developing field. A variety of imaging studies have been performed in essential tremor patients over the past decade. Our aim is to systematically review these neuroimaging studies in essential tremor and discuss the pathophysiology of essential tremor from a neuroimaging perspective.
Fig. 1.
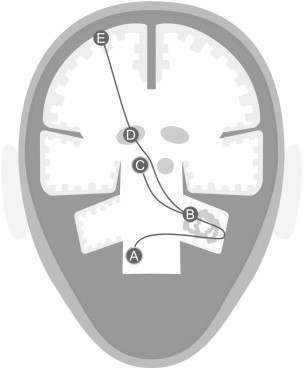
Tremor network: A) inferior olive; B) dentate nucleus; C) red nucleus; D) tha- lamus; E) motor cortex.
2. Methods
We queried the OvidSP Embase Classic+Embase and the Ovid MEDLINE® In-Process & Other Non-Indexed Citations from January 1st, 1947 to August 5th, 2013 using the terms ‘essential tremor’ and ‘familial tremor’ (Appendix A) in combination with the imaging keywords and their abbreviations stated in Table 1.
Table 1.
Keywords and abbreviations used in the literature search
| Keyword | Abbreviation |
|---|---|
| Magnetic Resonance Imaging | MRI |
| Voxel-Based Morphometry | VBM |
| Diffusion Weighted Imaging | DWI |
| Functional Magnetic Resonance Imaging | fMRI |
| Positron Emission Tomography | PET |
| Single-Photon Emission Computed Tomography | SPECT |
| Magnetic Resonance | - |
| Tomography | - |
| Scintigraphic Imaging | - |
Only original English-written articles that recruited both essential tremor patients and healthy controls were included. We identified brain regions and networks associated with essential tremor. Our main research questions for our systematic search were as follows: (1) are results of imaging studies congruent with areas within the known tremor network and valid in the scope of their imaging technique? (2) do the imaging studies help better understand the different hypotheses on the pathophysiology (neurodegeneration, GABA, oscillating network)? and (3) what recommendations can be set for future imaging research arising from current literature?
3. Results
A total of 375 abstracts of imaging studies were identified. We excluded abstracts in case they did not address a neuroimaging technique of interest or had deep brain stimulation as main topic. We also excluded studies that did not include patients with postural tremor and studies without a group of healthy controls. A total of 48 imaging studies met our inclusion criteria which account for a total of 713 essential tremor patients divided over 19 structural studies and 29 functional and receptor imaging studies (Fig. 2). Structural techniques include volumetry, white matter diffusion imaging, magnetic resonance spectroscopy, T2-FLAIR and T2*-relaxometry. Functional and molecular imaging methods include perfusion, glucose metabolism and receptor imaging.
Fig. 2.
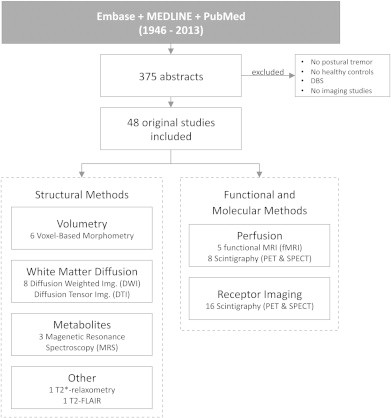
Included imaging studies are divided into structural methods and functional and molecular methods.
3.1. Structural imaging in essential tremor
Structural MRI, as used in current clinical practice, does not reveal significant abnormalities in individual essential tremor patients. Pathological studies however do indicate structural changes (Louis, 2010). More advanced imaging techniques in (sub)groups of patients might be able to reveal structural abnormalities in different brain regions indicative of neurodegenerative changes.
3.1.1. Voxel-based morphometry (VBM)
VBM is able to classify and quantify white and grey matter using MRI. It therefore allows analysis of local and total brain volume differences between patient groups (Whitwell and Josephs, 2007).
Six papers used VBM to investigate changes in white and grey matter in essential tremor patients compared to healthy controls (Table 2) (Bagepally et al., 2012; Benito-Leon et al., 2009; Cerasa et al., 2009; Daniels et al., 2006; Lin et al., 2013; Quattrone et al., 2008). Two studies did not show any differences after performing whole brain analysis with a 1.5T MRI in a total of 77 essential tremor patients and 59 healthy controls (Cerasa et al., 2009; Daniels et al., 2006; Quattrone et al., 2008). One of these studies reported grey matter volume reduction of the cerebellar vermis in a subgroup with presence of head tremor (n = 20) compared to healthy controls, but reported no differences between patients with and without (n = 30) head tremor (Cerasa et al., 2009; Quattrone et al., 2008). The second study investigated essential tremor by subdividing patients into those with only postural tremor (n = 14) and those with additional intention tremor (n = 13). Patients with predominant intention tremor showed compared to healthy controls an increase in grey matter in the temporoparietal regions, in the right middle occipital cortex, and in the higher order visuospatial processing areas (Daniels et al., 2006).
Table 2.
Voxel-based morphometry (VBM).
| Clinical features |
Imaging |
||||||
|---|---|---|---|---|---|---|---|
| ET and controls, age (SD) | ET | Cri | Results ET vs HC | Data analysis | |||
| Daniels et al. (2006), | 14 ET–PT 54.1(13.1) | 14 HC 51.1(14.6) | UE PT, UE IT | D | – | – | Whole brain, GM, WM |
| 1.5T | 13 ET–IT 61.8(10.3) | 13 HC 64.7(7.0) | ↑ | GM ET–IT: temporoparietal junction, unilateral occipital cortex | Pcor < 0.05 | ||
| Quattrone et al. (2008), | 30 ET-a 61.5(16.5) | 32 HC 66.2(8.1) | UE, head | D | – | – | Whole brain GM; ROI 3 lobules cerebellum |
| and Cerasa et al. (2009), 1.5T | 20 ET-h 70.6(7.6) | ↓ | GM h-ET: cerebellar anterior lobe and vermis | Pcor < 0.001 | |||
| Bagepally et al. (2012), 3T | 10 ET 53.1(12.7) 10 ET-h 26.5(5.5) |
17 HC 40.7(16.5) | UE, LE, head, voice |
D | ↓ | GM ET bilateral cerebellum hemispheres, vermis, frontal lobes, occipital lobes | Whole brain, GM, WM, ICV Punc < 0.001 |
| Benito-Leon et al. (2009), 3T | 19 ET 69.8(9.4) | 20 HC 68.9(10.0) | UE, head | Ls | ↓↑ | WM right cerebellum, left medulla, right parietal lobe, right limbic lobe |
Whole brain GM, WM Punc < 0.001 |
| ↓↑ | GM bilaterally cerebellum, bilateral parietal lobes, right frontal lobe, right insula |
||||||
| Lin et al. (2013), 3T | 10 ET 63.4(8.7) | 13 HC 65.3(11.1) | ? | – | ↓ | Caudate, middle temporal pole, insula, precuneus, STG | Whole brain GM (basic and DARTEL) |
| 10 PD 67.3(8.8) | ↑ | Middle temporal gyrus, precentral gyrus | Punc < 0.05 | ||||
a, arm tremor; BG, basal ganglia; cor, multiple comparison corrected; Cri, criteria; D, Deuschl et al. (1998); ET, essential tremor; GM, grey matter; h(ead), head tremor; HC, healthy controls; ICV, intracranial volume; IT, intention tremor; LE, lower extremity; Ls, Louis et al. (2001); Pcor, multiple comparison correction; PD, Parkinson's disease; PT, postural tremor; SD, standard deviation; ROI, region of interest; STG, superior temporal gyrus; UE, upper extremity; Punc, multiple comparison uncorrected; voice, voice tremor; vs, versus; WM, white matter.
The three most recent studies used 3T MRI, enabling higher spatial resolution compared to 1.5T MRI (Bagepally et al., 2012; Benito-Leon et al., 2009; Lin et al., 2013). In a total of 49 patients widespread white and grey matter changes in cerebellar and cerebral areas were found. In one of these studies, a post-hoc analysis showed no cerebellar differences but instead found widespread cerebral grey matter reduction in patients with head tremor (n = 10, age: 53.1 ± 15.5 years) compared to patients without head tremor (n = 10, age: 26.5 ± 5.5 years) (Bagepally et al., 2012).
In short, one of the six VBM studies revealed isolated volume reduction of the cerebellar vermis in a subgroup of essential tremor patients with head tremor (Quattrone et al., 2008). Other studies showed widespread cerebellar and/or cerebral abnormalities. The interpretation of such volume reductions remains uncertain. For example, volume reduction could be interpreted as regional atrophy in neurodegenerative disorders, but especially grey matter volume also strongly depends on age, training and cognitive functioning.
3.1.2. Diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI)
DWI is an MRI technique in which the image intensity of a voxel reflects the rate of water diffusion at that location, expressed as apparent diffusion coefficient (ADC) or mean diffusivity (MD) (Bammer and Fazekas, 2003). Diffusion tensor imaging (DTI) can be used to measure diffusion magnitude and to determine axonal direction in the central nervous system. DTI captures the amount of anisotropy of the water diffusion expressed as fractional anisotropy (FA) (Basser and Pierpaoli, 1996). DWI and DTI give an impression of axonal organization and might help to disentangle neuronal circuitries. In pathology–imaging correlations, a reduced FA is accompanied with an increased MD when axon and myelin density are affected (Moll et al., 2011). In essential tremor, based on the theory of white matter pathology in the cerebellum axonal organization could be disrupted leading to increased diffusion parameters (MD or ADC) and decreased directional diffusion (FA).
A total of eight diffusion studies in essential tremor were found. Three studies performed whole brain voxel-by-voxel comparison (Table 3) (Klein et al., 2011; Saini et al., 2012; Shin et al., 2008); the other studies used regions of interest (ROIs) involved in the tremor network. White matter pathology was determined in six studies with a total of 98 essential tremor patients and 92 healthy controls (Jia et al., 2011; Klein et al., 2011; Nicoletti et al., 2010; Prodoehl et al., 2013; Saini et al., 2012; Shin et al., 2008). Four studies investigating the cerebellum found either an increase in water diffusion (MD, ADC) or a decrease in preferential direction of diffusion (FA) in the inferior cerebellar peduncle, superior cerebellar peduncle and/or dentate nucleus (Klein et al., 2011; Nicoletti et al., 2010; Saini et al., 2012; Shin et al., 2008). Other regions mentioned were the pons (Saini et al., 2012; Shin et al., 2008), thalamus (Saini et al., 2012) and red nucleus (Jia et al., 2011; Shin et al., 2008). Nicoletti and colleagues divided essential tremor groups into short (<20 years) and long disease duration in order to relate clinical characteristics to diffusion parameters. FA mean values in the dentate nucleus were lower in patients with longer disease duration, however no linear correlation was observed in the whole group. Saini and colleagues did not find any significant correlations between diffusion measures and clinical characteristics (e.g. severity or duration of disease). Two studies investigated the tissue integrity of the basal ganglia, but neither observed significant differences (Jia et al., 2011; Martinelli et al., 2007). Moreover, two studies did not find differences in the cerebellum between essential tremor patients and healthy controls (Buijink et al., 2013; Martinelli et al., 2007). The absence of abnormalities in such studies could be due to their relatively small sample sizes, selection of ROIs (Buijink et al., 2013) and/or relatively short disease duration (median < 6 years) (Martinelli et al., 2007). Applying voxel-by-voxel analyses (Klein et al., 2011; Saini et al., 2012; Shin et al., 2008), widespread deviations were detected, not only in motor areas but also in non-motor areas like the frontal and temporoparietal areas. Prodoehl and colleagues suggested even the clinical use of diffusion imaging as they were able to differentiate between Parkinson's disease, atypical parkinsonism and essential tremor based on a combination of ROIs and their diffusion parameters (mainly basal ganglia and cerebellum) (Prodoehl et al., 2013).
Table 3.
Diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI).
| Clinical features |
Imaging |
|||||||
|---|---|---|---|---|---|---|---|---|
| ET and controls, age (SD) | ET | Cri | Results ET vs HC | Parameter | Data analysis | |||
| Martinelli et al. (2007), 1.5T DWI | 10 ET 66(11) | 10 HC 60(8) | UE, voice, head |
D | – | – | ADC b = 300, 600, 900 s/mm2 |
ROI: pons, MCP, cerebellar WM, DN, red nucleus, thalamus, caudate, putamen, pallidum, frontal and precentral WM Punc < 0.05 |
| Shin et al., (2008), 1.5T DTI | 10 ET 52.8(11.5) | 8 HC 51.3(11.1) | UE, head |
Fi | ↓ | Bilateral cerebellum, right pons, left retrorubral area of the midbrain and bilateral deep WM | FA b = 1000 s/mm2 |
WM voxel wise Punc < 0.05 |
| Nicoletti et al. (2010), | 25 ET 62.9(9.5) | 15 PD 46.6(6.3) | UE | D | ↑ | MD: SCP | FA, MD | ROI: red nucleus, MCP, SCP, |
| 1.5T DTI | 15 HC 62.4(5.4) | ↓ | FA: DN, SCP | b = 900 s/mm2 | DN, cerebellar WM, ventrolateral thalamus Punc < 0.001 |
|||
| Klein et al. (2011), 3T DTI | 14 ET 61.2(12.0) | 20 HC 60.2(8.1) | UE | D | ↑ | ROI: MD bilateral ICP Voxel wise/TBSS: Widespread MD |
FA, MD b = 1000 s/mm2 |
ROI: MCP, SCP, ICP FWE Pcor < 0.05 |
| ↓ | ROI: FA unilateral ICP | Voxel wise, TBSS TFCE Pcor < 0.05 | ||||||
| Jia et al. (2011), 3T DTI | 15 ET 65.1(11.4) | 15 HC 62.1(7.6) | UE | Ls | ↑ | ADC: red nucleus | ADC, FA b = 800 s/mm2 |
ROI: caudate, putamen, globus pallidus, thalamus, red nucleus, substantia nigra |
| Buijink et al. (2013), 3T DTI | 8 ET 48.4(17.8) | 5 HC 42.8(14.4) 7 FCMTE 39(12.2) |
UE | Ba | – | – | FA, MD b = 700 s/mm2 |
ROI cerebellum P < 0.05 |
| Saini et al. (2012), 3T DTI | 20 ET 38.2(16.5) | 17 HC 40.7(16.5) | UE, head, voice |
D | ↑ | ROI AD: ALIC, bilateral SCP, right ICP, CS ROI MD: right ALIC, left CS TBSS MD, RD: right frontoparietal WM TBSS RD: right frontoparietal WM, internal/external capsule TBSS AD: cerebral WM, thalamus, brainstem, internal/external capsule, cerebellar peduncles |
MD, RD, AD, FA b = 800 s/mm2 |
ROI: Corpus callosum, SCP, MCP, ICP, CS, ALIC Punc < 0.05 Voxel wise TBSS Pcor < 0.05 |
| ↓ | ROI FA: left SCP, right CS | |||||||
| Prodoehl et al. (2013), 3T DTI | 14 ET 61.6(11.0) | 15 PD 62.7(7.7) 14 MSAp 64.3(8.9) 12 PSP 70.7(5.6) 17 HC 62.9(9.0) |
UE | D | Combining measures to differentiate between the groups* | FA, RD, LD, MD b = 1000 s/mm2 |
ROI: Caudate, putamen, GP, SN, RN, SCP, MCP, ICP, DN | |
AD, axial diffusivity; ADC, apparent diffusion coefficient; ALIC, anterior limb of internal capsule; Ba, Bain et al. (2000); b-value, scaling value for attenuating effect; cor, multiple comparison corrected; Cri, criteria; CS, corticospinal tract; D, Deuschl and Elble (2009); DN, dentate nucleus; FA, fractional anisotropy; FCMTE, familial cortical myoclonic tremor with epilepsy: (F)ET, (familial) essential tremor; Fi, Findley (1996); FWE, family wise error; GP, globus pallidus; HC, healthy controls; head, head tremor; LD, longitudinal diffusivity; Ls, Louis et al. (2001); MCP SCP ICP, middle/superior/inferior cerebellar peduncle; MD, mean diffusivity; MSAp, multiple system atrophy with prominent parkinsonian signs; PD, Parkinson's disease; PSP, progressive supranuclear palsy; RD, radial diffusivity; RN, red nucleus; ROI, region of interest; SD, standard deviation; sn, substantia nigra; TBSS, tract-based spatial statistics; TFCE, threshold-free cluster enhancement; UE, upper extremity; Punc, multiple comparison uncorrected; voice, voice tremor; vs, versus; WM, white matter. *Not included in fig. 2
In summary, six out of eight studies found differences between the patient group and the control group using DWI and DTI. The abnormalities were primarily found in the cerebellar peduncles involved in the information flux and secondarily in the red nucleus. These studies also observed numerous deviations in non-motor areas.
Diffusion parameters are influenced by the distribution of the afferent and efferent fibres in a ROI. Changes in diffusion parameters are associated with pathology but do not necessarily indicate structural axonal loss or demyelination (Jones et al., 2013). The DWI and DTI studies mentioned above did not identify a particular diffusion parameter to be most sensitive to detect altered diffusion in essential tremor.
3.1.3. Magnetic resonance spectroscopy (MRS)
MRS is based on the same physical principles as conventional MRI, although it measures regional magnetic field variation due to different magnetic characteristics of neurochemistry (Barker, 2010). The studies below use the ratio between N-acetylaspartate (NAA) and total choline (Cho) or creatine (Cr). A reduction of NAA, or reduced NAA ratio, is associated with neurodegenerative processes (Tran et al., 2009).
Three studies with a total of 40 patients investigated possible metabolic dysfunction in essential tremor with the help of the MRS technique (Table 4) (Kendi et al., 2005; Louis et al., 2002; Pagan et al., 2003). ROIs included the cerebellum, thalamus and basal ganglia. Two studies specifically investigating the cerebellum found a decrease in the NAA ratio in the cerebellar cortex compared to healthy controls (Louis et al., 2002; Pagan et al., 2003). The first study also detected an inverse association between cerebellar cortical NAA ratio and tremor severity (Louis et al., 2002) whereas the second study did not (Pagan et al., 2003). Pagan and colleagues ruled out severe cerebellar atrophy (Pagan et al., 2003). There were no differences in the thalamus and basal ganglia between patients and healthy controls; however one study investigated lateralization in the thalamus and showed a decrease in NAA ratio contralateral to the predominant tremor side (Kendi et al., 2005).
Table 4.
Magnetic resonance spectroscopy (MRS).
| Clinical features |
Imaging |
||||||
|---|---|---|---|---|---|---|---|
| ET and controls, age (SD) | ET | Cri | Results ET vs HC | Data analysis | |||
| Louis et al. (2002), 1.5T | 16 ET 66(18) | 11HC 60(24) | UE | Ls | ↓ | Cerebellar cortex | ROI: Cerebellar cortex, cerebellar WM, vermis, thalamus, basal ganglia NAA/Cr ratio Slice thickness 15 mm P unc < 0.05 |
| Pagan et al. (2003), 1.5T | 10 ET 59.4(18.7) | 10 HC 57.2(17) | UE | Ba | ↓ | Both cerebellar hemispheres |
ROI: Vermis midline, dentate nuclei, cerebellar hemispheres, red nuclei, thalami NAA/Cho ratio, NAA/Cr ratio Slice thickness 15 mm P cor < 0.05 |
| Kendi et al. (2005), 1.5T | 14 ET 38.6(12.8) | 9 HC 35.4(11.7) | UE | Ls | – | – | ROI: Single voxel ROI thalami NAA/Cr, Cho/Cr Slice thickness 13 mm P unc < 0.05 |
Ba, Bain et al. (2000); cor, multiple comparison corrected; Cho, choline; Cr, creatine; Cri, criteria; ET, essential tremor; HC, healthy controls; NAA, N-acetylaspartate; head, head tremor; Ls, Louis et al. (2001); Med: ?, medication status unknown; ROI, region of interest; SD, standard deviation; UE, upper extremity; Punc, multiple comparison uncorrected; VBM, voxel based morphometry; voice, voice tremor; vs, versus; WM, white matter.
NAA is here expressed as a NAA ratio with help of cerebellar Cr or Cho levels. It is not possible to rule out an increase of total cerebellar metabolism, including these metabolites, in essential tremor (Pagan et al., 2003). Besides structural deficits, other explanations for the decrease in NAA ratio are abnormalities in neuronal cellular size or neuronal metabolic changes within the cerebellar hemispheres.
3.1.4. Other structural imaging
Two studies used T2/T2*-weighted contrasts in MRI to investigate essential tremor (Table 5) (Novellino et al., 2013; Oliveira et al., 2012). Quantitative T2*-relaxometry is able to determine brain iron concentration. An increased iron concentration has been associated with several neurodegenerative disorders (Salvador et al., 2010). One study in 24 essential tremor patients indicated significant iron accumulation in the bilateral pallidum, substantia nigra and right dentate nucleus (Novellino et al., 2013). Furthermore, a community-based aging study observed more total cerebellar white matter hyperintensity associated with cerebrovascular disease in 33 essential tremor patients compared to 507 controls with T2-weighted FLAIR MRI (Oliveira et al., 2012).
Table 5.
Other structural imaging.
| Clinical features |
Imaging |
||||||
|---|---|---|---|---|---|---|---|
| ET and controls, age (SD) or *median (range) | ET | Cri | Results ET vs HC | Data analysis | |||
| Oliveira et al. (2012), 1.5T | 33 ET *79(74–87) | 507 HC *78(75–82) | UE | Ls | ↑ | Total WMH Cerebellar WMH, temporal WMH |
T2-FLAIR MRI WMH in ROI: cerebellum, frontal lobe, basal ganglia, occipital, parietal, temporal lobes Pcor < 0.05 |
| Novellino et al. (2013), 3T | 24 ET 64.3(10.0) | 25 HC 64.2(9.3) | UE, head, dys |
D | ↑ | Bilateral globus pallidus, substantia nigra, right dentate nucleus (Punc < 0.001) Bilateral pallidum (FWE P < 0.05) |
Whole brain R2* (1/T2*) maps Punc < 0.001 and FWE P < 0.05 |
Cor, multiple comparison corrected; Cri, criteria; D, Deuschl et al. (1998); dys, dystonic features; ET, essential tremor; FWE, family wise error; head, head tremor; HC, healthy controls; Ls, Louis et al. (2001); ROI, region of interest; SD, standard deviation; UE, upper extremity; Punc, multiple comparison uncorrected; vs, versus; WMH, white matter hyperintensities.
3.2. Functional imaging
Functional imaging studies are able to associate essential tremor with brain activity in rest or during specific tasks. Differences in activation maps might indicate primary oscillations in parts of the outflow circuits or in the compensatory regions. Differences could also be caused by inhibitory or excitatory neurodegenerative changes.
3.2.1. Scintigraphic techniques
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are scintigraphic techniques. These are able to measure regional cerebral blood flow (rCBF), regional glucose metabolism or regional binding by employing different radiopharmaceuticals (Kessler, 2003). We divide the scintigraphic studies into perfusion imaging and glucose metabolism, and receptor imaging.
3.2.1.1. Perfusion imaging and glucose metabolism
Early rCBF scintigraphic studies applied task-related methods to show differences in brain perfusion between essential tremor patients and healthy controls. The PET studies that investigated the rCBF in essential tremor used either the diffusible radiopharmaceutical C215O or H215O. SPECT studies investigated rCBF with the radiopharmaceuticals 99mTc-hexamethyl propylene amine oxime (HMPAO) and 99mTc-ethyl cysteinate dimer bicisate (ECD). Importantly, quantitative perfusion PET and steady-state HMPAO SPECT studies show excellent correlations between uptake and actual perfusion (Andersen, 1989; Raichle et al., 1983). Glucose metabolism was investigated with 18F-FDG PET. 18F-FDG PET can be used to quantify regional glucose utilization. 18F-FDG is transported via the glucose transporter into the cell and is then phosphorylated into FDG-phosphate, which is not further metabolized but accumulates in the cell. PET can regionally quantify the amount of this accumulation. Usually, glucose utilization correlates well with regional synaptic activity (Otte and Halsband, 2006).
