Abstract
Background
Interstitial cystitis (IC) is a chronic bladder disorder of unknown etiology. Antiproliferative factor (APF), a peptide found in the urine of IC patients, has previously been shown to decrease incorporation of thymidine by normal bladder epithelial cells. This study was performed to determine the effect of APF on the cell cycle of bladder epithelial cells so as to better understand its antiproliferative activity.
Methods
Explant cultures from normal bladder biopsy specimens were exposed to APF or mock control. DNA cytometry was performed using an automated image analysis system. Cell cycle phase fractions were calculated from the DNA frequency distributions and compared by two-way analysis of variance (ANOVA).
Results
APF exposure produced statistically significant increases in the proportion of tetraploid and hypertetraploid cells compared to mock control preparations, suggesting a G2 and/or M phase cell cycle block and the production of polyploidy.
Conclusions
APF has a specific effect on cell cycle distributions. The presence of a peptide with this activity may contribute to the pathogenesis of interstitial cystitis through disruption of normal urothelial proliferation and repair processes.
Background
Interstitial cystitis (IC) is a chronic bladder disorder presenting with long-term symptoms of round-the-clock urinary frequency and/or pain in the absence of any other reasonable causation [1]. Although there are multiple hypotheses on the primary cause of IC [2-7], it remains a clinical enigma since there are no definitive data to support these theories [2-7]. A recent breakthrough in IC research has been the discovery of antiproliferative factor (APF) and its association with IC [8]. APF, a peptide purified from the urine of IC patients has been shown to decrease incorporation of thymidine by bladder epithelial cells, decrease levels of urinary heparin-binding epidermal growth factor-like growth factor (HB-EGF) and increase levels of epidermal growth factor (EGF) in vitro which reflects altered levels of these growth factors seen in IC patients [9,10]. Studies to characterize APF at the molecular level are on-going. To date, the activity corresponds to a single HPLC peak which contains a peptide with a molecular weight less than 3,000 Dalton [10]. Additionally, urine APF activity is found at a higher level in IC vs. non-IC patients [11]. APF is a reliable marker of IC and may be an important factor in the pathogenesis of IC [12]. This study was performed to determine the effect of APF on the cell cycle of bladder epithelial cells so as to better understand its antiproliferative activity.
Methods
Patients
The IC patient was a 28 year old white male who had previously undergone cystoscopy and fulfilled the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) diagnostic criteria for IC which included glomerulations upon cystoscopic examination as well as urgency and dysuria. The control was a 41 year old white female with no history of urinary tract pathology undergoing pelvic surgery. Informed consent was obtained from both subjects in accordance with guidelines of the Institutional Review Board of the University of Maryland School of Medicine.
Explant specimen procurement
Cystoscopy was performed with the patient under general anesthesia using non bacteriostatic normal saline as a bladder irrigant. Rigid cold cup biopsy forceps were used to acquire 4 mm2 pieces of transitional epithelium with submucosa for growth of primary bladder epithelial cells. Tissue specimens were transported from the operating room in sealed sterile containers containing sterile phosphate buffered saline (PBS, Biosource International. Camarillo, CA) at room temperature, removed and placed in DMEM-F12 (Cellgro, Herndon, VA) containing 10% fetal bovine serum, 1% antibiotic/antimycotic solution, 1% glutamine, 5 μg/ml EGF and 1 U/ml insulin (all from Sigma, St. Louis, MO), for growth of bladder epithelial cell explants, characterized by binding of AE-1/AE-3 pancytokeratin antibodies as previously described [13].
Antiproliferative Factor
APF was prepared from the supernatant of bladder epithelial cells explanted from one IC patient and purified using molecular weight fractionation, ion exchange chromatography, hydrophobic interaction chromatography, and reversed phase high performance liquid chromatography (HPLC), as previously described [10]. Mock preparations from culture media of cells from the non-IC control patient were processed using the same purification procedure. Mock preparations do not have any HPLC detectable peptide.
APF treatment
Normal bladder cell explant cultures were plated at 1 × 104 cells per well of a 6 well tissue culture plate in Eagle's minimal essential medium (MEM, Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, 1% antibiotic/antimycotic solution (Sigma, St. Louis, MO) 1% glutamine and incubated overnight at 37°C in a 5% CO2 atmosphere. The following day, cells were serum starved (MEM containing 1% glutamine and 1% antibiotic/antimycotic solution) and incubated overnight at 37°C. On the third day, HPLC-purified APF or mock preparation (10, 20, and 50 μl) was added to the medium and cells were further incubated at 37°C for 48 hours. On day 5, cells were harvested (trypsin/EDTA) and pelleted at 1000 × g centrifugation, resuspended in PBS and fixed using 95% ethanol while vortexing at low speed. Samples were then stored at 4°C until used.
DNA cytometry
All specimens were cytocentrifuged onto slides using a CytoTek Cytocentrifuge at 1,000 RPM for 5 minutes, fixed in 10% Böhm-Springer fixative, hydrolyzed for 45 minutes in 5 N hydrochloric acid and the DNA stained by Feulgen reaction using thionin according to Perceptronix Medical Incorporated (PMI) standard protocol. The Fuelgen stained nuclei were analyzed on a PMI Cytosavant image analysis instrument, which was programmed to scan each slide and acquire 2,000 single nuclei. Debris and cell clumps were rejected using optical density and morphometric features. DNA content was calculated from sum optical density. Diploid control cells were used for validation and identification of G1 cell populations for normalization. Morphometric features for selecting single, whole nuclei included area, sphericity, and eccentricity. After acquisition, cell image galleries were reviewed to ensure that only data from whole, single nuclei were retained in the frequency distribution file. The frequency and cumulative frequency of nuclei were plotted for the calculated sum optical density (DNA content) (Figures 1 and 2). The boundaries of the cell cycle phases were identified by the inflection points in the cumulative frequency distribution and cell subpopulation fractions were calculated.
