Abstract
The objectives of this prospective study in 62 moderate–severe TBI patients were to investigate volume change in cortical gray matter (GM), hippocampus, lenticular nucleus, lobar white matter (WM), brainstem and ventricles using a within subject design and repeated MRI in the early phase (1–26 days) and 3 and 12 months postinjury and to assess changes in GM apparent diffusion coefficient (ADC) in normal appearing tissue in the cortex, hippocampus and brainstem. The impact of Glasgow Coma Scale (GCS) score at admission, duration of post-traumatic amnesia (PTA), and diffusion axonal injury (DAI) grade on brain volumes and ADC values over time was assessed. Lastly, we determined if MRI-derived brain volumes from the 3-month scans provided additional, significant predictive value to 12-month outcome classified with the Glasgow Outcome Scale—Extended after adjusting for GCS, PTA and age.
Cortical GM loss was rapid, largely finished by 3 months, but the volume reduction was unrelated to GCS score, PTA, or presence of DAI. However, cortical GM volume at 3 months was a significant independent predictor of 12-month outcome. Volume loss in the hippocampus and lenticular nucleus was protracted and statistically significant first at 12 months. Slopes of volume reduction over time for the cortical and subcortical GGM were significantly different. Hippocampal volume loss was most pronounced and rapid in individuals with PTA > 2 weeks. The 3-month volumes of the hippocampus and lentiform nucleus were the best independent predictors of 12-month outcome after adjusting for GCS, PTA and age. In the brainstem, volume loss was significant at both 3 and 12 months. Brainstem volume reduction was associated with lower GCS score and the presence of DAI. Lobar WM volume was significantly decreased first after 12 months. Surprisingly DAI grade had no impact on lobar WM volume. Ventricular dilation developed predominantly during the first 3 months, and was strongly associated with volume changes in the brainstem and cortical GM, but not lobar WM volume.
Higher ADC values were detected in the cortex in individuals with severe TBI, DAI and PTA > 2 weeks, from 3 months. There were no associations between ADC values and brain volumes, and ADC values did not predict outcome.
Keywords: Post-traumatic amnesia, Diffuse axonal injury, Glasgow Coma Scale, ADC, Outcome
Highlights
-
•
Longitudinal study of brain volume changes following TBI
-
•
3 month MRI derived volumes are independent predictors of outcome at 12 months.
-
•
PTA, GCS and DAI have different impacts on different brain volumes.
-
•
Subcortical and cortical GM volume losses follow significantly different trajectories.
-
•
Significant changes in cortical ADC values develop slowly while volume changes are rapid.
1. Introduction
General cerebral atrophy is a common consequence of moderate to severe traumatic brain injury (TBI) (Trivedi et al., 2007; Ding et al., 2008; Sidaros et al., 2009; Warner et al., 2010a). This general atrophy is not predicted by focal lesion volume (Marcoux et al., 2008), and develops over at least 3 years with the bulk loss occurring in the first year (Sidaros et al., 2009; Blatter et al., 1997). However, there is limited knowledge on the trajectories of volume changes for different brain structures, especially in humans. There is evidence from rodent models of closed head injury that brain region and injury type determine the histopathological response, and hence degree of tissue loss and outcome (Fox et al., 1998; Immonen et al., 2009; Meaney and Smith, 2011). Longitudinal studies of structural changes following TBI in humans have so far only contained two, often poorly defined, time points of MRI (Ross, 2011), which is insufficient to describe trajectories of volume loss in different brain structures. Furthermore, the impact of different clinical characteristics related to TBI subtype classification, e.g. injury severity and presence of diffuse axonal injury (DAI)/traumatic axonal injury, on trajectory of volume changes in different brain structures has not been explored systematically.
Based on the animal literature we predicted that cortical volume declines first, mainly between the early and 3-month scans. To the best of our knowledge there are no human studies documenting longitudinal cortical volume changes postinjury. Rodent TBI studies consistently show that the histopathological response including neuronal necrosis and apoptosis is largely completed within 3 months after injury in the cortex (Conti et al., 1998; Rodriguez-Paez et al., 2005; Sato et al., 2001), although volume loss may continue for 12 months (Smith et al., 1997).
In the hippocampus, on the other hand, protracted neuronal loss lasting for at least 12 months is described in animals (Conti et al., 1998; Smith et al., 1997; Williams et al., 2001). In addition, prominent changes in synapse morphology and reduced hippocampal neurogenesis are reported (Gao et al., 2011; Scheff et al., 2005; Gao et al., 2008). Hippocampal volume loss is reported to be greatest during the first weeks, but continues for at least 12 months in rodents (Immonen et al., 2009; Smith et al., 1997). Thus, we predicted that hippocampal volume loss in humans following moderate–severe TBI would be protracted, and continue during the entire observation period.
For the lenticular nucleus less information is available, but quite disparate histopathological results are reported for other subcortical GM nuclei. In animals and humans, both very early and slowly evolving histopathological changes have been shown (Hicks et al., 1996; Greer et al., 2011; Tong et al., 2002). Patient studies have documented both volume loss (Warner et al., 2010b; Bendlin et al., 2008; Wilde et al., 2007) and no volume change in the lenticular nucleus (Salmond et al., 2005). Taken together the data suggest that lenticular nucleus volume most likely declines slowly, and we expected to see a significant reduction at 12 months.
Lobar WM volume changes are described as the slowest and take place over a very protracted time period due to Wallerian/Wallerian-like degradation being lengthy and incomplete in the brain compared to the peripheral nervous system (Vargas and Barres, 2007; Coleman, 2005; Coleman and Freeman, 2010). Significant lobar WM loss was therefore expected to develop between 3 and 12 months after injury.
Human studies have shown that the brainstem volume is reduced in the chronic phase after TBI (Sidaros et al., 2009; Kim et al., 2008), but animal studies have not focused on this region. We anticipated that brainstem volumes decreased slowly, similar to the lobar WM.
TBI leads to ventricular dilation in both humans and animals. One patient study reports that the period of ventricular dilation lasts until 2–7 months postinjury with no significant increase thereafter (Blatter et al., 1997). In rodents ventricular enlargement is described to take place until 6 months postinjury (Bouilleret et al., 2009; Liu et al., 2010). We therefore expected the ventricular volume increase to take place mainly between the early and 3-month scans. Ventricular dilation is considered to result primarily from WM volume loss (Anderson and Bigler, 1995), but if ventricular dilation develops early after TBI, GM volume loss may also contribute significantly. We therefore investigated the relationship between ventricular volume change over time and the volume changes in cortical GM, lobar WM and brainstem to establish the relative importance of volume loss in these structures to ventricular dilation.
Furthermore, the impact of clinical measures of TBI mechanism and severity, i.e. Glasgow Coma Scale (GCS), duration of post-traumatic amnesia (PTA), and DAI, on volume loss in different brain structures was assessed. Lower GCS scores were expected to lead to more severe loss of lobar WM and brainstem volumes, as unconsciousness postinjury is associated with deeper lesions (Jenkins et al., 1986) and increasing mechanical forces converging in the midbrain (Meaney and Smith, 2011). Longer duration of PTA (>2 weeks) was predicted to be associated with lower hippocampal volumes since PTA is a manifestation of hippocampal dysfunction and hence likely degree of injury. DAI was predicted to result in increased loss of lobar WM volumes, and increasing DAI severity was predicted to lead to greater brainstem volume loss. Based on postmortem evidence, DAI also gives rise to increased cortical thinning and could thus reduce cortical volume (Maxwell et al., 2010).
GM volume changes could be accompanied by changes in GM microstructure. The apparent diffusion coefficient (ADC) obtained from diffusion weighted imaging (DWI) is a measure of the degree of random isotropic diffusion. In GM diffusion is not restricted to a certain direction as in WM, and ADC values are considered to reflect cell number (neurons and for instance inflammatory cells), cell size (swelling vs. shrinkage), as well as other cytoarchitectonic features such as the composition and fraction of neuropil and extracellular matrix. It has previously been demonstrated that TBI patients with lesions visible on conventional MRI have increased ADC values in the brainstem the first month after TBI (Brandstack et al., 2011), while ADC values in cortex and subcortical GM increased between ~2 and ~3 months in moderate–severe TBI (Bendlin et al., 2008). Thus we expected the severe TBI patients to have increased ADC values in the normal appearing midbrain at 3 months. On the other hand, the ADC values in normal appearing cortical GM should increase more between 3 and 12 months in both moderate and severe TBI. Since DAI leads to loss of neurons, neuronal soma shrinkage, axonal changes and myelin loss in cortex (Greer et al., 2011; Coleman and Freeman, 2010; Maxwell et al., 2010), we expected that cortical ADC values would be higher in DAI than in non-DAI patients after 3 and 12 months. Moreover, we predicted that longer PTA would be associated with increased hippocampal ADC values due to increased cell loss.
The primary objectives of this prospective study in moderate and severe TBI patients were to investigate the trajectories of volume change in total cortical GM, hippocampus, lenticular nucleus, lobar WM, brainstem and ventricles using a within subject design and repeated MRI in the early phase and 3 and 12 months after injury and to relate the volume changes to clinical measures of TBI mechanism and severity; GCS score, PTA, and DAI. Changes in local diffusion properties in GM were studied to identify any associations between changes in tissue microstructure as described with ADC values and the clinical measures of TBI mechanism and severity; GCS score, PTA, and DAI. In addition the relationship between ADC values and brain volumes was examined. Our final aim was to determine if MRI-derived brain volumes and ADC values obtained after 3 months provided additional, significant predictive value to 12-month outcome after adjusting for GCS score, PTA and age.
2. Materials and methods
The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Social Science Data Services. Written consent was obtained from the patient or, for individuals underaged or incapacitated, their next of kin. Permission was obtained from the Norwegian Directorate of Health to use data from the deceased without consent from their next of kin.