Seven studies used SPECT and PET to determine the rCBF in essential tremor patients (Table 6). All four PET studies, having a total of 28 essential tremor patients and 24 healthy controls, conducted experiments in which the authors regarded the activation associated with tremor to be the summation of a postural motor task and a superimposed tremor. This experimental design was designed to untangle the processes of resting, postural stretching, tremor mimicking and passive wrist moving (Boecker et al., 1996; Colebatch et al., 1990; Jenkins et al., 1993; Wills et al., 1994). These four studies were consistent in their findings observing increased bilateral cerebellar activity during both action (tremor) and rest (no tremor) (Boecker et al., 1996; Colebatch et al., 1990; Jenkins et al., 1993; Wills et al., 1994). One study showed a suppression of the increased cerebellar activation with alcohol administration together with a reduction of the tremor amplitude (Wills et al., 1994). Two studies expanded their field of view to include the inferior olive (Boecker et al., 1996; Wills et al., 1994). Wills and colleagues did not detect altered olivary activity during rest nor during tremor (Wills et al., 1994). Boecker and colleagues detected an increase in medullary rCBF (region of inferior olive) after alcohol administration (Boecker et al., 1996). Other regions associated with the tremor were the thalamus (Jenkins et al., 1993; Wills et al., 1994), the striatum, the contralateral sensory motor cortex (Jenkins et al., 1993) and the red nucleus (Wills et al., 1994). Postural stretching and passively imposed movements in healthy controls were associated with strictly ipsilateral activation of the cerebellum activation and bilateral thalamus. Glucose metabolism was investigated with 18F-FDG reporting hypermetabolism in the medulla and both thalami, but not in the cerebellar cortex (Hallett and Dubinsky, 1993). Two studies using SPECT to determine rCBF in rest showed different results. One confirmed increased bilateral cerebellar activity (Czarnecki et al., 2011) whereas the other did not find any significant differences between essential tremor patients and healthy controls (Sahin et al., 2006).
Table 6.
Perfusion scintigraphy: positron emission tomography (PET) and single-photon emission computed tomography (SPECT).
| Clinical features |
Imaging |
|||||||
|---|---|---|---|---|---|---|---|---|
| ET and controls, age (SD) or *median (range) | ET | Cri | Tasks | Results | Data analysis | |||
| Colebatch et al. (1990), C215O PET: rCBF | 4 ET 56(?) | 4 HC 62(?) | UE | M | ET right arm posture vs rest | ↑ | Bilateral cerebellum, contralateral SMC, bilateral PMC | FOV axial 10.5 cm Punc < 0.05 |
| ET passively vs rest | ↑ | Contralateral SMC, bilateral PMC | ||||||
| Jenkins et al. (1993), C215O PET: rCBF | 11 ET *63.8(30–80) | 8 HC *57.1(29–73) | UE | Fin | ET vs CO in rest | ↑ | Bilateral cerebellum | FOV axial 10.13 cm Cerebellum FOV |
| ET right arm posture vs rest | ↑ | Bilateral cerebellum, contralateral striatal, thalami, SMC |
Pcor < 0.05 Rest Pcor < 0.025 |
|||||
| Hallett and Dubinsky (1993), 18F-FDG PET: rCMGlc | 8 ET *50(25–72) | 10 HC *44 (24–66) |
UE | – | ET vs CO in rest | ↑ | Medulla, thalami | ROI: Medulla, thalami, cerebellar cortices Punc < 0.05 |
| Wills et al. (1994), Wills et al. (1995), H215O PET: rCBF | 7 ET *49.4(28–74) | 6 HC *51.1(24–70) | UE | Fin | ET vs CO in rest | ↑ | Bilateral cerebellum, thalamus | FOV 10.65 cm Include ION Pcor < 0.05 Rest Punc < 0.05 |
| ET right arm posture vs rest | ↑ | Bilateral cerebellum, midbrain (red nucleus), bilateral thalamus | ||||||
| ↓ | Inferior temporal and occipital areas | |||||||
| Boecker et al. (1996), H215O PET: rCBF | 6 ET 54(14) | 6 HC 45(18) | UE | Fin | ET vs CO in rest | ↑ |
Before ethanol: bilateral cerebellum After ethanol: less reduction in cerebellar rCBF, medulla oblongata |
Axial FOV10.65 cm Including ION P < 0.001 |
| ET right arm posture vs rest | ↑ |
Before ethanol: bilateral cerebellum, cerebellar vermis After ethanol: cerebellar vermis |
||||||
| Sahin et al. (2006), Technetium-99m- HMPAO SPECT: rCBF | 11 ET ? | 9 HC ? | UE, head, voice |
Ba | ET vs CO in rest | − | – | ROI: 47 cortical, and cerebellar ROI cortex/occipital ratio |
| Czarnecki et al. (2011), 99mTc-ethyl cysteinate SPECT: rCBF | 5 ET 67.4(7.1) | 5 PT 52.2(10.2) 5 HC *61(55–66) |
UE | D | ET vs CO in rest | ↑ | Bilateral cerebellar, left inferior frontal gyrus |
Pcor < 0.02 Rest Punc < 0.05 |
| ET tremor inducing motor task vs rest | ↑ | SMA, contralateral motor cortex | ||||||
| ↓ | Cerebellum, visual cortex | |||||||
Ba, Bain et al. (2000); cor, multiple comparison corrected; Cri, criteria; D, Deuschl et al. (1998); ET, essential tremor; Fin, Findley et al. (1993); FOV, field of view; HC, healthy controls; head, head tremor; ION, inferior olive nucleus; M, Marsden CD et al. (1983); PMC, premotor cortices; PT, psychogenic tremor; rCBF, regional blood flow; rCMGlc, regional cerebral glucose metabolism; RN, red nucleus; SMA, supplementary motor area; SMC, sensory motor cortex; Punc, multiple comparison uncorrected; UE, upper extremity; voice, voice tremor; vs, versus.
One SPECT study focused on cognitive functioning and related the rCBF with cognitive performances in patients and healthy controls. This study determined differences in test performances but showed no difference in rCBF values (Sahin et al., 2006).
The most consistent finding throughout all scintigraphic rCBF studies is the increased cerebellar activity, mostly bilateral, during posture and rest. One study, using metabolic glucose rate, did not show any increased metabolism in the cerebellum. Here, the absence of cerebellar changes could be related to the analysis technique applied. Relatively large ROIs were included and normalized with respect to mean hemispheric uptake that was higher in the essential tremor group compared to controls (although not statistically significant). PET has been of great influence to form current hypotheses concerning essential tremor. Because of the better resolution of new PET systems, there is a need for renewed functional PET studies in essential tremor.
3.2.1.2. Receptor imaging
A total of fifteen studies were found conducting receptor imaging (Table 7). SPECT was mainly used for determining striatal dopamine transporter binding. Currently, 123I-FP-CIT SPECT is used in clinical practice to determine nigrostriatal dysfunction in Parkinson's disease (PD) and to differentiate PD from other tremor disorders (Booij et al., 1999). Two relatively small studies indicated a decrease in striatal dopamine binding in essential tremor patients who also exhibited parkinsonian symptoms (Lee et al., 1999; Schwartz et al., 2004).
Table 7.
Receptor scintigraphy: positron emission tomography (PET) and single-photon emission computed tomography (SPECT).
| Clinical features |
Imaging |
||||||
|---|---|---|---|---|---|---|---|
| Not included in Fig. 2 |
ET and controls, age (SD) or *median (range) | ET | Cri | Results ET vs HC | Data analysis | ||
| Brooks et al. (1992), 18F-DOPA PET dopa transporter | 8 ET 60(10) | 30 HC 63(10) 16 PD 56(12) 12 sPT 59(19) 11 rest 58(11) |
UE, head, jaw, legs, lips, rest | – | – | – | Striatal uptake with occipital reference P < 0.05 |
| Asenbaum et al. (1998), SPECT 123I-beta-CIT dopamine transporter | 32 ET *63(31–83) | 30 HC *45(21–75) 29 PD *61(39–81) |
UE, rest, mouth, head, legs | Fi | – | – | Striatal uptake with cerebellar reference P < 0.05 |
| Lee et al. (1999), SPECT 123I-IPT dopamine transporter | 9 PT 60.0(11.4) 6 PT + RT 68.3(10.3) |
21 HC 61.8(9.7) 11 PD 60.8(6.5) |
UE, jaw, voice, head, tongue, lip, rest | – | – | – | Basal ganglia uptake with occipital reference |
| ↓ | Striatal uptake | P < 0.05 | |||||
| Parkinson study group (2000), 123I-beta-CIT SPECT dopamine transporter | 14 ET 69(10) | 43 PD 68(8) 17 PSP 72(6) 22 HC 67(8) |
UE | Fa | – | – | Striatal uptake with cerebellar reference P < 0.05 |
| Benamer et al. (2000), 123I-FP-CIT SPECT dopamine transporter | 27 ET 64.1(8.8) | 35 HC 61(8.7) 158 Pdism 62.8(9.0) | UE | Fin | − | − | Striatal uptake with cerebellar reference P < 0.05 |
| Antonini et al. (2001), 11C-FE-CIT PET dopamine transporter | 5 ET 60(8) | 31 PD 55(13) 8 HC 52(16) |
UE | – | – | – | Striatal uptake with cerebellar reference |
| Schwartz et al. (2004), 123I-FP-CIT SPECT dopamine transporter | 10 ET 68.5(6.5) | Threshold HC | UE, VMC | D | ↓ | Striatal uptake | Striatal uptake with threshold method |
| Wang et al. (2005), 99mTc-Trodat-1 SPECT dopamine transporter | 12 ET 52.1(14.1) | 27 PD 54.3(10.3) 10 HC 52.5(10.7) |
UE | Fin | – | – | Striatal uptake with occipital reference p< 0.05 |
| Breit et al. (2006), 11C-dMP PET dopamine transporter | 6 ET 60(5) | 10 HC 58(5) 20 PD 63(8) |
UE | Fin | − | − | Dorsal putamen, caudate with occipital reference |
| Isaias et al. (2008) and Isaias et al. (2010), 123I-FP-CIT SPECT dopamine transporter | 32 ET 70(7) | 31 HC 64(10) 47 PD 62(10) |
UE, head | D | ↓ | Striatal uptake | Striatal uptake with occipital reference P < 0.05 |
| Doepp et al. (2008), 123I-FP-CIT SPECT dopamine transporter | 25 ET 64(12) | 46 PD 64(10) 50 HC 60(15) |
UE, rest | L | – | – | Striatal uptake with occipital reference P < 0.05 |
| Boecker et al. (2010), 11C-flumazenil PET (GABA) | 8 ET 65.5(8.0) | 11 HC 56.6(4.3) | UE, head |
Fin | ↑ | Dentate nucleus, ventrolateral thalamus, LPC | Whole brain ROI tremor network template FWE P < 0.05 |
| Roselli et al. (2010), 123I-FP-CIT SPECT dopamine and serotonin transporter | 15 ET 70.6(5.7) | 15 DLB 76.6(2.3) 15 PSP 66.6(8.2) 15 PD 78.6(5.8) 9 HC 74.6(2.8) |
UE | D | – | – | Midbrain and striatal uptake with occipital reference, MRI alignment P < 0.05 |
| Di et al. (2012), 123I-FP-CIT SPECT dopamine transporter | 15 ET 52.5(19.5) | 21 PD 59.9(13.4) 14 DYS 52.7(16.5) 17 HC 55.3(13.7) |
UE | D | – | – | Striatal uptake with occipital reference P < 0.05 |
| Gerasimou et al. (2012), 123I-FP-CIT SPECT dopamine transporter | 28 ET 64(15) | 28 HC 63(11) 33 PD ? |
UE | D | ↓ | Striatal uptake (less severely than in PD) |
Striatal uptake with occipital reference P < 0.05 |
BG, basal ganglia; Cri, criteria; D, Deuschl et al. (1998); DLB, Dementia with Lewy bodies; DYS, dystonia; ET, essential tremor; Fa, Fahn S and Elton RL (1987); Fi, Findley (1996); Fin, Findley et al. (1993); FWE, familial wise error-corrected; HC, healthy controls; L, Louis et al. (1998); LPC, lateral premotor cortex; PD, Parkinson's disease; Pdism, parkinsonism; PSP, progressive supranuclear palsy; PT, postural tremor; rest, rest tremor; sn, substantia nigra; sPT, sporadic postural tremor; UE, upper extremity; VIM, ventral intermediate thalamus; VMC, impaired visuomotor coordination; vs, versus.
Moreover, two studies reported a mild reduction in striatal dopamine transporter binding in 60 ‘pure’ essential tremor patients compared to 59 healthy controls although less severe compared to 80 Parkinson's disease patients (Gerasimou et al., 2012; Isaias et al., 2008). The reduced dopaminergic uptake pattern differed between the studies. Alternatively, the majority of DAT-SPECT and DOPA-PET studies did not show any deviations in striatal uptake in a total of 168 essential tremor patients (Brooks et al., 1992; Breit et al., 2006; Antonini et al., 2001; Doepp et al., 2008; Wang et al., 2005; Asenbaum et al., 1998; Benamer et al., 2000; Di et al., 2012; Isaias et al., 2010; Roselli et al., 2010; Parkinson study group, 2000). The two largest amongst these studies, with groups of 27 and 32 essential tremor patients, included patients fulfilling the clinical criteria and without any signs of parkinsonism (Asenbaum et al., 1998; Benamer et al., 2000).
One study investigated the GABAergic function, mediating inhibition in motor control, with the help of PET and the GABAA receptor antagonist 11C-flumazenil (Boecker et al., 2010). Boecker and colleagues found increased tracer binding in the unilateral cerebellum (dentate nucleus), unilateral ventrolateral thalamus and the unilateral premotor cortex in 8 patients with essential tremor compared to 11 controls.
Another study investigated serotonin transporter binding (Roselli et al., 2010). Using 123I-FP-CIT SPECT, Roselli and colleagues found an increased tracer binding in the midbrain of patients with essential tremor compared to those with Parkinson's disease and other parkinsonian syndromes (Roselli et al., 2010). However, serotonin transporter binding in essential tremor was comparable to uptake in healthy controls.
3.2.2. Functional magnetic resonance imaging (fMRI)
In fMRI, an increase in regional blood oxygenation is used as a proxy for local neuronal activity. The MRI signal is weighted by the difference in magnetic properties of oxygenated and deoxygenated blood. Difference between change in blood flow and oxygen use results in the Blood Oxygen Level-Dependent (BOLD) signal (Ogawa, 2012). With fMRI it is possible to relate cognitive and motor tasks to specific brain activations. Connections between activated regions in rest can be evaluated by employing resting state-fMRI that is based on spontaneous BOLD signal fluctuations (Biswal, 2012).
Of the five fMRI studies in essential tremor, all except one (Popa et al., 2013) performed whole-brain analyses (Table 8). The first fMRI study was performed in 12 essential tremor patients and 15 healthy controls (Bucher et al., 1997). Tasks were designed to specifically relate tremor with brain activation patterns. These included resting, postural stretching and passive movements of the wrist (excluding proprioceptive afferent activity). The control group was asked to mimic the tremor. Essential tremor patients showed significant increased activations in the bilateral cerebellum and ipsilateral red nucleus during tremor compared to tremor mimicking. Thalamic activity was present during tremor, during tremor mimicking and during passive movement. Olivary activation was not evident.
Table 8.
Functional MRI (fMRI).
| Clinical features |
Imaging |
|||||||
|---|---|---|---|---|---|---|---|---|
| ET and controls, age (SD) | ET | Cri | Tasks | Results ET vs HC | Data analysis | |||
| Bucher et al. (1997), 1.5T | 12 ET 61.1(11.9) | 15 HC 58.2(9.8) | UE | Fin | ET (most effected arm vs rest) and HC (mimicking tremor right arm vs rest) | ↑ | Bilateral cerebellum Bilateral red nucleus |
Whole brain Pcor < 0.05 |
| Cerasa et al. (2010), 1.5T | 12 ET 62.2(12.4) | 12 HC 59.8(10.7) | UE | D | Stroop task | ↑ | Left dorsolateral prefrontal, bilateral inferior parietal cortex* | Whole brain FDR Pcor < 0.05 |
| Passamonti et al. (2011), 3T | 15 ET 61.6(9.3) | 15 HC 60.4(7.3) | UE | D | High vs low load verbal working memory | ↑ | Cerebellum* | Whole brain Connectivity seed cerebellum FWE Pcor < 0.05 |
| Popa et al. (2013), 3T | 11 ET 51.5(11.8) | 11 HC 51.8(14.0) | UE, head, voice | D | − | ↓ | CTC pathways* | rsfMRI connectivity ROI: Cerebellum-MC vs Cerebellum-DBN 1 Hz rTMS Pcor < 0.05 |
| Fang et al. (2013), 3T | 20 ET 50.3(14.2) | 20 HC 50.3(14.2) | UE, LE, head, voice | L | – | ↓ | Bilateral cerebellar lobes, bilateral thalamus (mediodorsal and ventral intermediate thalamic nucleus), insular lobe* | Regional homogeneity rsfMRI Pcor < 0.05 |
| ↑ | Bilateral prefrontal, parietal cortices, left primary cortex* | |||||||
Cor, multiple comparison corrected; Cri, criteria; CTC, cerebello-thalamo-cortical pathways; cor, multiple comparison corrected; D, Deuschl et al. (1998); DBN, default brain network; EMG, electromyography; ET, essential tremor; FDR, false discovery rate; Fin, Findley et al. (1993); HC, healthy controls; head, head tremor; L, Louis et al. (1998); LE, lower extremity; lSMA, left supplementary motor area; MC, motor cortex; PMA, primary motor area; PSA, primary sensory area; ReHo, regional homogeneity; ROI, region of interest; rs, resting state; rTMS, repetitive transcranial magnetic stimulation; UE, upper extremity; Punc, multiple comparison uncorrected; voice, voice tremor; vs, versus. *Not included in fig. 2
Two studies performed resting state fMRI (rs-fMRI) in essential tremor (Fang et al., 2013; Popa et al., 2013). The first study performed independent component analysis (ICA) on rs-fMRI data in 11 patients, and showed changes in the cerebello-thalamo-cortical network induced by repetitive transcranial magnetic stimulation (rTMS) of the cerebellum. Low cerebello-thalamo-cortical connectivity recovered after 5 days of consecutive rTMS taking the default brain network as the control network (Popa et al., 2013). The second study used the regional homogeneity (ReHo) measurement of rs-fMRI which investigates regional functional connectivity by calculating temporal correlation of adjacent voxels (Fang et al., 2013). This study found a decreased ReHo in the cerebellum (anterior and posterior lobes) and the thalamus of tremor patients compared to healthy controls. Non-motor areas, like the prefrontal, the parietal and the insular cortices showed increased ReHo values.
Two other studies with a total of 27 patients concentrated on cognitive functioning in essential tremor (Cerasa et al., 2010; Passamonti et al., 2011). Despite similar performances between patients and healthy controls in both studies (verbal working memory and Stroop task), essential tremor patients showed increased cerebellar activation compared to controls while executing verbal working memory tasks (Passamonti et al., 2011). During the Stroop task patients showed an increased activity in the parieto-prefrontal areas involved in the execution of attentional tasks (Cerasa et al., 2010).
In conclusion, one fMRI study reported increased cerebellar activity related to tremor. Using rs-fMRI a decreased functional connectivity in the cerebello-thalamo-cortical network has been determined. How to interpret BOLD changes in the cerebellar cortex is less certain than changes in the neocortex because of its complex anatomical architecture (Diedrichsen et al., 2010).
4. Discussion
The imaging studies mainly point to the involvement of the cerebellum in essential tremor. Other identified areas and nuclei have mostly been mentioned as parts of the tremor network and include the brainstem, the red nucleus, the thalamus, the basal ganglia and widespread abnormalities (Fig. 3). This will be further discussed in Part I of this section. With respect to the different hypotheses regarding the underlying disease mechanisms in essential tremor, some imaging findings are more consistent with general neurodegenerative changes while others are more consistent with localized abnormalities of the GABAergic system. This will be discussed in Part II. Patient selection and recommendations for future imaging in essential tremor are discussed in Part III of this section.
Fig. 3.
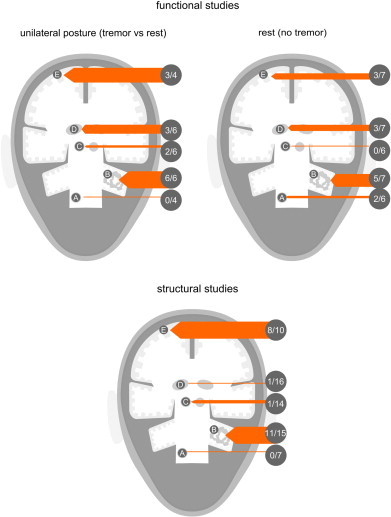
The tremor network weighted, based on number of studies finding abnormal- ities against total amount of studies that investigated the region. A) Inferior olive; B) dentate nucleus; C) red nucleus; D) thalamus; E) widespread abnormalities.
4.1. Part I: are results of imaging studies congruent with existing theories on the ‘tremor network’?
A majority of the structural and functional findings agree with the pathological involvement of the cerebellum in essential tremor. Although in many neuroimaging studies the cerebellum is the predominant region of interest, studies performing whole brain analysis also indicate cerebellar changes. It is difficult to point to specific areas in the cerebellum because of its complex anatomical architecture and the limitations of analysis techniques applied. Nonetheless, abnormalities in the dentate nucleus, vermis, superior cerebellar peduncle and inferior cerebellar peduncle were associated with essential tremor (Jia et al., 2011; Klein et al., 2011; Nicoletti et al., 2010; Shin et al., 2008). Clinically, several symptoms point to the involvement of the cerebellum in essential tremor, such as intentional tremor, eye movement abnormalities (Helmchen et al., 2003), affected tandem gait (Stolze et al., 2001) and abnormal eye–hand coordination (Trillenberg et al., 2006). Suppression of essential tremor has been reported after lesioning the cerebellum (Dupuis et al., 1989). Cerebellar involvement is also confirmed in neuropathological studies (Louis et al., 2007; Louis et al., 2013b; Louis, 2010). It is however not clear whether the cerebellar symptoms and pathological changes point to primary involvement of the cerebellum in tremor generation, or merely indicate secondary cerebellar changes. Based on the current imaging studies, it is also difficult to differentiate between primary and secondary changes.
A number of functional studies pointed to thalamic involvement during tremor (Bucher et al., 1997; Jenkins et al., 1993; Wills et al., 1994). Studies that investigated passive movement found thalamic activations and suggested afferent or proprioceptive input to be the main cause of these activations (Bucher et al., 1997; Jenkins et al., 1993; Wills et al., 1994). Stereotactic surgery of the ventral intermediate nucleus (VIM)of the thalamus has been proven to be an effective treatment for essential tremor (Flora et al., 2010). Intraoperative microelectrode recording show only thalamic tremor activity during postural tremor and not in rest (Hua et al., 2005). Currently, the role of the thalamus in essential tremor is not entirely clear. Probably, it is mostly seen as a relay station rather than a primary tremor generator.