2.1. Subjects
124 moderate to severe (GCS score ≤ 13) TBI patients aged 14–65 years were admitted to the Neurosurgical Department, St. Olav's Hospital, Trondheim University Hospital, Norway, in the period October 10, 2004–October 10, 2007. St. Olav's Hospital is the only level 1 trauma center in a region of 680,000 inhabitants. 17 of these patients did not participate in MRI study (did not consent, could not be examined with MRI or did not participate for other reasons). An additional 20 patients died before acquisition of follow-up MRI scans. Longitudinal MRI data was thus available for 87 TBI patients. Only patients with at least 2 successful segmentations of the T1-weighted 3D brain volumes were included in the study, therefore another 25 patients were excluded.
For each T1-weighted 3D scan the segmentation quality was assessed visually and scans were excluded if hematomas were included as brain tissue, or GM, WM or ventricles were incorrectly delineated.
See Fig. 1 for details about patient inclusion and exclusion and Fig. 2 for an example of successful NeuroQuant segmentation. Six scans were excluded due to insufficient segmentation quality. Five of these were acquired in the early phase, and segmentation failed due to hematomas segmented as brain tissue (n = 3), incorrect delineation of WM and GM of injured frontal lobe (n = 1), and excessive motion (n = 1). One 3-month scan failed due to an MRI technical mistake.
Fig. 1.
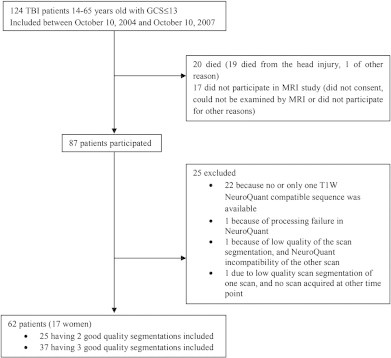
TBI patients with GCS ≤ 13 that could not be explained by other factors than traumatic head injury, admitted to the Neurosurgical Department, St. Olav's Hospital, Trondheim University Hospital, Norway in the period 2004–2007. Flow chart of the inclusion and exclusion criteria, and the final number of patients participating in the current study.
Fig. 2.
Successful NeuroQuant segmentation of repeated MRIs obtained in a 21-year-old patient with moderate traumatic brain injury at a) 9 days postinjury and b) 3 months and c) 12 months postinjury.
Unsuccessful segmentations resulted in exclusion of two TBI patients. Furthermore, in four TBI patients the number of scans was reduced from three to two.
In total 161 segmented T1-weighted 3D scans from 62 moderate to severe TBI patients were included in the final sample. 37 patients had three and 25 patients had two scans successfully segmented.
2.2. MRI and image processing
Scanning was performed at three different time points: early phase (0–26 days postinjury) and 3 months and 12 months postinjury. The early phase scan was acquired as soon as the clinical condition of the patients allowed it.
2.2.1. Scan protocol
MRI acquisition was performed on a 1.5 T Siemens Symphony Sonata scanner (Siemens, Erlangen, Germany) with an eight-channel head coil. Imaging was performed following a standardized protocol, where slice positioning for transversal scans was parallel with the anterior–posterior commissural line. A T1-weighted 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence was obtained in the sagittal plane with TR = 7.1 ms; TE = 3.45 ms; flip angle = 7°; TI = 1000 ms; FOV = 256 × 256; acquisition matrix of 256 × 192 × 128, reconstructed to 256 × 256 × 128, giving a reconstructed voxel resolution of 1.00 × 1.00 × 1.33 mm. For diffusion-weighted imaging a single-shot spin echo planar imaging sequence with 19 slices, slice thickness 5 mm, TR = 3300 ms; TE = 110 ms; NEX = 4; acquisition matrix size 256 × 256; FoV = 230; in-plane resolution 0.9 × 0.9 mm and baseline images (b = 0 s/mm2) plus varying diffusion gradient strength along each of three orthogonal directions with b = 500 and 1000 s/mm2 was used. Diffusion trace maps were computed from the isotropic diffusion images and used to estimate ADC values. FLAIR, T2, and T2*-weighted hemosequences were also acquired.
2.2.2. Segmentation of the T1-weighted 3D brain scans
Fully automated segmentation was performed using NeuroQuant (CorTechs Labs, La Jolla, CA, US) which is a FDA-cleared tool for clinical evaluation of hippocampal atrophy in mild cognitive impairment and Alzheimer's disease (Brewer et al., 2012). The output from NeuroQuant segmentation contains volumes of the total lobar WM, total cortical GM, lateral, third, fourth, and inferior lateral ventricles, cerebellum, hippocampus, amygdala, caudate, putamen, pallidum, thalamus and brainstem given as both absolute volume in cm3 and relative to the intracranial volume (ICV). In this study only volumes relative to ICV (%ICV) are used. The ICV-corrected volumes of the segmented structures from the left and right hemispheres were combined. The ICVs in the early phase and 12-month scan were significantly different (linear mixed model; mean ± standard error: early phase 1740.06 ± 19.98 cm3; at 12 months 1706.10 ± 19.74 cm3; p < 0.001). ICV is estimated from the total brain volume in NeuroQuant, and may therefore be influenced by brain edema in the early phase and brain atrophy in the chronic phase. Differences between ICV measurements based on total brain volume in the early and chronic phases are not surprising in TBI patients (Ding et al., 2008; Sidaros et al., 2009). Therefore, volumes of segmented structures were normalized and expressed as proportions of the ICV estimated from the 3-month scan.
Because of the large number of segmented brain structures, we chose to analyze the brain structures with low frequency of segmentation mistakes. The ICV-corrected volumes of cortical GM, hippocampus, brainstem, lenticular nucleus, lobar WM, and ventricles were compared between the early, 3-month and 12-month scans. Putamen and pallidum were evaluated together as they form the lenticular nucleus. The lenticular nucleus was used since it was better delineated than the thalamus in the TBI patients. Total ventricular volume was calculated as the sum of third, fourth and lateral ventricular volumes. We evaluated the ventricular volumes together since all ventricles have been shown to dilate in TBI (Warner et al., 2010b; Kim et al., 2008).
In addition the volume change over time was calculated for all structures. For the solid structures the following was used: early phase volume minus volume at 3 months, and early phase volume minus volume at 12 months. For the ventricles: volume at 3 months minus early phase volume, and volume at 12 months minus early phase volume. This was done in order for all mean changes to be positive.
Total brain atrophy from the early phase to 3 and 12 months was estimated using linear mixed model based on the sum of the volume changes of the solid structures (cortical GM, hippocampus, lenticular nucleus, lobar WM, and brainstem) subtracting the ventricular volume change (as ventricular volume change is defined as reversed, please see above).
2.2.3. ADC measurements in GM
ADC measurements were performed in PACS using Spectra Workstation IDS5 11.4.1 in all 62 patients. Ten circle-shaped ROIs with radius of 2.7 mm were positioned in apparently healthy GM bilaterally in the cortex, hippocampus and the brainstem (Fig. 3). These ROIs were chosen because they are located to regions where ADC value changes have been reported earlier (see Introduction), the regions are readily identifiable, and the ROIs are located to parts of GM with minimal WM contamination. All ROIs were positioned in apparently healthy tissue within the structure of interest. In patients with visible focal pathology on MRI the ROI was moved to the closest healthy tissue if necessary. The cortical ROIs in the superior frontal sulcus and postcentral sulcus were positioned at the sulcal depth in order to avoid measuring ADC in GM close to the skull with possible minor contusions. The cortical ROIs were placed on the same slice, just above the most dorsal slice displaying the lateral ventricles. In the posterior insular gyrus ROIs were set on the slice where the head of the caudate nucleus had its greatest extent. ROIs in the hippocampus and brainstem were positioned on the most caudal slice where mesencephalon was still visible. In the hippocampus the ROIs were located as laterally as possible in the anterior part of the hippocampal body in order to avoid placing the ROIs within the hippocampal head which frequently suffers from contusions in TBI. For all ROIs, the ADC values from the left and right hemispheres were averaged. The image analyst (V.B.) was blinded to clinical information. Intra-rater reliability with regard to ROI positioning and subsequent ADC measurements was assessed in 14 scans which were reanalyzed after >1 month from the first assessment. Intraclass correlation coefficients (ICC) using two-way random single measures were used to calculate reliability.
Fig. 3.
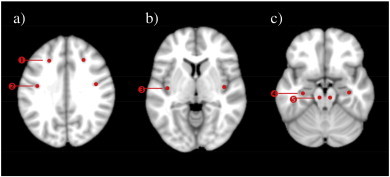
ROI placement for ADC measurements in a)  superior frontal sulcus ROI,
superior frontal sulcus ROI,  postcentral sulcus ROI; b)
postcentral sulcus ROI; b)  insular gyrus ROI; c)
insular gyrus ROI; c)  hippocampal ROI,
hippocampal ROI,  brainstem ROI. Circle-shaped ROIs with radius of 2.7 mm were positioned in apparently healthy tissue. In TBI patients with visible focal pathology, the ROI was moved to the closest apparently healthy GM tissue within the structure of interest.
brainstem ROI. Circle-shaped ROIs with radius of 2.7 mm were positioned in apparently healthy tissue. In TBI patients with visible focal pathology, the ROI was moved to the closest apparently healthy GM tissue within the structure of interest.
2.3. Injury-related variables
GCS score was recorded at hospital admittance, or before intubation in case of a pre-hospital intubation. All patients included had GCS ≤ 13 that could not be explained by other factors than head injury, as explained in previous publications from this cohort (Skandsen et al., 2010; Moen et al., 2012). The head injury severity scale (HISS) is based on GCS, where GCS ≤ 8 is considered severe while GCS 9–13 as moderate head injury (Stein and Spettell, 1995).
GCS scores were available for all included patients.
PTA was recorded and dichotomized into PTA > 2 weeks and PTA ≤ 2 weeks which corresponds to moderate and moderate–severe Mississippi PTA intervals, respectively. Mississippi PTA intervals have been shown to be more accurate predictors of TBI outcome than the traditional Russell intervals (Nakase-Richardson et al., 2011). Data on PTA were missing in 4 patients. These were excluded from analyses including PTA.