Only two neuroimaging studies showed abnormalities in the inferior olive area. One study showed an increased glucose metabolism (Hallett and Dubinsky, 1993) while the other showed an increase in medullary rCBF after alcohol administration (Boecker et al., 1996). Involvement of the inferior olive in tremor generation in the literature comes from animal models that suggest the inferior olive to function as an intrinsic pacemaker (Long et al., 2002) and the suggestion that the inferior olive would be prone to intrinsic calcium channel-dependent neuronal oscillations (Park et al., 2010). Recently, the first post-mortem study focusing on microscopic changes of the inferior olivary nucleus did not indicate any structural differences (Louis et al., 2013a). Lack of imaging evidence that points to the involvement of the olivary nucleus might be due to its anatomical location picking up physiological noise and/or the use of incorrect functional parameters.
Abnormalities in the red nucleus are reported in two diffusion studies and one fMRI study. (Bucher et al., 1997; Jia et al., 2011; Shin et al., 2008). Because of its tight functional connection with the cerebellum and inferior olive, the red nucleus is hard to study in isolation.
Most scintigraphic studies looking into the basal ganglia found no dopaminergic deficits in essential tremor (Antonini et al., 2001; Asenbaum et al., 1998; Benamer et al., 2000; Breit et al., 2006; Brooks et al., 1992; Doepp et al., 2008; Isaias and Antonini, 2010; Wang et al., 2005) while two studies measured slight abnormalities (Gerasimou et al., 2012; Isaias et al., 2008). One study reported iron accumulation in the basal ganglia (Novellino et al., 2013). The basal ganglia are not often primarily associated with tremor generation in essential tremor.
In current literature, non-motor symptoms have been increasingly associated with essential tremor (Janicki et al., 2013). Especially in structural VBM and DWI studies non-motor areas like frontal and temporoparietal areas, which are involved in cognitive and visuospatial processing, are associated with essential tremor (Bagepally et al., 2012; Benito-Leon et al., 2009; Daniels et al., 2006; Klein et al., 2011; Klein et al., 2011; Lin et al., 2013; Lin et al., 2013; Saini et al., 2012; Shin et al., 2008; Shin et al., 2008). Increased grey matter volume could imply compensatory processes such as those found in the visuospatial processing areas (Lin et al., 2013). Diffusion studies however showed a widespread increase of white matter diffusion, opting for extensive white matter dysfunction. One rs-fMRI study found abnormalities in non-motor areas with functional connectivity analysis (Fang et al., 2013). The cognitive deficits in essential tremor affect attention, working memory and executive functions. These cognitive performances are known to be associated with regional cerebellar abnormalities (Bermejo-Pareja and Puertas-Martin, 2012; Egner and Hirsch, 2005). One fMRI study investigated essential tremor during the Stroop task, assessing attentional control and evaluating executive functions, and found the involvement of several ‘cognitive’ cortical areas but no cerebellar areas (Cerasa et al., 2010). The authors hypothesized that the increased cortical activity suggests an increase in cognitive effort due to a more subtle cerebellar dysfunction.
4.2. Part II: do the imaging techniques help to understand the pathophysiology of essential tremor better?
Essential tremor has been considered to be primarily a neurodegenerative disorder, with progressive cell loss, in part of the literature. Some pathoanatomical cerebellar investigations support structural changes. Post-mortem studies showed an increased number of Purkinje cell axonal swellings (torpedoes) and actual Purkinje cell loss in the cerebellum (Louis et al., 2007; Louis et al., 2013b; Louis, 2010), but others have not been able to confirm these findings (Rajput et al., 2012b; Symanski et al., 2014). Neighbouring non-Purkinje cell abnormalities like abnormal axonal processes of basket cells and Bergman gliosis were found in other post-mortem studies (Erickson-Davis et al., 2010; Shill et al., 2008). Several structural imaging studies observed widespread abnormalities, changes not only included cerebellar areas but also frontal and temporoparietal areas (Bagepally et al., 2012; Klein et al., 2011; Lin et al., 2013; Saini et al., 2012; Shin et al., 2008). These findings might support a more generalized neurodegenerative nature of essential tremor based on widespread alterations in the brain rather than specific cerebellar cell loss. Other structural imaging studies showed an isolated reduction in cerebellar volume(Quattrone et al., 2008) and an increase in cerebellar diffusion supporting a local ‘purkinjopathy’ (Jia et al., 2011; Klein et al., 2011; Nicoletti et al., 2010; Shin et al., 2008). Metabolic changes, shown with MRS, support regional neuronal loss in the cerebellar cortex (Louis et al., 2002; Pagan et al., 2003). Regional deficits however, do not necessarily indicate the primary site of pathology.
In essential tremor the limited symptomatology and the absence of a consistent neuropathological substrate lead one to suspect disease mechanisms other than neurodegeneration. Drugs that facilitate GABA transmission, like primidone, gabapentin and benzodiazepines, are effective treatments in essential tremor (Zesiewicz et al., 2005). Moreover, the explicit role of ethanol, which is a GABAA receptor agonist and considered to reduce tremor in essential tremor patients, implies the involvement of the GABA system in essential tremor (Boecker et al., 1996; Zeuner et al., 2003). The GABAergic hypothesis which is the abnormal functioning of the inhibitory neurotransmitter GABA is most often considered in light of neurodegenerative Purkinje cell loss. Alternatively, one could consider abnormalities of GABA receptor subtypes or other isolated biochemical changes related to GABA function (Boecker et al., 2010; Kralic et al., 2005; Mally et al., 1996). Post-mortem investigations in the dentate nucleus showed reduction of GABAA and GABAB receptor expression. Additional findings indicated loss of GABAB receptor messenger RNA transcripts in the dentate nucleus cells which could account for the loss of GABA receptors (Paris-Robidas et al., 2012). In genetic animal research, GABAA-alpha1 receptor knockout mice presented phenotypic similarities with essential tremor (Kralic et al., 2005). Our literature search identified PET/SPECT studies that are in accordance with the GABA hypothesis (Boecker and Brooks, 1998). These studies showed obvious cerebellar overactivity during tremor manifestation and rest, and activities that normalized after ethanol intake along with the tremor reduction. Two PET studies specifically investigated the GABAergic system. These reported a diffuse increased binding at the benzodiazepine receptor site (part of the GABAA complex) in the ventrolateral thalamus, the premotor cortex and the cerebellum (dentate nucleus) (Boecker et al., 2010; Gironell et al., 2012). The authors suggested that an increased availability of benzodiazepine receptor sites might reflect a reactive receptor upregulation due to a localized GABAergic deficit or reflect functional abnormalities at the receptor level (Boecker et al., 2010; Gironell et al., 2012). Gironell and colleagues showed a positive relationship between tremor severity and cerebellar GABAA receptor binding (Gironell et al., 2012). Theoretically, cerebellar functional disturbances could cause secondary cerebellar damage (Deuschl and Elble, 2009). Therefore, the GABA hypothesis does not disagree with Purkinje cell loss or other morphological changes in essential tremor. These results warrant further research to determine the role of GABA in essential tremor. Currently, GABA MRS imaging is a growing field which might lead to meaningful insights in essential tremor (Mullins et al., 2012).
Another common hypothesis is the oscillating network hypothesis that implies excessive neuronal oscillations throughout the whole cerebellar circuit resulting in entrainment. This theory is nowadays evolving towards a theory that holds multiple oscillations interacting in the tremor network (Deuschl and Elble, 2009; Helmich et al., 2013). Oscillations are mostly investigated with high temporal resolution methods relating EEG and thalamic local field potential to tremor measured by EMG with coherence analysis (Pedrosa et al., 2012; Raethjen et al., 2007). There are theories that point to the inferior olive as the origin of the faulty oscillations (Hallett, 2014). PET and fMRI studies show a constant overactivity of the cerebellum. This might be either due to abnormalities in the cerebellum or due to an abnormal cerebellar afferent input from the inferior olive (Boecker et al., 1996; Boecker and Brooks, 1998; Bucher et al., 1997; Colebatch et al., 1990; Wills et al., 1994). We know that the inferior olive is under the modulating influence of GABA; a deficiency in the GABAergic system could increase synchronicity. The olivary involvement is not clearly determined in functional imaging studies, although it does not seem to show structural changes. It is important to conduct imaging research across multiple regions of the brain using functional and effective (causal) connectivity techniques. For example, dynamic causal modelling in fMRI reveals temporal dependency of activity between areas. By combining imaging techniques with modalities that offer high temporal resolution it might be possible to capture the dynamic behaviour of the oscillations.
4.3. Part III: what recommendations can be set for future imaging research arising from current literature?
4.3.1. Patient selection
In some studies essential tremor patients showed additional neurological symptoms. For example, inclusion of essential tremor patients with concomitant parkinsonism could explain DAT deficits in two studies (Lee et al., 1999; Schwartz et al., 2004). Essential tremor not only might be confused with other tremulous disorders, but also might be a heterogeneous disorder. This may explain the variability in clinical measures, epidemiological measures, conflicting pathological findings, differences in medication response, and yet indecisive data on genetics (Fekete and Jankovic, 2011). Also, inconsistent neuroimaging findings implicate a spectrum of disorders with (initially or eventually) similar phenotypes but different structural or functional changes. The classical essential tremor criteria are evolving and three sub-classifications have been proposed: sporadic, hereditary and senile essential tremors (tremor onset after age of 65 years) (Deuschl and Elble, 2009). Future imaging research could benefit using these sub-classifications with appropriate group sizes. Inclusion based on more stringent clinical selection like disease duration will lead to more firm conclusions.
4.3.2. Imaging limitations and recommendations with respect to imaging techniques
Although the spatial resolution of the clinical imaging techniques is moderate to good (e.g. fMRI 2–3 mm, SPECT 6–10 mm, PET 3–6 mm), small regions that are of interest like nuclei in the brainstem still may not be detectable (Diedrichsen et al., 2010; Draganski and Bhatia, 2010). In the near future, more accurate techniques like 7T MRI will become available offering new opportunities in neuroimaging research. fMRI sequences are known for their limited temporal resolution taking up to 2–3 s for a whole brain scan, while tremor frequency varies between 4 and 12 Hz. To capture tremor modulations it is possible to combine techniques, like multimodal EEG/EMG–fMRI. An alternative option is to confine the analysis to a specific region in the brain and register with high temporal resolution. In order to directly relate essential tremor to the BOLD signal one study introduced EMG–fMRI (Contarino et al., 2012). Simultaneous EMG–fMRI was performed in 6 essential tremor patients who had undergone unilateral thalamotomy. An EMG based regressor revealed ipsilateral cerebellar and contralateral thalamic activations. Also, other EMG–fMRI studies have proven to be useful for imaging movement disorders (van der Meer et al., 2010; van Rootselaar et al., 2007; van Rootselaar et al., 2008). In addition, the fact that brain activations may be related not only to pathological output, but also to afferent input as a result of the ongoing movements, is often not taken into account. By applying an external perturbation, for instance TMS or another perturbation technique with well-known properties, it is possible to challenge the system and perhaps distinguish cause-and-effect (Popa et al., 2013).
The analysis techniques are also improving. These were originally developed for cerebral cortical areas. Recently, specific toolboxes have been developed to specifically investigate the cerebellum and brainstem (Beissner et al., 2014; Diedrichsen et al., 2010; Diedrichsen et al., 2011). In some cases in functional imaging studies it is useful to add an extra condition as localizer, targeting functionally specific ROIs (Poldrack, 2007). Furthermore, analysis techniques move towards network detection and focus more on connectivity circuits instead of individual structures.
So far, only 123I-FP-CIT SPECT has gained a recognized role in clinical practice for differentiating essential tremor and parkinsonian diseases (Benamer et al., 2000; Cuberas-Borros et al., 2011). The clinical impact of other imaging techniques is modest. Imaging results in individual patients are variable and these techniques cannot (yet) be adopted in clinical practice.
5. Conclusion
In essence, contemporary clinical diagnosis of essential tremor is based on clinical assessment of the phenomenological characteristics and its course. Essential tremors' high prevalence, its unclear association with additional comorbidity and its variation in clinical presentation cause misdiagnosis. Neuroimaging results are congruent with areas within the tremor network. Functional imaging studies that provide insights in regional cerebral blood flow and metabolism point to the cerebello-thalamo-cortical outflow pathways, with cerebellar involvement as the most consistent finding. Structural imaging studies show more widespread alterations. Theories on the pathophysiology of essential tremor, including the neurodegenerative, GABAergic and oscillatory network hypotheses, are not mutually exclusive and should be further elucidated. GABAergic dysfunction is perhaps the primary factor involved in the pathophysiological hypotheses and is worthwhile investigating more intensively. Inconsistencies of study results can be attributed to patient selection. Future research requires more stringent inclusion criteria and application of tailored analysis techniques that focus on connectivity in brain networks. The rapid developments of imaging techniques in combination with other tremor recording modalities could lead to a diagnostic imaging paradigm for essential tremor in the future.
Appendix A. Supplementary materials
Supplementary materials for Neuroimaging essentials in essential tremor: A systematic review.
References
- Andersen A.R. 99mTc-D,L-hexamethylene-propyleneamine oxime (99mTc-HMPAO): basic kinetic studies of a tracer of cerebral blood flow. Cerebrovascular and Brain Metabolism Reviews. 1989;1:288–318. http://www.ncbi.nlm.nih.gov/pubmed/2701656 [PubMed] [Google Scholar]
- Antonini A., Moresco R.M., Gobbo C., De N.R., Panzacchi A., Barone P., Calzetti S., Negrotti A., Pezzoli G., Fazio F. The status of dopamine nerve terminals in Parkinson's disease and essential tremor: a PET study with the tracer [11-C]FE-CIT. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2001;22:47–48. doi: 10.1007/s100720170040. 11487195 [DOI] [PubMed] [Google Scholar]
- Asenbaum S., Pirker W., Angelberger P., Bencsits G., Pruckmayer M., Brucke T. [123I]beta-CIT and SPECT in essential tremor and Parkinson's disease. Journal of Neural Transmission (Vienna, Austria: 1996) 1998;105:1213–1228. doi: 10.1007/s007020050124. 9928890 [DOI] [PubMed] [Google Scholar]
- Bagepally B.S., Bhatt M.D., Chandran V., Saini J., Bharath R.D., Vasudev M.K., Prasad C., Yadav R., Pal P.K. Decrease in cerebral and cerebellar gray matter in essential tremor: a voxel-based morphometric analysis under 3 T MRI. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2012;22:275–278. doi: 10.1111/j.1552-6569.2011.00598.x. 21447032 [DOI] [PubMed] [Google Scholar]
- Bain P., Brin M., Deuschl G., Elble R., Jankovic J., Findley L., Koller W.C., Pahwa R. Criteria for the diagnosis of essential tremor. Neurology. 2000;54:S7. 10854345 [PubMed] [Google Scholar]
- Bammer R., Fazekas F. Diffusion imaging of the human spinal cord and the vertebral column. Topics in Magnetic Resonance imaging: TMRI. 2003;14:461–476. doi: 10.1097/00002142-200312000-00004. 14872166 [DOI] [PubMed] [Google Scholar]
- Barker P.B. Clinical MR Spectroscopy: Techniques and Applications. Cambridge University Press; Cambridge: 2010. p. 264. [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. 8661285 [DOI] [PubMed] [Google Scholar]
- Beissner F., Schumann A., Brunn F., Eisentrager D., Bar K.J. Advances in functional magnetic resonance imaging of the human brainstem. Neuroimage. 2014;86:91–98. doi: 10.1016/j.neuroimage.2013.07.081. 23933038 [DOI] [PubMed] [Google Scholar]
- Benamer T.S., Patterson J., Grosset D.G., Booij J., de Bruin K., van Royen E., Speelman J.D., Horstink M.H., Sips H.J., Dierckx R.A., Versijpt J., Decoo D., Van Der Linden C., Hadley D.M., Doder M., Lees A.J., Costa D.C., Gacinovic S., Oertel W.H., Pogarell O., Hoeffken H., Joseph K., Tatsch K., Schwarz J., Ries V. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Movement Disorders: Official Journal of the Movement Disorder Society. 2000;15(3):503–510. 10830416 [PubMed] [Google Scholar]
- Benito-Leon J., Alvarez-Linera J., Hernandez-Tamames J.A., Alonso-Navarro H., Jimenez-Jimenez F.J., Louis E.D. Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. Journal of the Neurological Sciences. 2009;287:138–142. doi: 10.1016/j.jns.2009.08.037. 19717167 [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F., Puertas-Martin V. Cognitive features of essential tremor: a review of the clinical aspects and possible mechanistic underpinnings. Tremor and Other Hyperkinetic Movements. 2012;1 doi: 10.7916/D89W0D7W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B. Resting state fMRI: a personal history. Neuroimage. 2012;62:938–944. doi: 10.1016/j.neuroimage.2012.01.090. 22326802 [DOI] [PubMed] [Google Scholar]
- Boecker H., Brooks D.J. Functional imaging of tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 1998;13(Suppl. 3):64–72. doi: 10.1002/mds.870131311. http://www.ncbi.nlm.nih.gov/pubmed/9827597 [DOI] [PubMed] [Google Scholar]
- Boecker H., Weindl A., Brooks D.J., Ceballos-Baumann A.O., Liedtke C., Miederer M., Sprenger T., Wagner K.J., Miederer I. GABAergic dysfunction in essential tremor: an 11C-flumazenil PET study. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 2010;51:1030–1035. doi: 10.2967/jnumed.109.074120. 20554735 [DOI] [PubMed] [Google Scholar]
- Boecker H., Wills A.J., Ceballos-Baumann A., Samuel M., Thompson P.D., Findley L.J., Brooks D.J. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Annals of Neurology. 1996;39:650–658. doi: 10.1002/ana.410390515. 8619551 [DOI] [PubMed] [Google Scholar]
- Bonuccelli U. Essential tremor is a neurodegenerative disease. Journal of Neural Transmission (Vienna, Austria: 1996) 2012;119:1383–1387. doi: 10.1007/s00702-012-0878-8. 23011236 [DOI] [PubMed] [Google Scholar]
- Booij J., Tissingh G., Winogrodzka A., van Royen E.A. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. European Journal of Nuclear Medicine. 1999;26:171–182. doi: 10.1007/s002590050374. 9933352 [DOI] [PubMed] [Google Scholar]
- Breit S., Reimold M., Reischl G., Klockgether T., Wullner U. [(11)C]d-threo-methylphenidate PET in patients with Parkinson's disease and essential tremor. Journal of Neural Transmission (Vienna, Austria: 1996) 2006;113:187–193. doi: 10.1007/s00702-005-0311-7. 15959851 [DOI] [PubMed] [Google Scholar]
- Brooks D.J., Playford E.D., Ibanez V., Sawle G.V., Thompson P.D., Findley L.J., Marsden C.D. Isolated tremor and disruption of the nigrostriatal dopaminergic system: an 18F-DOPA PET study. Neurology. 1992;42:1554–1560. doi: 10.1212/wnl.42.8.1554. 1641153 [DOI] [PubMed] [Google Scholar]
- Bucher S.F., Seelos K.C., Dodel R.C., Reiser M., Oertel W.H. Activation mapping in essential tremor with functional magnetic resonance imaging. Annals of Neurology. 1997;41:32–40. doi: 10.1002/ana.410410108. 9005863 [DOI] [PubMed] [Google Scholar]
- Buijink A.W., Caan M.W., Tijssen J.M., Hoogduin N.M.M., van Rootselaar A.F. Decreased cerebellar fiber density in cortical myoclonic tremor but not in essential tremor. Cerebellum (London, England) 2013;12(2):199–204. doi: 10.1007/s12311-012-0414-2. 22961557 [DOI] [PubMed] [Google Scholar]
- Cerasa A., Messina D., Nicoletti G., Novellino F., Lanza P., Condino F., Arabia G., Salsone M., Quattrone A. Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR. American Journal of Neuroradiology. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. 19342539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A., Passamonti L., Novellino F., Salsone M., Gioia M.C., Morelli M., Paglionico S., Giofre L., Arabia G., Quattrone A. Fronto-parietal overactivation in patients with essential tremor during Stroop task. Neuroreport. 2010;21:148–151. doi: 10.1097/WNR.0b013e328335b42c. 20010442 [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Findley L.J., Frackowiak R.S., Marsden C.D., Brooks D.J. Preliminary report: activation of the cerebellum in essential tremor. Lancet. 1990;336:1028–1030. doi: 10.1016/0140-6736(90)92489-5. 1977019 [DOI] [PubMed] [Google Scholar]
- Contarino M.F., Groot P.F., van der Meer J.N., Bour L.J., Speelman J.D., Nederveen A.J., van den Munckhof P., Tijssen M.A., Schuurman P.R., van Rootselaar A.F. Is there a role for combined EMG–fMRI in exploring the pathophysiology of essential tremor and improving functional neurosurgery? PloS One. 2012;7:e46234. doi: 10.1371/journal.pone.0046234. 23049695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuberas-Borros G., Lorenzo-Bosquet C., Aguade-Bruix S., Hernandez-Vara J., Pifarre-Montaner P., Miquel F., Alvarez-Sabin J., Castell-Conesa J. Quantitative evaluation of striatal I-123-FP-CIT uptake in essential tremor and Parkinsonism. Clinical Nuclear Medicine. 2011;36:991–996. doi: 10.1097/RLU.0b013e3182291a7b. 21975386 [DOI] [PubMed] [Google Scholar]
- Czarnecki K., Jones D.T., Burnett M.S., Mullan B., Matsumoto J.Y. SPECT perfusion patterns distinguish psychogenic from essential tremor. Parkinsonism & Related Disorders. 2011;17:328–332. doi: 10.1016/j.parkreldis.2011.01.012. 21317018 [DOI] [PubMed] [Google Scholar]
- Daniels C., Peller M., Wolff S., Alfke K., Witt K., Gaser C., Jansen O., Siebner H.R., Deuschl G. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology. 2006;67:1452–1456. doi: 10.1212/01.wnl.0000240130.94408.99. 17060572 [DOI] [PubMed] [Google Scholar]
- Deuschl G., Bain P., Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Movement Disorders: Official Journal of the Movement Disorder Society. 1998;13(Suppl. 3):2–23. doi: 10.1002/mds.870131303. 9827589 [DOI] [PubMed] [Google Scholar]
- Deuschl G., Elble R. Essential tremor — neurodegenerative or nondegenerative disease towards a working definition of ET. Movement Disorders: Official Journal of the Movement Disorder Society. 2009;24:2033–2041. doi: 10.1002/mds.22755. 19750493 [DOI] [PubMed] [Google Scholar]
- Di G.D., Camardese G., Bentivoglio A.R., Cocciolillo F., Guidubaldi A., Pucci L., Bruno I., Janiri L., Giordano A., Fasano A. Dopaminergic dysfunction and psychiatric symptoms in movement disorders: a 123I−FP-CIT SPECT study. European Journal of Nuclear Medicine and Molecular imaging. 2012;39:1937–1948. doi: 10.1007/s00259-012-2232-7. 22976499 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Maderwald S., Kuper M., Thurling M., Rabe K., Gizewski E.R., Ladd M.E., Timmann D. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. 20965257 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Verstynen T., Schlerf J., Wiestler T. Advances in functional imaging of the human cerebellum. Current Opinion in Neurology. 2010;23:382–387. doi: 10.1097/WCO.0b013e32833be837. http://www.ncbi.nlm.nih.gov/pubmed/20581682 [DOI] [PubMed] [Google Scholar]
- Doepp F., Plotkin M., Siegel L., Kivi A., Gruber D., Lobsien E., Kupsch A., Schreiber S.J. Brain parenchyma sonography and 123I−FP-CIT SPECT in Parkinson's disease and essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2008;23:405–410. doi: 10.1002/mds.21861. 18067184 [DOI] [PubMed] [Google Scholar]
- Draganski B., Bhatia K.P. Brain structure in movement disorders: a neuroimaging perspective. Current Opinion in Neurology. 2010;23:413–419. doi: 10.1097/WCO.0b013e32833bc59c. 20610992 [DOI] [PubMed] [Google Scholar]
- Dupuis M.J., Delwaide P.J., Boucquey D., Gonsette R.E. Homolateral disappearance of essential tremor after cerebellar stroke. Movement Disorders: Official Journal of the Movement Disorder Society. 1989;4:183–187. doi: 10.1002/mds.870040210. 2733709 [DOI] [PubMed] [Google Scholar]
- Egner T., Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. 15627596 [DOI] [PubMed] [Google Scholar]
- Erickson-Davis C.R., Faust P.L., Vonsattel J.P., Gupta S., Honig L.S., Louis E.D. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. Journal of Neuropathology and Experimental Neurology. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. 20142764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Elton R.L. (1987), Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. in: Recent Developments in Parkinson's Disease S. Fahn (Eds.), MCCDGMe, Florham Park, NJ: Macmillan Health Care Information.