DAI classification was performed by experienced senior neuroradiologists based on the early FLAIR, diffusion-, T2- and T2*-weighted scans, for details see Skandsen et al. (2010). DAI was classified into grade 1; traumatic lesions confined to lobar WM, grade 2; lesions also detected in the corpus callosum, and in grade 3; presence of brainstem lesions (Gentry, 1994). DAI classification was available for all included patients.
2.4. Outcome assessment
Global outcome was assessed by telephone interview 12 months after injury using the structured interview for Glasgow Outcome Scale—Extended (GOSE) (Jennett and Bond, 1975; Jennett et al., 1981). To reduce the potential error associated with the telephone setting (LeGrand et al., 2007), relatives or caregivers also provided information, in two-thirds of the cases, and the best source of information was used (based on the judgment of the authors) for details see Skandsen et al. (2010). Outcome data were available for all included individuals, but missing in 5 non-included patients. Patients were dichotomized into disability (GOSE < 7) and good recovery (GOSE ≥ 7) after 12 months.
2.5. Statistical analyses
2.5.1. Characteristics of included and excluded TBI patients.
The Mann–Whitney U-test, χ2-test, independent samples T-test and the Wilcoxon U-test were used to compare demographic and clinical data.
2.5.2. Longitudinal brain volume changes
Significant changes in the volume of each of the brain structures at each time point was calculated with a linear mixed effects model.
The relationships between ventricular volume change and volume changes of lobar WM, cortical GM and brainstem were explored using linear regression separately for the periods early to 3 months and early to 12 months. There were separate regression models for lobar WM, cortical GM, and brainstem volume changes as the volume changes were expected to be correlated.
Impact of TBI subgroup on brain volumes was investigated in the following dichotomized groups; moderate vs. severe TBI, PTA ≤ 2 weeks vs. > 2 weeks, and DAI vs. non-DAI. The volumes were compared over time within each group and between the dichotomized groups. For DAI a subdivision into four DAI groups, i.e. non-DAI = 0, and DAI grades 1, 2, and 3 were performed and the effect of degree of DAI was assessed with one-way ANOVA.
A significant F-value was followed by a paired Student's T-tests within the group, and with independent samples T-test between the dichotomized groups. The resulting p-values were Bonferroni corrected for number of time points evaluated for each structure separately.
The following analyses of volume differences between the different injury mechanism groups were performed:
2.5.2.1. Moderate vs. severe TBI
Differences between cortical GM, hippocampal, lenticular nucleus, lobar WM, brainstem and ventricular volumes in the early phase and at 3 and 12 months were investigated. Since DAI was more frequent in severe TBI, the presence of DAI was included in the analysis of lobar WM volume differences between moderate and severe TBI.
2.5.2.2. Moderate vs. severe TBI
Differences between cortical GM, hippocampal, and ventricular volumes in the early phase and at 3 and 12 months were investigated.
2.5.2.3. DAI vs. non-DAI
Differences in cortical GM, lobar WM, brainstem, and ventricular volumes in the early phase and at 3 and 12 months were investigated.
2.5.2.4. DAI grades 0–3
The effect of degree of DAI (no DAI, DAI grade 1, 2 or 3) as an ordinal variable on brainstem and total ventricular volumes was assessed in the early phase and at 3 and 12 months.
2.5.3. Differences in volume changes over time between brain structures
The presence of statistically significant differences in rate of volume loss between different brain structures was evaluated in a linear mixed model with random intercept since this model fitted the data best, i.e. had the lowest Akaike's information criterion. There were not enough observations to build a model exploring non-linear changes over time. The following comparisons were done: cortical GM vs. hippocampus, cortical GM vs. lobar WM, hippocampus vs. lentiform nucleus, and hippocampus vs. lobar WM matter. For these analyses significant differences in the interaction term between volume loss over time and brain structure were calculated, and the estimated difference in slope with 95% confidence intervals was reported.
2.5.4. Outcome prediction
Hierarchical regression analysis was performed to determine if 3-month MRI-derived volumes contributed significantly to 12-month GOSE outcome after taking into account the established outcome predictors GCS score, PTA (PTA ≤ 2 weeks = 0, PTA > 2 weeks = 1) and age which were entered in the first step as the base model with which all subsequent models were compared. The MRI measures were cortical GM, hippocampal, lenticular nucleus and ventricular volumes at 3 months. In addition we used the Rotterdam CT scores (Maas et al., 2005) obtained from the worst CT scan, to see if this neuroimaging modality provided additional predictive value (Yuh et al., 2012). Overall variance explained (R2) and statistical significance were calculated for every model. R2-change and significance of F-value change were calculated as differences between the base model and all subsequent models.
2.5.5. Longitudinal analysis of ADC changes in GM
Intraclass correlation coefficients (ICC) using two way random single measures were used to calculate reliability of the ROI ADC values (Gwet, 2008).
A linear mixed effects model was used in the statistical comparisons of the ADC values in the different ROIs over time in the entire TBI group, and between the TBI subgroups; moderate vs. severe TBI, PTA ≤ 2 weeks vs. >2 weeks, and DAI vs. non-DAI. The effect of degree of DAI (non-DAI = 0, DAI grades 1, 2, and 3) was assessed with ANOVA. Significant F-values were followed by paired Student's T-test or independent sample T-test. The resulting p-values were Bonferroni corrected as described earlier. Associations between ADC values and corresponding volumes at 3 and 12 months were examined using Pearson correlation.
A similar hierarchical regression analysis as described for the volumes above was performed to establish if cortical ADC values from the superior frontal and post-central sulci ROIs at 3 months were independent significant predictors of 12 month outcome.
Clinical and demographic data (age, GCS and GOSE scores) are given as mean ± standard deviation, and volumes and ADC values as mean ± standard error. The threshold for statistical significance was p < 0.05 for all analyses, including analyses applying Bonferroni correction for multiple comparisons.
3. Results
3.1. TBI patient characteristics (Table 1)
Table 1.
TBI patient characteristics.
| All (n = 62) | Moderatea (n = 36) | Severeb (n = 26) | ||
|---|---|---|---|---|
| Age (years) | 32.5 ± 15.2 | 34.3 ± 15.8 | 30.1 ± 14.4 | |
| GCS | 9.1 ± 3.4 | 11.5 ± 1.6* | 5.7 ± 1.8* | |
| Mechanism of injury | Road accident | 34 (55%) | 19 (53%) | 15 (58%) |
| Fall injury | 23 (37%) | 13 (36%) | 10 (38%) | |
| Other | 4 (6%) | 3 (8%) | 1 (4%) | |
| Unknown | 1 (2%) | 1 (3%) | 0 | |
| Post-traumatic amnesia | >2 weeks | 19 (31%) | 5 (14%)* | 14 (54%)* |
| ≤2 weeks | 39 (63%) | 30 (83%)* | 9 (35%)* | |
| Diffuse axonal injury | No | 19 (31%) | 17 (47%)* | 2 (8%)* |
| Yes | 41 (66%) | 19 (53%)* | 22 (85%)* | |
| Grade 1 | 13 (21%) | 5 (14%)* | 8 (31%)* | |
| Grade 2 | 17 (27%) | 11 (31%)* | 6 (23%)* | |
| Outcome at 12 months | Grade 3 | 11 (18%) | 3 (8%)* | 8 (31%)* |
| With disability (GOSE < 7) | 27 (44%) | 12 (33%)* | 15 (58%)* | |
| Good recovery (GOSE 7–8) | 35 (56%) | 24 (67%)* | 11 (42%)* | |
| Days between injury and MRIc | Scan 1 | 10.2 ± 6.7 | 9.0 ± 6.2 | 12.1 ± 7.3 |
| Scan 2 | 98.4 ± 15.2 | 98.2 ± 14.3 | 98.6 ± 16.7 | |
| Scan 3 | 373.5 ± 26.3 | 377.1 ± 30.1 | 369.0 ± 20.3 | |
GCS 9–13.
GCS 3–8.
In days.
Significant difference between moderate and severe TBI.
Numbers of patients (percentages) or means ± standard deviations are shown. Percentages were calculated considering the total number of patients in each column.
GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Scale—Extended; MRI, magnetic resonance imaging.
There were no significant differences with regard to the clinical variables between included and excluded TBI patients who survived for 12 months. Of the included patients 58% suffered moderate and 42% severe TBI. See Table 1 for details.
Comparison between the included males (n = 45) and females (n = 17) showed that GCS score, HISS, and PTA and GOSE score at 12 months were similar between the sexes.
Injury mechanism, age and number of days between the head injury and the MRI scans were similar in the moderate and severe TBI patients. There were significantly more patients with PTA > 2 weeks, any grade of DAI and poor 12-month outcome in the severe than in the moderate TBI group.
3.2. Longitudinal brain structure volume changes in the entire TBI group (Figs. 4 and 5)
Fig. 4.
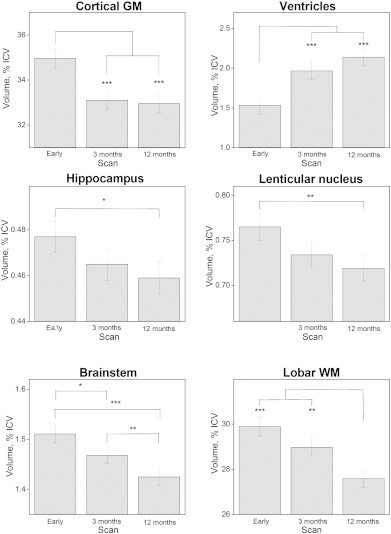
Longitudinal changes in mean ICV-corrected brain volumes with standard errors in the early phase (0–26 days post-injury, scan 1) and 3 months (scan 2), and 12 months (scan 3) postinjury. Volumes of the left and right sides of the structures were summed and evaluated together. ***p < 0.001, **p < 0.01, *p < 0.05.
Fig. 5.