- Fang W., Lv F., Luo T., Cheng O., Liao W., Sheng K., Wang X., Wu F., Hu Y., Luo J., Yang Q.X., Zhang H. Abnormal regional homogeneity in patients with essential tremor revealed by resting-state functional MRI. PloS One. 2013;8:e69199. doi: 10.1371/journal.pone.0069199. 23869236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete R., Jankovic J. Revisiting the relationship between essential tremor and Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 2011;26:391–398. doi: 10.1002/mds.23512. 21462256 [DOI] [PubMed] [Google Scholar]
- Findley L.J. Classification of tremors. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society. 1996;13:122–132. doi: 10.1097/00004691-199603000-00003. 8849967 [DOI] [PubMed] [Google Scholar]
- Findley L., Koller W., LeWitr P. (1993), Classification and definition of tremor, Lord Walton of Detchanr ed., pp. 22-23. Indications for and clinical implications of botulinum toxin therapy. London: Royal Society of Medicine Services Limited.
- Flora E.D., Perera C.L., Cameron A.L., Maddern G.J. Deep brain stimulation for essential tremor: a systematic review. Movement Disorders: Official Journal of the Movement Disorder Society. 2010;25:1550–1559. doi: 10.1002/mds.23195. 20623768 [DOI] [PubMed] [Google Scholar]
- Gerasimou G., Costa D.C., Papanastasiou E., Bostanjiopoulou S., Arnaoutoglou M., Moralidis E., Aggelopoulou T., Gotzamani-Psarrakou A. SPECT study with I-123-ioflupane (DaTSCAN) in patients with essential tremor. Is there any correlation with Parkinson's disease? Annals of Nuclear Medicine. 2012;26:337–344. doi: 10.1007/s12149-012-0577-4. http://www.ncbi.nlm.nih.gov/pubmed/22382608 [DOI] [PubMed] [Google Scholar]
- Gironell A., Figueiras F.P., Pagonabarraga J., Herance J.R., Pascual-Sedano B., Trampal C., Gispert J.D. GABA and serotonin molecular neuroimaging in essential tremor: a clinical correlation study. Parkinsonism & Related Disorders. 2012;18:876–880. doi: 10.1016/j.parkreldis.2012.04.024. 22595620 [DOI] [PubMed] [Google Scholar]
- Hallett M. Tremor: pathophysiology. Parkinsonism & Related Disorders. 2014;20(Suppl. 1):S118–S122. doi: 10.1016/S1353-8020(13)70029-4. 24262161 [DOI] [PubMed] [Google Scholar]
- Hallett M., Dubinsky R.M. Glucose metabolism in the brain of patients with essential tremor. Journal of the Neurological Sciences. 1993;114:45–48. doi: 10.1016/0022-510x(93)90047-3. 8433096 [DOI] [PubMed] [Google Scholar]
- Helmchen C., Hagenow A., Miesner J., Sprenger A., Rambold H., Wenzelburger R., Heide W., Deuschl G. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain: a Journal of Neurology. 2003;126:1319–1332. doi: 10.1093/brain/awg132. 12764054 [DOI] [PubMed] [Google Scholar]
- Helmich R.C., Toni I., Deuschl G., Bloem B.R. The pathophysiology of essential tremor and Parkinson's tremor. Current Neurology and Neuroscience Reports. 2013;13:378. doi: 10.1007/s11910-013-0378-8. 23893097 [DOI] [PubMed] [Google Scholar]
- Isaias I.U., Antonini A. Single-photon emission computed tomography in diagnosis and differential diagnosis of Parkinson's disease. Neuro-degenerative Diseases. 2010;7:319–329. doi: 10.1159/000314498. 20616566 [DOI] [PubMed] [Google Scholar]
- Isaias I.U., Canesi M., Benti R., Gerundini P., Cilia R., Pezzoli G., Antonini A. Striatal dopamine transporter abnormalities in patients with essential tremor. Nuclear Medicine Communications. 2008;29:349–353. doi: 10.1097/MNM.0b013e3282f4d307. 18317299 [DOI] [PubMed] [Google Scholar]
- Isaias I.U., Marotta G., Hirano S., Canesi M., Benti R., Righini A., Tang C., Cilia R., Pezzoli G., Eidelberg D., Antonini A. Imaging essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2010;25:679–686. doi: 10.1002/mds.22870. 20437537 [DOI] [PubMed] [Google Scholar]
- Jain S., Se L., Louis E.D. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Archives of Neurology. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. 16908735 [DOI] [PubMed] [Google Scholar]
- Janicki S.C., Cosentino S., Louis E.D. The cognitive side of essential tremor: what are the therapeutic implications? Therapeutic Advances in Neurological Disorders. 2013;6:353–368. doi: 10.1177/1756285613489591. 24228071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins I.H., Bain P.G., Colebatch J.G., Thompson P.D., Findley L.J., Frackowiak R.S., Marsden C.D., Brooks D.J. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Annals of Neurology. 1993;34:82–90. doi: 10.1002/ana.410340115. 8517685 [DOI] [PubMed] [Google Scholar]
- Jia L., Jia-Lin S., Qin D., Qing L., Yan Z. A diffusion tensor imaging study in essential tremor. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2011;21:370–374. doi: 10.1111/j.1552-6569.2010.00535.x. 21091815 [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knosche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. 22846632 [DOI] [PubMed] [Google Scholar]
- Kendi A.T., Tan F.U., Kendi M., Erdal H.H., Tellioglu S. Magnetic resonance spectroscopy of the thalamus in essential tremor patients. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2005;15:362–366. doi: 10.1177/1051228405279039. 16254402 [DOI] [PubMed] [Google Scholar]
- Kessler R.M. Imaging methods for evaluating brain function in man. Neurobiology of Aging. 2003;24(Suppl. 1):S21–S35. doi: 10.1016/s0197-4580(03)00047-2. 12829104 [DOI] [PubMed] [Google Scholar]
- Klein J.C., Lorenz B., Kang J.S., Baudrexel S., Seifried C., van de Loo S., Steinmetz H., Deichmann R., Hilker R. Diffusion tensor imaging of white matter involvement in essential tremor. Human Brain Mapping. 2011;32:896–904. doi: 10.1002/hbm.21077. 20572209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic J.E., Criswell H.E., Osterman J.L., O'Buckley T.K., Wilkie M.E., Matthews D.B., Hamre K., Breese G.R., Homanics G.E., Morrow A.L. Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice. Journal of Clinical investigation. 2005;115:774–779. doi: 10.1172/JCI23625. 15765150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Kim Y.D., Im J.H., Kim H.J., Rinne J.O., Bhatia K.P. 123I-IPT brain SPECT study in essential tremor and Parkinson's disease. Neurology. 1999;52:1422–1426. doi: 10.1212/wnl.52.7.1422. 10227629 [DOI] [PubMed] [Google Scholar]
- Lin C.H., Chen C.M., Lu M.K., Tsai C.H., Chiou J.C., Liao J.R., Duann J.R. VBM reveals brain volume differences between Parkinson's disease and essential tremor patients. Frontiers in Human Neuroscience. 2013;7:247. doi: 10.3389/fnhum.2013.00247. http://www.ncbi.nlm.nih.gov/pubmed/23785322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M.A., Deans M.R., Paul D.L., Connors B.W. Rhythmicity without synchrony in the electrically uncoupled inferior olive. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. http://www.ncbi.nlm.nih.gov/pubmed/12486184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.D. Essential tremors: a family of neurodegenerative disorders? Archives of Neurology. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. http://www.ncbi.nlm.nih.gov/pubmed/19822775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.D. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem inquiry. Lancet Neurology. 2010;9:613–622. doi: 10.1016/S1474-4422(10)70090-9. 20451458 [DOI] [PubMed] [Google Scholar]
- Louis E.D., Babij R., Cortes E., Vonsattel J.P., Faust P.L. The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Movement Disorders: Official Journal of the Movement Disorder Society. 2013;28:779–786. doi: 10.1002/mds.25400. http://www.ncbi.nlm.nih.gov/pubmed/23483605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.D., Babij R., Lee M., Cortes E., Vonsattel J.P. Quantification of cerebellar hemispheric Purkinje cell linear density: 32 ET cases versus 16 controls. Movement Disorders: Official Journal of the Movement Disorder Society. 2013;28:1854–1859. doi: 10.1002/mds.25629. 23925732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.D., Faust P.L., Vonsattel J.P., Honig L.S., Rajput A., Robinson C.A., Rajput A., Pahwa R., Lyons K.E., Ross G.W., Borden S., Moskowitz C.B., Lawton A., Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain: a Journal of Neurology. 2007;130:3297–3307. doi: 10.1093/brain/awm266. 18025031 [DOI] [PubMed] [Google Scholar]
- Louis E.D., Ferreira J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2010;25:534–541. doi: 10.1002/mds.22838. 20175185 [DOI] [PubMed] [Google Scholar]
- Louis E.D., Ford B., Frucht S., Barnes L.F., Tang M., Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Annals of Neurology. 2001;49:761–769. doi: 10.1002/ana.1022. 11409428 [DOI] [PubMed] [Google Scholar]
- Louis E.D., Ford B., Lee H., Andrews H., Cameron G. Diagnostic criteria for essential tremor: a population perspective. Archives of Neurology. 1998;55:823–828. doi: 10.1001/archneur.55.6.823. 9626774 [DOI] [PubMed] [Google Scholar]
- Louis E.D., Shungu D.C., Chan S., Mao X., Jurewicz E.C., Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neuroscience Letters. 2002;333:17–20. doi: 10.1016/s0304-3940(02)00966-7. 12401550 [DOI] [PubMed] [Google Scholar]
- Mally J., Baranyi M., Vizi E.S. Change in the concentrations of amino acids in CSF and serum of patients with essential tremor. Journal of Neural Transmission (Vienna, Austria: 1996) 1996;103:555–560. doi: 10.1007/BF01273153. 8811501 [DOI] [PubMed] [Google Scholar]
- Marsden C.D., Obeso J.A., Rothwell J.C. Benign essential tremor is not a single entity. In: Yahr M.D., editor. Current Concepts in Parkinson's Disease. Excerpta Medica; Amsterdam: 1983. pp. 31–46. [Google Scholar]
- Martinelli P., Rizzo G., Manners D., Tonon C., Pizza F., Testa C., Scaglione C., Barbiroli B., Lodi R. Diffusion-weighted imaging study of patients with essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2007;22:1182–1185. doi: 10.1002/mds.21287. 17469200 [DOI] [PubMed] [Google Scholar]
- Moll N.M., Rietsch A.M., Thomas S., Ransohoff A.J., Lee J.C., Fox R., Chang A., Ransohoff R.M., Fisher E. Multiple sclerosis normal-appearing white matter: pathology-imaging correlations. Annals of Neurology. 2011;70:764–773. doi: 10.1002/ana.22521. 22162059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins P.G., McGonigle D.J., O'Gorman R.L., Puts N.A., Vidyasagar R., Evans C.J., Edden R.A. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. http://www.ncbi.nlm.nih.gov/pubmed/23246994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti G., Manners D., Novellino F., Condino F., Malucelli E., Barbiroli B., Tonon C., Arabia G., Salsone M., Giofre L., Testa C., Lanza P., Lodi R., Quattrone A. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–994. doi: 10.1212/WNL.0b013e3181d5a460. 20308683 [DOI] [PubMed] [Google Scholar]