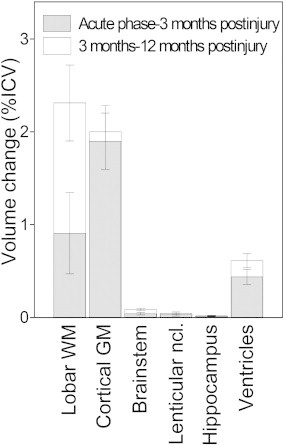
Illustration of the volume changes over time. Volume changes (as %ICV) early phase–3 months and 3 months–12 months postinjury. The differences between different time points are calculated using linear mixed model. Means ± error bars are plotted.
During the first 12 months after TBI significant volume differences were found for all analyzed structures (Fig. 4). Cortical GM experienced a rapid volume reduction and was significantly reduced at 3 months compared to the early scan, but did not change significantly after that. The hippocampal and lenticular nucleus volumes decreased more slowly and the volume was not significantly reduced before 12 months postinjury. Lobar WM volume was significantly reduced first at 12 months compared to both the early and the 3-month scan. Brainstem volumes decreased significantly between the early and 3-month scans and also between the 3-month and 12-month scans. The ventricular volume expanded from the early phase, but a significant increase was present first at 12-months. Statistical comparison of the interaction between slope of volume loss over time for the different brain structures demonstrated that cortical GM loss followed a significantly different trajectory compared to the hippocampus (estimated difference in slope was 1.2 (CI: 0.7–1.8), p < 0.0001), but not compared to lobar WM (estimated difference in slope was −0.04 (CI: −0.9–0.8); p = 0.9). The hippocampus and lenticular nucleus had similar trajectories of volume loss (estimated difference in slope was −0.01 (CI: −0.3–0.0); p = 0.1). For lobar WM and hippocampus significantly different trajectories of volume loss were present (estimated difference in slope was 1.2 (CI: 0.7–1.7); p < 0.0001).
Ventricular dilation was strongly associated with change in brainstem volumes at 3 months (R2 = 0.246, p = 0.001) and 12 months (R2 = 0.488, p < 0.001), and total cortical GM volume at 3 months (R2 = 0.143, p = 0.012) and 12 months (R2 = 0.331, p < 0.001). Ventricular dilation was not significantly associated with lobar WM volume changes at any time point.
The total brain volume decline was 3.8 ± 0.8% of ICV between the early and 3-month scans, and 5.7 ± 0.8% of ICV between the early and 12-month scans.
3.3. The impact of clinical TBI subtypes on brain volumes (Figs. 6 and 7)
Fig. 6.
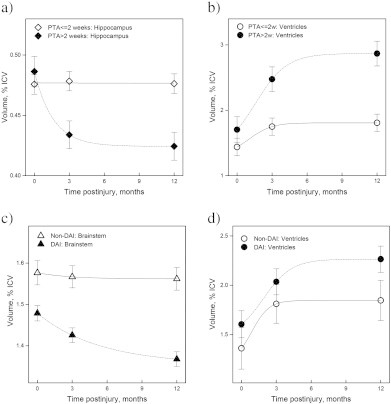
Mean volumes of different brain structures plotted for: a) hippocampus and b) ventricles of patients with PTA > and ≤2 weeks. c) Brainstem and d) ventricles of DAI and non-DAI patients. For statistical evaluation of volume differences see Materials and methods and Results.
Fig. 7.
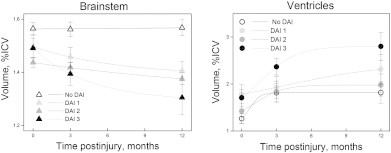
The association between the longitudinal changes in brainstem and ventricular ICV-corrected volumes for the non-DAI and the three DAI groups (DAI grades 1, 2, and 3). Figures show means and standard errors. Left and right side volumes were summed and evaluated together. Significant differences in brainstem and ventricular volumes between the non-DAI and the three DAI groups were found at all time points. Please note that the units on the y axis differ between the plots, and that the x axis does not cross the y axis at 0.
3.3.1. TBI severity
Lobar WM and brainstem volume at 12 months were smaller in the severe compared to the moderate TBI group (lobar WM: 26.34 ± 0.58% ICV vs. 28.60 ± 0.51% ICV, p = 0.012; brainstem: 1.36 ± 0.03% ICV vs. 1.47 ± 0.02% ICV, p = 0.005) while cortical GM, ventricular, hippocampal and lenticular nucleus volumes were similar. The difference in lobar WM volume at 12 months was significant also after correcting for the presence of DAI (p = 0.012).
Moreover, hippocampal volume change between the early and 3-month scans was larger in the severe group compared to the moderate group (0.03 ± 0.01 %ICV vs. 0.00 ± 0.01%ICV, p = 0.046). For the other structures, there were no differences in volume changes over time between moderate and severe TBI.
3.3.2. PTA duration (Fig. 6a, b)
Patients with PTA > 2 weeks had smaller hippocampi at 3 and 12 months, and larger ventricles at 3 and 12 months compared to patients with PTA ≤ 2 weeks. Cortical GM volumes did not differ significantly between the PTA groups.
Hippocampal volume change over time was larger in the PTA > 2 weeks than in the PTA ≤ 2 weeks group between the early and 3-month scans (0.05 ± 0.01 %ICV vs. 0.00 ± 0.01 %ICV, p = 0.003). Cortical GM and ventricular volume changes over time did not differ between the PTA groups.
3.3.3. DAI groups (Figs. 6c, d and 7)
Fig. 8.
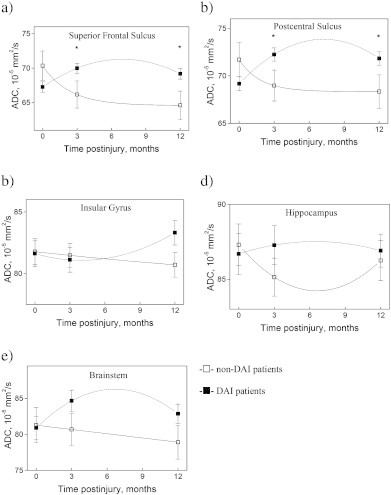
Longitudinal changes in mean ADC values with standard deviations in non-DAI (□) and DAI (▪) patients in the superior frontal sulcus (a), postcentral sulcus (b), insular gyrus (c), hippocampus (d) and brainstem (e). Right and left side values were averaged. Statistical significant differences between non-DAI and DAI groups are marked with *. Significant differences were found between: a) DAI (1) and DAI (2), p = 0.009; non-DAI (2) and DAI (2), p = 0.028; non-DAI (3) and DAI (3), p = 0.001. b) DAI (1) and DAI (2), p = 0.006; non-DAI (2) and DAI (2), p = 0.020; non-DAI (3) and DAI (3), p = 0.004. The p-values were corrected for multiple comparisons. Please note that the units on the y axis differ between the plots, and that the x axis does not cross the y axis at 0.
Smaller brainstem volumes were present at all time points in the DAI group compared to the non-DAI group. No differences in cortical GM, lobar WM or ventricular volumes were present between the DAI and non-DAI groups.
The non-DAI group had larger brainstem volumes than the DAI grade 2 group in the early phase; than the DAI grade 2 and 3 groups at 3 months, and the DAI grade 1, 2 and 3 groups at 12 months. In DAI 3 brainstem volumes decreased significantly between the early and 3 month scans, and further till 12 months. In DAI 2 brainstem volume decrease was present first after 12 months. In DAI 1 brainstem volumes decreased between the early scan and 12 months and between the 3 and 12 month scans. In the non-DAI group, brainstem volumes did not change with time.
No significant differences between the DAI groups were observed in ventricular volumes.
For the non-DAI, DAI 2 and DAI 3 groups the ventricular volume decreased between the early and 3 month scans, and between the early and 12 month scans. DAI 1 ventricular volume decreased between the early and 12 month scans.
3.4. Outcome prediction based on 3-month MRI volumes (Table 2)
Table 2.
Hierarchical regression analysis of 12-month outcome prediction using the 3-month brain structure volumes with GCS and PTA as base model.
| Models | R2 | R2-change | Significance of F change |
|---|---|---|---|
| Base model | 0.385 | ||
| CortGM 3 months | 0.515 | 0.053 | 0.023 |
| Ventricles 3 months | 0.467 | 0.005 | 0.494 |
| Hippocampus 3 months | 0.535 | 0.073 | 0.007 |
| Lenticular ncl. 3 months | 0.529 | 0.067 | 0.010 |
| Rotterdam CT score | 0.465 | 0.003 | 0.596 |
Base predictors: GCS, PTA duration (0 = PTA ≤ 2 weeks; 1 = PTA > 2 weeks), and age
CortGM predictors: GCS, PTA duration, age, and cortical GM volume at 3 months
Ventricular predictors: GCS, PTA duration, age, and ventricular volume at 3 months
Hippocampal predictors: GCS, PTA duration, age, and hippocampal volume at 3 months
Lenticular nucleus predictors: GCS, PTA duration, age, and lenticular nucleus volume at 3 months
Rotterdam CT score predictors: GCS, PTA duration, age, and Rotterdam CT score
CortGM, cortical gray matter; GCS, Glasgow Coma Scale; PTA, posttraumatic amnesia duration.
The hierarchical regression analyses with PTA, GCS scores and age in the base model demonstrated that the MRI-derived brain volumes of cortical GM, hippocampus, and lenticular nucleus at 3 months explained significantly more of the final outcome than the base model (Table 2). The ventricular 3-month volume did not add to the 12-month outcome prediction, and neither did the Rotterdam CT score.
3.5. Analysis of ADC value changes in GM
3.5.1. Intra-rater reliability of ADC measurements in the ROIs
ICC of repeated measurements were 0.74 for superior frontal sulcus, 0.86 for postcentral sulcus, 0.69 for insular gyrus, 0.88 for hippocampus and 0.89 for brainstem.
3.5.2. Overall changes in entire TBI group
No ADC value changes over time were found in any ROI in the entire TBI group.
3.5.3. The impact of clinical TBI subtypes on ADC value changes
3.5.3.1. TBI severity (Table 3a)
Table 3.