- Novellino F., Cherubini A., Chiriaco C., Morelli M., Salsone M., Arabia G., Quattrone A. Brain iron deposition in essential tremor: a quantitative 3-Tesla magnetic resonance imaging study. Movement Disorders: Official Journal of the Movement Disorder Society. 2013;28:196–200. doi: 10.1002/mds.25263. 23238868 [DOI] [PubMed] [Google Scholar]
- Ogawa S. Finding the BOLD effect in brain images. Neuroimage. 2012;62:608–609. doi: 10.1016/j.neuroimage.2012.01.091. 22309802 [DOI] [PubMed] [Google Scholar]
- Oliveira A.P., Brickman A.M., Provenzano F.A., Muraskin J., Louis E.D. White matter hyperintensity burden on magnetic resonance imaging in essential tremor. Tremor and Other Hyperkinetic Movements (New York, N.Y.) 2012;2 doi: 10.7916/D8K64GS0. http://www.ncbi.nlm.nih.gov/pubmed/23439769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte A., Halsband U. Brain imaging tools in neurosciences. Journal of Physiology, Paris. 2006;99:281–292. doi: 10.1016/j.jphysparis.2006.03.011. 16713203 [DOI] [PubMed] [Google Scholar]
- Pagan F.L., Butman J.A., Dambrosia J.M., Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;60:1344–1347. doi: 10.1212/01.wnl.0000065885.15875.0d. 12707440 [DOI] [PubMed] [Google Scholar]
- Paris-Robidas S., Brochu E., Sintes M., Emond V., Bousquet M., Vandal M., Pilote M., Tremblay C., Di P.T., Rajput A.H., Rajput A., Calon F. Defective dentate nucleus GABA receptors in essential tremor. Brain: a Journal of Neurology. 2012;135:105–116. doi: 10.1093/brain/awr301. 22120148 [DOI] [PubMed] [Google Scholar]
- Park Y.G., Park H.Y., Lee C.J., Choi S., Jo S., Choi H., Kim Y.H., Shin H.S., Llinas R.R., Kim D. Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10731–10736. doi: 10.1073/pnas.1002995107. 20498062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson study group. A multicenter assessment of dopamine transporter imaging with DOPASCAN/SPECT in parkinsonism. Parkinson Study Group. Neurology. 2000;55:1540–1547. doi: 10.1212/wnl.55.10.1540. 11094111 [DOI] [PubMed] [Google Scholar]
- Passamonti L., Novellino F., Cerasa A., Chiriaco C., Rocca F., Matina M.S., Fera F., Quattrone A. Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain: A Journal of Neurology. 2011;134:2274–2286. doi: 10.1093/brain/awr164. 21747127 [DOI] [PubMed] [Google Scholar]
- Pedrosa D.J., Reck C., Florin E., Pauls K.A., Maarouf M., Wojtecki L., Dafsari H.S., Sturm V., Schnitzler A., Fink G.R., Timmermann L. Essential tremor and tremor in Parkinson's disease are associated with distinct “tremor clusters” in the ventral thalamus. Experimental Neurology. 2012;237:435–443. doi: 10.1016/j.expneurol.2012.07.002. 22809566 [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. http://www.ncbi.nlm.nih.gov/pubmed/18985121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa T., Russo M., Vidailhet M., Roze E., Lehericy S., Bonnet C., Apartis E., Legrand A.P., Marais L., Meunier S., Gallea C. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: an open label trial. Brain Stimulation. 2013;6:175–179. doi: 10.1016/j.brs.2012.04.009. 22609238 [DOI] [PubMed] [Google Scholar]
- Prodoehl J., Li H., Planetta P.J., Goetz C.G., Shannon K.M., Tangonan R., Comella C.L., Simuni T., Zhou X.J., Leurgans S., Corcos D.M., Vaillancourt D.E. Diffusion tensor imaging of Parkinson's disease, atypical parkinsonism, and essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2013;13:1816–1822. doi: 10.1002/mds.25491. http://www.ncbi.nlm.nih.gov/pubmed/23674400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrone A., Cerasa A., Messina D., Nicoletti G., Hagberg G.E., Lemieux L., Novellino F., Lanza P., Arabia G., Salsone M. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR. American Journal of Neuroradiology. 2008;29:1692–1697. doi: 10.3174/ajnr.A1190. 18653686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raethjen J., Govindan R.B., Kopper F., Muthuraman M., Deuschl G. Cortical involvement in the generation of essential tremor. Journal of Neurophysiology. 2007;97:3219–3228. doi: 10.1152/jn.00477.2006. 17344375 [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Martin W.R., Herscovitch P., Mintun M.A., Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 1983;24:790–798. http://www.ncbi.nlm.nih.gov/pubmed/6604140 [PubMed] [Google Scholar]
- Rajput A.H., Adler C.H., Shill H.A., Rajput A. Essential tremor is not a neurodegenerative disease. Neurodegenerative Disease Management. 2012;2:259–268. doi: 10.2217/nmt.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput A.H., Robinson C.A., Rajput M.L., Robinson S.L., Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism & Related Disorders. 2012;18(5):626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Roselli F., Pisciotta N.M., Pennelli M., Aniello M.S., Gigante A., De Caro M.F., Ferrannini E., Tartaglione B., Niccoli-Asabella A., Defazio G., Livrea P., Rubini G. Midbrain SERT in degenerative parkinsonisms: a 123I−FP-CIT SPECT study. Movement Disorders: Official Journal of the Movement Disorder Society. 2010;25:1853–1859. doi: 10.1002/mds.23179. 20669272 [DOI] [PubMed] [Google Scholar]
- Sahin H.A., Terzi M., Ucak S., Yapici O., Basoglu T., Onar M. Frontal functions in young patients with essential tremor: a case comparison study. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:64–72. doi: 10.1176/jnp.18.1.64. 16525072 [DOI] [PubMed] [Google Scholar]
- Saini J., Bagepally B.S., Bhatt M.D., Chandran V., Bharath R.D., Prasad C., Yadav R., Pal P.K. Diffusion tensor imaging: tract based spatial statistics study in essential tremor. Parkinsonism & Related Disorders. 2012;18:477–482. doi: 10.1016/j.parkreldis.2012.01.006. 22297126 [DOI] [PubMed] [Google Scholar]
- Salvador G.A., Uranga R.M., Giusto N.M. Iron and mechanisms of neurotoxicity. International Journal of Alzheimer's Disease. 2010;2011:720658. doi: 10.4061/2011/720658. http://www.ncbi.nlm.nih.gov/pubmed/21234369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Groshar D., Inzelberg R., Hocherman S. Dopamine-transporter imaging and visuo-motor testing in essential tremor, practical possibilities for detection of early stage Parkinson's disease. Parkinsonism & Related Disorders. 2004;10:385–389. doi: 10.1016/j.parkreldis.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shill H.A., Adler C.H., Sabbagh M.N., Connor D.J., Caviness J.N., Hentz J.G., Beach T.G. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. 18413570 [DOI] [PubMed] [Google Scholar]
- Shin D.H., Han B.S., Kim H.S., Lee P.H. Diffusion tensor imaging in patients with essential tremor. AJNR. American Journal of Neuroradiology. 2008;29:151–153. doi: 10.3174/ajnr.A0744. 17921227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolze H., Petersen G., Raethjen J., Wenzelburger R., Deuschl G. The gait disorder of advanced essential tremor. Brain: a Journal of Neurology. 2001;124:2278–2286. doi: 10.1093/brain/124.11.2278. 11673328 [DOI] [PubMed] [Google Scholar]
- Symanski C., Shill H.A., Dugger B., Hentz J.G., Adler C.H., Jacobson S.A., Driver-Dunckley E., Beach T.G. Essential tremor is not associated with cerebellar Purkinje cell loss. Movement Disorders. 2014;18(5):626–628. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- Tran T., Ross B., Lin A. Magnetic resonance spectroscopy in neurological diagnosis. Neurologic Clinics. 2009;27(1):21–60. doi: 10.1016/j.ncl.2008.09.007. 19055974 [DOI] [PubMed] [Google Scholar]
- Trillenberg P., Fuhrer J., Sprenger A., Hagenow A., Kompf D., Wenzelburger R., Deuschl G., Heide W., Helmchen C. Eye–hand coordination in essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2006;21:373–379. doi: 10.1002/mds.20729. 16211601 [DOI] [PubMed] [Google Scholar]
- van der Meer J.N., Tijssen M.A., Bour L.J., van Rootselaar A.F., Nederveen A.J. Robust EMG–fMRI artifact reduction for motion (FARM) Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2010;121:766–776. doi: 10.1016/j.clinph.2009.12.035. 20117046 [DOI] [PubMed] [Google Scholar]
- van Rootselaar A.F., Maurits N.M., Renken R., Koelman J.H., Hoogduin J.M., Leenders K.L., Tijssen M.A. Simultaneous EMG–functional MRI recordings can directly relate hyperkinetic movements to brain activity. Human Brain Mapping. 2008;29:1430–1441. doi: 10.1002/hbm.20477. 17979119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rootselaar A.F., Renken R., de Jong B.M., Hoogduin J.M., Tijssen M.A., Maurits N.M. fMRI analysis for motor paradigms using EMG-based designs: a validation study. Human Brain Mapping. 2007;28:1117–1127. doi: 10.1002/hbm.20336. 17274019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jiang Y.P., Liu X.D., Chen Z.P., Yang L.Q., Liu C.J., Xiang J.D., Su H.L. 99mTc-TRODAT-1 SPECT study in early Parkinson's disease and essential tremor. Acta Neurologica Scandinavica. 2005;112:380–385. doi: 10.1111/j.1600-0404.2005.00517.x. 16281920 [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Josephs K.A. Voxel-based morphometry and its application to movement disorders. Parkinsonism & Related Disorders. 2007;13(Suppl. 3):S406–S416. doi: 10.1016/S1353-8020(08)70039-7. 18267273 [DOI] [PubMed] [Google Scholar]
- Wills A.J., Jenkins I.H., Thompson P.D., Findley L.J., Brooks D.J. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Annals of Neurology. 1994;36:636–642. doi: 10.1002/ana.410360413. 7944296 [DOI] [PubMed] [Google Scholar]
- Wills A.J., Jenkins I.H., Thompson P.D., Findley L.J., Brooks D.J. A positron emission tomography study of cerebral activation associated with essential and writing tremor. Archives of Neurology. 1995;52:299–305. doi: 10.1001/archneur.1995.00540270095025. 7872885 [DOI] [PubMed] [Google Scholar]
- Zesiewicz T.A., Elble R., Louis E.D., Hauser R.A., Sullivan K.L., Dewey R.B., Jr., Ondo W.G., Gronseth G.S., Weiner W.J. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. 15972843 [DOI] [PubMed] [Google Scholar]
- Zeuner K.E., Molloy F.M., Shoge R.O., Goldstein S.R., Wesley R., Hallett M. Effect of ethanol on the central oscillator in essential tremor. Movement Disorders: Official Journal of the Movement Disorder Society. 2003;18:1280–1285. doi: 10.1002/mds.10553. 14639668 [DOI] [PubMed] [Google Scholar]
- Hua S.E., Lenz F.A., Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. Journal of neurophysiology. 2005;93(1):117–127. doi: 10.1152/jn.00527.2004. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15317839 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials for Neuroimaging essentials in essential tremor: A systematic review.