Longitudinal ADC values of TBI subgroups and comparison between the clinical TBI subtypes.
| a) Moderate vs. severe TBI. | ||||
|---|---|---|---|---|
| Moderate TBI | Severe TBI | p-Value | ||
| Superior frontal sulcus | ||||
| Early phase | 68.2 ± 1.1 | 68.1 ± 1.3 | NS | |
| 3 months | 66.9 ± 1.0 | 71.5 ± 1.2 | 0.011 | |
| 12 months | 65.0 ± 1.1 | 70.7 ± 1.2 | 0.005 | |
| Postcentral sulcus | ||||
| Early phase | 70.2 ± 0.9 | 69.6 ± 1.1 | NS | |
| 3 months | 69.9 ± 0.9 | 73.2 ± 1.0 | NS | |
| 12 months | 68.9 ± 1.0 | 73.2 ± 1.0 | 0.009 | |
| Insular gyrus | ||||
| Early phase | 81.5 ± 1.1 | 81.9 ± 1.3 | NS | |
| 3 months | 80.7 ± 1.0 | 81.9 ± 1.2 | NS | |
| 12 months | 81.4 ± 1.1 | 83.9 ± 1.2 | NS | |
| Hippocampus | ||||
| Early phase | 86.1 ± 1.4 | 88.0 ± 1.7 | NS | |
| 3 months | 85.8 ± 1.3 | 87.8 ± 1.5 | NS | |
| 12 months | 85.1 ± 1.4 | 88.7 ± 1.5 | NS | |
| Brainstem | ||||
| Early phase | 80.6 ± 1.7 | 81.5 ± 2.1 | NS | |
| 3 months | 80.5 ± 1.6 | 87.5 ± 1.9 | 0.016 | |
| 12 months | 80.3 ± 1.7 | 83.5 ± 1.9 | NS | |
| b) Patients with PTA ≤ 2 weeks vs. >2 weeks | ||||
| PTA ≤ 2 weeks | PTA > 2 weeks | p-Value | ||
| Superior frontal sulcus | ||||
| Early phase | 68.2 ± 1.0 | 67.9 ± 1.5 | NS | |
| 3 months | 68.0 ± 1.0 | 70.4 ± 1.5 | NS | |
| 12 months | 66.3 ± 1.1 | 70.8 ± 1.5 | 0.037 | |
| Postcentral sulcus | ||||
| Early phase | 70.0 ± 0.9 | 69.8 ± 1.4 | NS | |
| 3 months | 70.4 ± 0.9 | 72.9 ± 1.3 | NS | |
| 12 months | 69.9 ± 1.0 | 72.6 ± 1.3 | NS | |
| Insular gyrus | ||||
| Early phase | 81.7 ± 0.9 | 81.5 ± 1.4 | NS | |
| 3 months | 81.9 ± 0.9 | 79.5 ± 1.3 | NS | |
| 12 months | 81.8 ± 1.0 | 84.0 ± 1.3 | NS | |
| Hippocampus | ||||
| Early phase | 87.0 ± 1.1 | 86.2 ± 1.6 | NS | |
| 3 months | 86.0 ± 1.1 | 85.0 ± 1.5 | NS | |
| 12 months | 86.1 ± 1.1 | 87.8 ± 1.5 | NS | |
| Brainstem | ||||
| Early phase | 80.8 ± 1.5 | 81.3 ± 2.2 | NS | |
| 3 months | 81.8 ± 1.4 | 83.8 ± 2.1 | NS | |
| 12 months | 81.2 ± 1.5 | 82.6 ± 2.1 | NS | |
ADC in 10−5 m2/s. Values given as means ± standard deviations.
Early phase scans were acquired 0–26 days postinjury.
Statistical differences between moderate vs. severe or PTA ≤ 2 vs. >2 weeks were Bonferroni-corrected for every structure separately.
ADC, apparent diffusion coefficient; NS, non-significant; TBI, traumatic brain injury.
The severe TBI group had higher ADC values in the brainstem at 3 months, the superior frontal sulcus at 3 and 12 months, and the postcentral sulcus at 12 months compared to the moderate TBI group. No significant differences in ADC values between moderate and severe TBI groups were found at any time point for the insula or hippocampus.
In the severe TBI group the ADC values increased over time in the superior frontal sulcus from the early phase to 3 months (p = 0.043), and the postcentral sulcus from the early phase to both 3 and 12 months (early phase < 3 months, p = 0.028; early phase < 12 months, p = 0.027). There were no differences in brainstem, insula or hippocampal ADC values between any time points. There were no differences in ADC values over time in any ROI in the moderate TBI group.
3.5.3.2. PTA duration (Table 3b)
At 12 months the ADC values in the superior frontal sulcus were higher in patients with PTA > 2 weeks than in those with PTA ≤ 2 weeks, while no differences were found in the other ROIs. In the PTA > 2 weeks group ADC values in insula increased between 3 and 12 months (p = 0.046). In the group with PTA ≤ 2 weeks no differences in ADC values overtime were found in any ROI.
3.5.4. DAI groups (Figs. 8 and 9)
Fig. 9.

The associations between longitudinal changes in mean ADC values for the non-DAI group (non-DAI = DAI 0) and the three DAI groups (DAI grades 1, 2, 3). Longitudinal analysis within each DAI group was performed separately for both ROIs using a linear mixed model. Significant F-values were followed by the Student's T-test. The resulting p-values were Bonferroni corrected separately for every ROI. At each time point, differences between DAI subgroups were evaluated using one-way ANOVA, and significant F-values were followed by independent samples T-tests, the resulting p-values were Bonferroni corrected for the three time points separately. Significant differences between different time points for each DAI group were confirmed and marked as: @—significantly different from scan 2; $—significantly different from scan 3 (***p < 0.001, **p < 0.01, *p < 0.05).
In the superior frontal sulcus and postcentral sulcus ADC values were higher in TBI patients with DAI than in non-DAI patients at 3 and 12 months. The ADC values increased significantly from the early phase to 3 months in the superior frontal (p = 0.009) and the postcentral sulcus (p = 0.005) in the DAI group. No differences were found in ADC values or ADC value change over time in the insula, hippocampus or brainstem ROIs between the non-DAI and the DAI groups, or within the DAI grades.
The different DAI grades were associated with significant differences in cortical GM ADC values (Fig. 9). In DAI 3, the superior frontal sulcus ADC values were higher than those in the non-DAI group at 3 months, and compared to both non-DAI and DAI 1 at 12 months. In DAI 2 the ADC values were higher in the superior frontal sulcus at 3 and 12 months than those in the non-DAI group. The DAI 3 group had higher ADC values in the postcentral sulcus at both 3 and 12 months than non-DAI and DAI 1 groups. In the superior frontal sulcus ADC values decreased between 3 and 12 months (p = 0.018) in DAI 1, and increased between the early phase and both 3 and 12 months in DAI 2 (early phase < 3 months, p = 0.036; early phase < 12 months, p = 0.018). There were no significant changes over time within the non-DAI and the DAI 3 groups.
The ADC values increased between the early phase and 3 months in the DAI 2 group (p = 0.020) in the postcentral sulcus. In DAI 3, the ADC values were significantly increased both at 3 and 12 months compared to the early phase (early phase < 3 months, p = 0.003; early phase < 12 months, p = 0.003). ADC values in postcentral sulcus did not change with time in the non-DAI and DAI 1 groups.
3.5.5. ROI ADC values vs. brain structure volumes.
There were no correlations between the ADC values in the cortex, hippocampus or brainstem and the volumes of these structures at 3 and 12 months.
3.5.6. ADC and outcome prediction
ADC values of superior frontal and postcentral sulci from 3 months did not add to the 12-month outcome prediction (Table 4).
Table 4.
Hierarchical regression analysis of 12-month outcome prediction using the 3-month ADC values with GCS, PTA and age as base model.
| Models | R2 | R2-change | Significance of F change |
|---|---|---|---|
| Base model | 0.462 | ||
| Superior frontal sulcus model | 0.479 | 0.017 | 0.208 |
| Postcentral sulcus model | 0.480 | 0.018 | 0.193 |
Base predictors: GCS, PTA duration, and age.
Superior frontal sulcus model: GCS, PTA duration, age, and superior frontal sulcus ADC value at 3 months
Postcentral sulcus model: GCS, PTA duration, age, and postcentral sulcus ADC value at 3 months
ADC, apparent diffusion coefficient; GCS, Glasgow Coma Scale; PTA, posttraumatic amnesia duration.
4. Discussion
This is to the best of our knowledge the largest number of moderate and severe TBI patients included in a prospective, longitudinal MRI study of brain volumes and diffusion changes where data have been systematically collected at 3 different time points, i.e. early phase and 3 and 12 months postinjury. Moreover, the patient group included in this study was representative for the entire group of TBI patients surviving for 1 year. The results not only confirmed most of our predictions, but also revealed new, unexpected findings.
4.1. Longitudinal volume changes
Significant cortical GM volume loss took place between the early and 3-month scans without any further significant decline at 12 months. This is the first direct evidence for cortical GM volume loss being an early occurrence after TBI in humans, similar to results in animal models (Rodriguez-Paez et al., 2005; Smith et al., 1997; Liu et al., 2010). The rapid cortical volume loss probably stems from a combination of neuronal necrosis and apoptosis initiated at time of injury and peaking early postinjury (Conti et al., 1998). Supporting these observations in animals, human PET studies show early and widespread neurodegeneration in intact cortex following TBI (Wu et al., 2004; Bergsneider et al., 2001). We did not find any evidence for GCS scores, duration of PTA or presence of DAI being associated with cortical volume loss. The effect of DAI and PTA on cortical volumes may, however, be localized to specific cortical regions for instance connected to affected WM tracts or the medial temporal lobe, and hence not be reflected in total cortical volumes.
The volume loss in the hippocampus was significantly slower than that in the cortex and not significantly different from the early and 3 month scans before 12 months postinjury. This slow, continuous loss of hippocampal volume after TBI in humans concurs with animal studies (Immonen et al., 2009; Smith et al., 1997). However, both animal data (Immonen et al., 2009; Smith et al., 1997) and autopsy data from humans (Maxwell et al., 2003) demonstrate significant early neuronal loss in the hippocampus. The lack of a significant decline in hippocampal volume between the early and 3 month-scans in the current study may be due to the early scans being obtained at ~10.2 days after injury. At that time point some volume loss may already have taken place. Alternatively, MRI-based hippocampal volume loss may take longer to develop than histological identified cell loss. Still, our result concurs with findings in cross-sectional studies where decreased hippocampal volume is detected first after 3 months in moderate–severe TBI patients compared to matched controls (Wilde et al., 2007; Bigler et al., 1997). The current data also agree with results from longitudinal studies with two time points, which show that hippocampal volumes decline over a protracted period from 1 week to 2.5 years (Warner et al., 2010b; Ng et al., 2008). The slowly evolving hippocampal volume loss is consistent with the protracted apoptotic neuronal death described in this region (Williams et al., 2001), coupled with glial proliferation (Gao and Chen, 2013) which may counteract some of the volume change, and impaired neurogenesis (Gao et al., 2008) which may affect volume when accumulated over time. The hippocampus is critical for declarative memory (Squire et al., 2004; Eichenbaum et al., 2007), and hippocampal damage is known to produce symptoms of anterograde and retrograde amnesia (Cipolotti and Bird, 2006). It was thus not surprising to find that patients with PTA > 2 weeks had significantly lower hippocampal volumes at both 3 and 12 months than patients with PTA ≤ 2 weeks. Moreover, hippocampal volume loss was markedly more pronounced from the early to 3 month scans in the PTA > 2 weeks group. These results demonstrated that PTA reflects hippocampal neuronal injury and that more severe PTA is associated with more rapid and extensive neuronal death in this region.
Significant volume loss developed over 12 months in the lenticular nucleus. Decreased lenticular nucleus volume has been reported in cross-sectional and longitudinal studies of TBI in children and adults ≥1 year after injury (Wilde et al., 2007; Anderson and Bigler, 1995; Primus et al., 1997). The histopathological changes in the lenticular nucleus include apoptosis, neuronal loss and/or shrinkage due to transneuronal degeneration and loss of myelinated axons as described in animal studies (Tong et al., 2002; Ng et al., 1994). Based on the current results these processes take place over a protracted period. The present data do not support the notion that basal ganglia respond more rapidly and briefly to TBI than the hippocampus (Hicks et al., 1996). Rather, the current study showed that the trajectory of volume loss was similar in the subcortical GM structures, i.e. the lenticular nucleus and hippocampus. Moreover, the subcortical GM structures were shown to differ significantly from the cortex with regard to the slopes of volume loss suggesting important differences in the histopathological responses between these GM structures to TBI.
As predicted lobar WM volume loss decreased mainly in the late phase, being significant first at 12 months. This concurs with the slowly evolving pathological response in WM (Vargas and Barres, 2007; Coleman, 2005; Coleman and Freeman, 2010). The extended period of volume loss found in lobar WM in humans in the current study is most likely the macroscopic consequence of the protracted histological changes in WM. Previous longitudinal studies have only been able to show a significant decline in WM volumes between an early (1–2 months) and a later time point (6–12 months) after mild to severe TBI (Ding et al., 2008; Sidaros et al., 2009; Warner et al., 2010b). Surprisingly the trajectory of WM loss and cortical GM loss did not differ significantly although the slope of WM loss was significantly different from the hippocampus, lenticular nucleus, and brainstem. This finding could reflect a significant interaction between WM volume loss and cortical volume loss over time at the individual level. Another explanation could be that volume losses in cortical GM and lobar WM volumes were the least linear. The lack of a significant interaction between volume losses over time between these two regions could hence be due to the model not being able to take nonlinearities into account.
Lobar WM volume loss was associated with injury severity as patients with severe TBI had significantly reduced lobar WM volumes compared to the moderate TBI group. This difference was present also after correcting for the presence of DAI. Unexpectedly, DAI was not associated with decreased lobar WM volume. This may be due to DAI classifications being based on visible lesions on conventional MRI which may not fully describe WM involvement in TBI.
The brainstem was the only structure in which volume loss was significant between every scan session, thus demonstrating particularly pervasive and enduring consequences of TBI to this region. Previous MRI studies have reported brainstem volume loss in cross-sectional and longitudinal studies with two time points in both children and adults with moderate and/or severe TBI (Sidaros et al., 2009; Warner et al., 2010b; Fearing et al., 2008). WM injury probably contributed to the early brainstem volume decline since patients with DAI grade 3 had the fastest and largest reduction of brainstem volume, present from 3 months. Also the group with severe TBI had smaller brainstem volumes, but this was detectable first after 12 months, appearing at the same time as reduced lobar WM volumes in this group. The WM tracts in the brain stem thus appear to be particularly sensitive to TBI.
Ventricular volumes were significantly increased at 3 months, followed by a non-significant increase at 12 months. This result is a refinement of the previously described time frame of significant ventricular dilation which was shown to last until ˜2–7 months postinjury (Blatter et al., 1997). Ventricular dilation at 3 and 12 months was associated with brainstem and cortical GM volumes. Neither lobar WM volumes nor the presence of DAI was associated with larger ventricular volumes. These results demonstrate the importance of deep WM loss in particular, followed by cortical GM to ventricular dilation in TBI, rather than hemispheric WM loss.
For the entire TBI group mean brain atrophy was ~4% of ICV after 3 months and ~6% of ICV after 12 months, demonstrating that the bulk volume loss occurred within the first 3 months. Still, a notable protracted component to the atrophy was present. No directly comparable human studies exist, but endpoint brain volume atrophy in the chronic phase after TBI is reported to be from 1.4% to 8% in cross-sectional and longitudinal studies in mild to severe TBI (Trivedi et al., 2007; Ding et al., 2008; Sidaros et al., 2009).
4.2. Longitudinal ADC changes
ADC values increased over time in the individuals with the most serious brain injuries, i.e. severe TBI, PTA > 2 weeks, and DAI, and there were no associations between ADC values and volumes. Participants with severe TBI had higher ADC values in deep, radiologically unaffected sulcal cortical GM, which increased significantly throughout the 12 month period, even when total cortical volume remained similar between 3 and 12 months. Thus it appears that small-scale remodeling of cortex after TBI persisted for a longer period than volumetric changes. Moreover, a significant contribution from DAI to the increase in cortical ADC values was present. More severe DAI grades gave rise to increased cortical ADC values developing at a more rapid timescale. In DAI the cortical ADC changes may be connected to delayed neuronal death, soma shrinkage, loss of cortical axons and myelination developing over an extended time period in this condition (Goetz et al., 2004; Hergan et al., 2002).
As expected there were increased ADC values at 3 months in the brainstem in severe TBI, but not in moderate TBI. This finding lends further support to the notion that deep brain injury is significantly more pronounced in severe than moderate TBI. PTA > 2 weeks was not associated with increased hippocampal ADC values. Both increased and decreased hippocampal ADC values are reported after TBI in animals and humans (Bendlin et al., 2008; Obenaus et al., 2007; Van putten et al., 2005). We predicted that PTA > 2 weeks would lead to increased ADC values due to increased cell loss, but did not find support for this assumption in the data. It is possible that the pathophysiological mechanisms leading to PTA are more heterogeneous than we expected and/or that volume loss combined with glial proliferation, inflammation (Woodcock and Morganti-Kossmann, 2013; Lin and Wen, 2013; Giunta et al., 2012) and/or neurogenesis (Gao et al., 2008; Greer et al., 2011; Gao and Chen, 2013) counteracted any effect of neuronal loss on ADC values.
4.3. MRI-derived 3-month volumes for 12-month outcome prediction
The hierarchical regression analysis demonstrated that MRI-derived volumes at 3 months contributed independently to 12-month outcome prediction even adjusting for duration of PTA, GCS scores and age. This finding demonstrates a significant clinical potential for MRI volumes obtained at 3 months for outcome prediction. Rotterdam CT score, on the other hand, did not have additional predictive value. Furthermore, cortical ADC values at 3 months did not improve outcome prediction.
4.4. Methodological considerations
This is the first study using a fully automated method, NeuroQuant, for deriving brain volumes from MRI scans in patients with TBI. Our results show that NeuroQuant performed very well in moderate–severe TBI patients even in the presence of different types of injuries and postoperative changes. Its limitations were restricted to segmentation problems in the early phase due to hematomas and contusions, and excessive movement during scanning. For the 3-month scans no segmentations failed. Since several volumes derived from the 3-month scan were independent predictors of outcome at 12 months, MRI at 3 months appears to be of clinical value in the assessment of the brain injury and prediction of later function. However, for the individual patient the presence of focal injury (contusions, hemorrhages) also plays a significant role for outcome, but it was beyond the scope of the present paper to evaluate the interaction between focal injuries and the atrophy in different brain structures.
It should be noted that the volume change measures obtained using independent NeuroQuant segmentations would not be expected to have the spatial specificity and power to detect subtle change that many across time point registration methods might provide (Holland et al., 2009; Fox et al., 2001; Thompson et al., 2004). On the other hand, NeuroQuant has previously been shown to have results comparable to that of hand segmentation of subregional volumes by anatomical experts (Brewer et al., 2009), and thus provides an identifiable and translatable measure of structure volume. As a FDA-cleared tool used at more than 100 clinical sites and applied to over 30,000 individuals in routine clinical practice, the tool yields standardized data that are directly comparable to results obtained in the clinic. Further, by providing independent segmentations of each time point, this approach is inverse-consistent and not subject to registration bias that may inflate measures of change provided by registration-based techniques (Holland et al., 2012). Thus, the approach yields a conservative measure of the regional brain volume changes associated with TBI.
Reliability of the ADC measurements was high to very high, but only performed by a single rater and interrater variability could not be assessed as Ozturk et al. (2008) recommend. There are several ways of analyzing ADC values, and we choose manually positioned ROIs to try to optimize ROI localization to avoid partial volume effects as slice thickness was 5 mm. However, more isotropic voxels and/or smaller voxels would be preferable to avoid partial volume effects, which potentially could have made it easier to detect statistical significant differences between groups and over time. For all the GM ROIs, the effect sizes of the differences between ADC values for the different TBI groups were very large. This was also seen in the regions where there were no statistical significant differences. Taken together, our results show that that variability in ADC measures is substantial in the material, and that ROI positioning probably plays some part in this, but that the largest contribution comes from individual differences within and between subjects. ADC values in GM may change slightly during normal aging. However, according to earlier studies, brain ADC values of healthy subjects remain constant during the age period 20–60 years (Klimas et al., 2013; Watanabe et al., 2013). As most of the subjects included in the present study are in this age range, we assumed that the diffusion changes revealed are TBI-associated. Including a matched control group may have helped in the interpretation of the ADC values, which relies on inferences from histopathology. Newer diffusion based MRI methods such as restriction spectrum imaging (White et al., 2013) may be better suited for characterizing tissue microstructural changes in TBI in the future.
The large number of analyses conducted across multiple brain regions increased the susceptibility to type I errors in the current study.
5. Conclusion
In summary, the effect of TBI on brain volumes differed between brain structures, and injury subgroups (GCS score, PTA, DAI) were associated with specific patterns of volume loss. Changes in cortical diffusion properties were detected in the more severe TBI subgroups in particular in patients with DAI grade 3, where diffusion properties increased in value and encompassed more cortical regions with time. Volume changes and diffusion changes were not associated. Cortical GM, hippocampus and lenticular nucleus volumes at 3 months were all very significant independent predictors of 12-month outcome even after adjusting for PTA duration, GCS scores and age. Thus TBI induced early and widespread structural brain volume loss which has significant impact on outcome in the chronic phase in moderate and severe TBI patients.
Acknowledgments
The authors would like to thank Stine Borgen Lund and Beate Holmqvist Karlsen for management of the database and the GOSE interviews, Kjell Arne Kvistad, Jana Rydland and Marit Folvik for MRI readings, Anders M. Dale for helpful advice, and the staff of CorTechs Labs, Inc. for their assistance with NeuroQuant installation and upgrading.
References
- Anderson C.V., Bigler E.D. Ventricular dilation, cortical atrophy, and neuropsychological outcome following traumatic brain injury. Journal of Neuropsychiatry and Clinical Neurosciences. 1995;7:42–48. doi: 10.1176/jnp.7.1.42. 7711490 [DOI] [PubMed] [Google Scholar]
- Bendlin B.B., Ries M.L., Lazar M., Alexander A.L., Dempsey R.J., Rowley H.A. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. 18556217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsneider M., Hovda D.A., McArthur D.L., Etchepare M., Huang S., Sehati N. Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. Journal of Head Trauma Rehabilitation. 2001;16:135–148. doi: 10.1097/00001199-200104000-00004. 11275575 [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Blatter D.D., Anderson C.V., Johnson S.C., Gale S.D., Hopkins R.O. Hippocampal volume in normal aging and traumatic brain injury. AJNR. American Journal of Neuroradiology. 1997;18:11–23. 9010515 [PMC free article] [PubMed] [Google Scholar]
- Blatter D.D., Bigler E.D., Gale S.D., Johnson S.C., Anderson C.V., Burnett B.M. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. AJNR. American Journal of Neuroradiology. 1997;18:1–10. 9010514 [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V., Cardamone L., Liu Y.R., Fang K., Myers D.E., O'Brien T.J. Progressive brain changes on serial manganese-enhanced MRI following traumatic brain injury in the rat. Journal of Neurotrauma. 2009;26:1999–2013. doi: 10.1089/neu.2009.0943. 19604101 [DOI] [PubMed] [Google Scholar]
- Brandstack N., Kurki T., Hiekkanen H., Tenovuo O. Diffusivity of normal-appearing tissue in acute traumatic brain injury. Clinical Neuroradiology. 2011;21:75–82. doi: 10.1007/s00062-011-0058-5. 21394634 [DOI] [PubMed] [Google Scholar]
- Brewer J.B., Magda S., Airriess C., Smith M.E. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR. American Journal of Neuroradiology. 2009;30:578–580. doi: 10.3174/ajnr.A1402. 19112065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L., Bird C.M. Amnesia and the hippocampus. Current Opinion in Neurology. 2006;19:593–598. doi: 10.1097/01.wco.0000247608.42320.f9. 17102699 [DOI] [PubMed] [Google Scholar]
- Coleman M.P. Axon degeneration mechanisms: commonality amid diversity. Nature Reviews Neuroscience. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman M.P., Freeman M.R. Wallerian degeneration, wld(s), and nmnat. Annual Review of Neuroscience. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. 20345246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A.C., Raghupathi R., Trojanowski J.Q., McIntosh T.K. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. 9671657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K., Marquez de la Plata C., [!(%xInRef|ce:surname)!] M., Wang J.Y., Mumphrey M., Moore C., Harper C. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. Journal of Neurotrauma. 2008;25:1433–1440. doi: 10.1089/neu.2008.0683. 19072588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. 17417939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearing M.A., Bigler E.D., Wilde E.A., Johnson J.L., Hunter J.V., Li Xiaoqi. Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. Journal of Child Neurology. 2008;23:729–737. doi: 10.1177/0883073808314159. 18658073 [DOI] [PubMed] [Google Scholar]
- Fox G.B., Fan L., Levasseur R.A., Faden A.I. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. Journal of Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. 9726259 [DOI] [PubMed] [Google Scholar]
- Fox N.C., Crum W.R., Scahill R.I., Stevens J.M., Janssen J.C., Rossor M.N. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–205. doi: 10.1016/S0140-6736(01)05408-3. 11476837 [DOI] [PubMed] [Google Scholar]
- Gao X., Chen J. Moderate traumatic brain injury promotes neural precursor proliferation without increasing neurogenesis in the adult hippocampus. Experimental Neurology. 2013;239C:38–48. doi: 10.1016/j.expneurol.2012.09.012. 23022454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Deng P., Xu Z.C., Chen J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PloS One. 2011;6:e24566. doi: 10.1371/journal.pone.0024566. 21931758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Enikolopov G., Chen J. Direct isolation of neural stem cells in the adult hippocampus after traumatic brain injury. Journal of Neurotrauma. 2008;25:985–995. doi: 10.1089/neu.2008.0460. 18665804 [DOI] [PubMed] [Google Scholar]
- Gentry L.R. Imaging of closed head injury. Radiology. 1994;191:1–17. doi: 10.1148/radiology.191.1.8134551. 8134551 [DOI] [PubMed] [Google Scholar]
- Giunta B., Obregon D., Velisetty R., Sanberg P.R., Borlongan C.V., Tan J. The immunology of traumatic brain injury: a prime target for Alzheimer's disease prevention. Journal of Neuroinflammation. 2012;9:185. doi: 10.1186/1742-2094-9-185. 22849382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz P., Blamire A., Rajagopalan B., Cadoux-hudson T.O.M., Young D., Styles P. Increase in apparent diffusion coefficient in normal appearing white matter following human traumatic brain injury correlates with injury severity. Journal of Neurotrauma. 2004;21:645–654. doi: 10.1089/0897715041269731. 15253793 [DOI] [PubMed] [Google Scholar]
- Greer J.E., McGinn M.J., Povlishock J.T. Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31:5089–5105. doi: 10.1523/JNEUROSCI.5103-10.2011. 21451046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwet, K.L., Intrarater reliability, in: Wiley Encycl., Published Online, 2008
- Hergan K., Schaefer P.W., Sorensen A.G., Gonzalez R.G., Huisman T.A.G.M. Diffusion-weighted MRI in diffuse axonal injury of the brain. European Radiology. 2002;12:2536–2541. doi: 10.1007/s00330-002-1333-2. 12271396 [DOI] [PubMed] [Google Scholar]
- Hicks R., Soares H., Smith D., McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathologica. 1996;91:236–246. doi: 10.1007/s004010050421. 8834535 [DOI] [PubMed] [Google Scholar]
- Holland D., Brewer J.B., Hagler D.J., Fennema-notestine C., Dale A.M. Subregional neuroanatomical change as a biomarker for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;107:20954–20959. doi: 10.1073/pnas.0906053106. 19996185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., McEvoy L.K., Dale A.M. Unbiased comparison of sample size estimates from longitudinal structural measures in ADNI. Human Brain Mapping. 2012;33:2586–2602. doi: 10.1002/hbm.21386. 21830259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen R.J., Kharatishvili I., Gröhn H., Pitkänen A., Gröhn O.H.J. Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage. 2009;45:1–9. doi: 10.1016/j.neuroimage.2008.11.022. 19101638 [DOI] [PubMed] [Google Scholar]
- Jenkins A., Teasdale G., Hadley M.D., Macpherson P., Rowan J.O. Brain lesions detected by magnetic resonance imaging in mild and severe head injuries. Lancet. 1986;2:445–446. doi: 10.1016/s0140-6736(86)92145-8. 2874424 [DOI] [PubMed] [Google Scholar]
- Jennett B., Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;305:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Jennett B., Snoek J., Bond M.R., Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. Journal of Neurology, Neurosurgery, and Psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. 6453957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Avants B., Patel S., Whyte J., Coslett B.H., Pluta J. Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. Neuroimage. 2008;39:1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. 17999940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas A., Drzazga Z., Kluczewska E., Hartel M. Regional ADC measurements during normal brain aging in the clinical range of b values: a DWI study. Clinical imaging. 2013;37:637–644. doi: 10.1016/j.clinimag.2013.01.013. 23462734 [DOI] [PubMed] [Google Scholar]
- LeGrand S.A., Hindman B.J., Dexter F., Moss L.G., Todd M.M. Reliability of a telephone-based Glasgow Outcome Scale assessment using a structured interview in a heterogenous population of patients and examiners. Journal of Neurotrauma. 2007;24:1437–1446. doi: 10.1089/neu.2007.0293. 17892406 [DOI] [PubMed] [Google Scholar]
- Lin Y., Wen L. Inflammatory response following diffuse axonal injury. International Journal of Medical Sciences. 2013;10:515–521. doi: 10.7150/ijms.5423. 23532682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.R., Cardamone L., Hogan R.E., Gregoire M.-C., Williams J.P., Hicks R.J. Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 2010;51:1788–1795. doi: 10.2967/jnumed.110.078626. 21051651 [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Hukkelhoven C.W.P.M., Marshall L.F., Steyerberg E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the Computed Tomographic Classification and Combinations of Computed Tomographic Predictors. Neurosurgery. 2005;57:1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. 16331165 [DOI] [PubMed] [Google Scholar]
- Marcoux J., McArthur D.A., Miller C., Glenn T.C., Villablanca P., Martin N.A. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate–pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Critical Care Medicine. 2008;36:2871–2877. doi: 10.1097/CCM.0b013e318186a4a0. 18766106 [DOI] [PubMed] [Google Scholar]
- Maxwell W.L., Dhillon K., Harper L., Espin J., MacIntosh T.K., Smith D.H. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. Journal of Neuropathology and Experimental Neurology. 2003;62:272–279. doi: 10.1093/jnen/62.3.272. 12638731 [DOI] [PubMed] [Google Scholar]
- Maxwell W.L., MacKinnon M.-A., Stewart J.E., Graham D.I. Stereology of cerebral cortex after traumatic brain injury matched to the Glasgow outcome score. Brain: a Journal of Neurology. 2010;133:139–160. doi: 10.1093/brain/awp264. 19897544 [DOI] [PubMed] [Google Scholar]
- Meaney D., Smith D. Biomechanics of concussion. Clinics in Sports Medicine. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. 21074079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen K.G., Skandsen T., Folvik M., Brezova V., Kvistad K.A., Rydland J. A longitudinal MRI study of traumatic axonal injury in patients with moderate and severe traumatic brain injury. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83:1193–1200. doi: 10.1136/jnnp-2012-302644. 22933813 [DOI] [PubMed] [Google Scholar]
- Nakase-Richardson R., Sherer M., Seel R.T., Hart T., Hanks R., Arango-Lasprilla J.C. Utility of post-traumatic amnesia in predicting 1-year productivity following traumatic brain injury: comparison of the Russell and Mississippi PTA classification intervals. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:494–499. doi: 10.1136/jnnp.2010.222489. 21242285 [DOI] [PubMed] [Google Scholar]
- Ng H., Mahaliyana R.D., Poonb W.S. The pathological spectrum of diffuse axonal injury in blunt head trauma: assessment with axon and myelin strains. Clinical Neurology and Neurosurgery. 1994;34:24–31. doi: 10.1016/0303-8467(94)90025-6. 8187378 [DOI] [PubMed] [Google Scholar]
- Ng K., Mikulis D.J., Glazer J., Kabani N., Till C., Greenberg G. Magnetic resonance imaging evidence of progression of subacute brain atrophy in moderate to severe traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2008;89:S35–S44. doi: 10.1016/j.apmr.2008.07.006. 19081440 [DOI] [PubMed] [Google Scholar]
- Obenaus A., Robbins M., Blanco G., Galloway N.R., Snissarenko E., Gillard E. Multi-modal magnetic resonance imaging alterations in two rat models of mild neurotrauma. Journal of Neurotrauma. 2007;24:1147–1160. doi: 10.1089/neu.2006.0211. 17610354 [DOI] [PubMed] [Google Scholar]
- Ozturk A., Sasson A.D., Farrell J.A.D., Landman B.A., da Motta A.C.B.S., Aralasmak A. Regional differences in diffusion tensor imaging measurements: assessment of intrarater and interrater variability. AJNR. American Journal of Neuroradiology. 2008;29:1124–1127. doi: 10.3174/ajnr.A0998. 18356471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus E.A., Bigler E.D., Anderson C.V., Johnson S.C., Mueller R.M., Blatter D. Corpus striatum and traumatic brain injury. Brain Injury: [BI] 1997;11:577–586. doi: 10.1080/026990597123278. 9251866 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Paez A.C., Brunschwig J.P., Bramlett H.M. Light and electron microscopic assessment of progressive atrophy following moderate traumatic brain injury in the rat. Acta Neuropathologica. 2005;109:603–616. doi: 10.1007/s00401-005-1010-z. 15877231 [DOI] [PubMed] [Google Scholar]
- Ross D. Review of longitudinal studies of MRI brain volumetry in patients with traumatic brain injury. Brain Injury: [BI] 2011;25:1271–1278. doi: 10.3109/02699052.2011.624568. 22077533 [DOI] [PubMed] [Google Scholar]
- Salmond C.H., Chatfield D.A., Menon D.K., Pickard J.D., Sahakian B.J. Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain: a Journal of Neurology. 2005;128:189–200. doi: 10.1093/brain/awh352. 15548553 [DOI] [PubMed] [Google Scholar]
- Sato M., Chang E., Igarashi T., Noble L.J. Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain Research. 2001;917:45–54. doi: 10.1016/s0006-8993(01)02905-5. 11602228 [DOI] [PubMed] [Google Scholar]
- Scheff S.W., Price D.A., Hicks R.R., Baldwin S.A., Robinson S., Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. Journal of Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. 16004576 [DOI] [PubMed] [Google Scholar]
- Sidaros A., Skimminge A., Liptrot M.G., Sidaros K., Engberg A.W., Herning M. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44:1–8. doi: 10.1016/j.neuroimage.2008.08.030. 18804539 [DOI] [PubMed] [Google Scholar]
- Skandsen T., Kvistad K.A., Solheim O., Strand I.H., Folvik M., Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. Journal of Neurosurgery. 2010;113:556–563. doi: 10.3171/2009.9.JNS09626. 19852541 [DOI] [PubMed] [Google Scholar]
- Smith D.H., Chen X.-H., Pierce J.E.S., Wolf J.A., Trojanowski J.Q., Graham D.I. Progressive atrophy and neuron death for one year following brain trauma in the Rat. Journal of Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. 9383090 [DOI] [PubMed] [Google Scholar]
- Squire L.R., Stark C.E.L., Clark R.E. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. 15217334 [DOI] [PubMed] [Google Scholar]
- Stein S.C., Spettell C. The Head Injury Severity Scale (HISS): a practical classification of closed-head injury. Brain Injury: [BI] 1995;9:437–444. doi: 10.3109/02699059509008203. 7550215 [DOI] [PubMed] [Google Scholar]
- Thompson P.M., Hayashi K.M., De Zubicaray G.I., Janke A.L., Rose S.E., Semple J. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. 15275931 [DOI] [PubMed] [Google Scholar]
- Tong W., Igarashi T., Ferriero D.M., Noble L.J. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Experimental Neurology. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. 12093087 [DOI] [PubMed] [Google Scholar]
- Trivedi M.A., Ward M.A., Hess T.M., Gale S.D., Dempsey R.J., Rowley H.A. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: relationship with duration of coma. Journal of Neurotrauma. 2007;24:766–771. doi: 10.1089/neu.2006.0205. 17518532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Putten H.P., Bouwhuis M.G., Muizelaar J.P., Lyeth B.G. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. Journal of Neurotrauma. 2005;22:857–872. doi: 10.1089/neu.2005.22.857. 16083353 [DOI] [PubMed] [Google Scholar]
- Vargas M.E., Barres B.A. Why is Wallerian degeneration in the CNS so slow? Annual Review of Neuroscience. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. 17506644 [DOI] [PubMed] [Google Scholar]
- Warner M.A., Marquez de la Plata C., Spence J., Wang J.Y., Harper C., Moore C. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. Journal of Neurotrauma. 2010;27:2121–2130. doi: 10.1089/neu.2010.1429. 20874032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M.A., Youn T.S., Davis T., Chandra A., Marquez de la Plata C., Moore C. Regionally selective atrophy after traumatic axonal injury. Archives of Neurology. 2010;67:1336–1344. doi: 10.1001/archneurol.2010.149. 20625067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Sakai O., Ozonoff A., Kussman S., Jara H. Age-related apparent diffusion coefficient changes in the normal brain. Radiology. 2013;266:575–582. doi: 10.1148/radiol.12112420. 23143020 [DOI] [PubMed] [Google Scholar]
- White N.S., Leergaard T.B., D'Arceuil H., Bjaalie J.G., Dale A.M. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Human Brain Mapping. 2013;34:327–346. doi: 10.1002/hbm.21454. 23169482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A., Bigler E.D., Hunter J.V., Fearing M.A., Scheibel R.S., Newsome M.R. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Developmental Medicine and Child Neurology. 2007;49:294–299. doi: 10.1111/j.1469-8749.2007.00294.x. 17376141 [DOI] [PubMed] [Google Scholar]
- Williams S., Raghupathi R., Mcintosh M.M.T.K., Saatman K.E., Graham D.I. In situ DNA fragmentation occurs in white matter up to 12 months after head injury in man. Acta Neuropathologica. 2001;102:581–590. doi: 10.1007/s004010100410. 11761718 [DOI] [PubMed] [Google Scholar]
- Woodcock T., Morganti-Kossmann M.C. The role of markers of inflammation in traumatic brain injury. Frontiers in Neurology. 2013;4:18. doi: 10.3389/fneur.2013.00018. 23459929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-M., Huang S.-C., Hattori N., Glenn T.C., Vespa P.M., Yu C.-L. Selective metabolic reduction in gray matter acutely following human traumatic brain injury. Journal of Neurotrauma. 2004;21:149–161. doi: 10.1089/089771504322778613. 15000756 [DOI] [PubMed] [Google Scholar]
- Yuh E.L., Cooper S.R., Ferguson A.R., Manley G.T. Quantitative CT improves outcome prediction in acute traumatic brain injury. Journal of Neurotrauma. 2012;29:735–746. doi: 10.1089/neu.2011.2008. 21970562 [DOI] [PMC free article] [PubMed] [Google Scholar]