Figure 1.
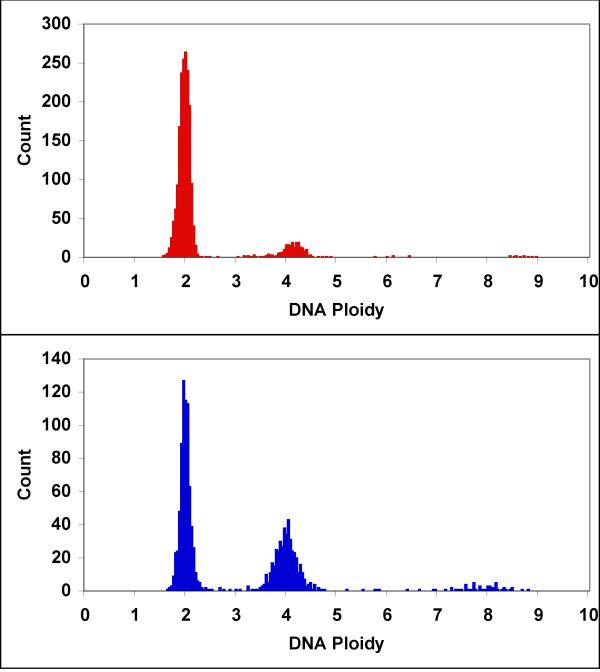
Representative DNA frequency distributions DNA frequency distributions from 10 μl mock treated (top panel) and 10 μl APF treated (bottom panel) normal bladder epithelial cell cultures show an APF associated increase in the proportion of cells with tetraploid DNA value. The presence of cells with octaploid DNA values suggests the cultures contain a mixture of cycling diploid and tetraploid cells.
Figure 2.
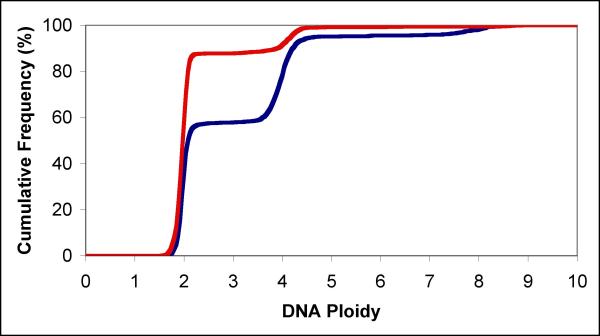
Cumulative DNA frequency distributions Cumulative DNA frequency distributions for 10 μl mock treated (red line) and 10 μl APF (blue line) treated normal bladder epithelial cells were used to calculate cell cycle sub-population fractions.
Statistical analysis
ANOVA analysis was performed using Statview (SAS Institute, Cary, North Caroline) with cell cycle fractions dependence on the independent variables treatment (APF or Mock) and dose (volume of treatment).
Results
Primary cultures of normal bladder epithelium were treated in replicate with 10, 20 or 50 μl of APF or mock preparation per 3 ml well. DNA histograms from one replicate of APF treated (10 μl) and one mock treated sample are presented in Figure 1. The histograms clearly show that APF treatment increased the proportion of cells with tetraploid DNA values. There is also a distinct population of cells at the octaploid level. Cell cycle analysis from DNA frequency distributions is relatively straightforward when dealing with a cycling diploid population. The presence of what appears to be a significant cycling tetraploid population in the APF treated cells complicates the analysis. While there are sophisticated algorithms for estimating the cell cycle phases for each of two populations they are largely built on the assumption that the two populations are independent. To determine whether APF may contribute to the formation of tetraploid cells by producing a G2 or M phase block of the cell cycle for both diploid and tetraploid cells, the DNA frequency distributions for all samples were analyzed to extract the proportion of cells with diploid, diploid S, tetraploid, tetraploid S, and octaploid DNA values. Cumulative frequency distributions were calculated and then plotted (Figure 2). Cell sub-populations were derived from these cumulative frequency distributions.
Cell population fractions for all samples appear in Table 1. As presented, the sum of all fractions is 100% and an increase in the percentage of cells in one group due to treatment will reduce the percentage in other groups. Diploid percentages are lower for APF treated samples than for Mock treated samples at all treatment levels. ANOVA analysis (STATVIEW Statistical Analysis software) of the effect of treatment as a factor and dose as a covariate on the diploid percentage yielded p-values of less than 0.0001 for both. Similarly there were statistically significant increases in Diploid S (p = 0.0041), Tetraploid (p = 0.0005), Tetraploid S (p = 0.0015), and Octaploid (p = 0.0098) fractions. Dose was significant only for the decrease in the Diploid population and an increase in the Tetraploid fraction (p < 0.0001). Little difference was seen between the 10 and 20 μl doses for either Mock or APF treated samples, but cells treated with 50 μl of APF or the mock preparation were clearly different from the lower level treatments. This experiment has been replicated five times with similar results.
Table 1.
Cell population fractions (%). Cumulative frequency distributions were examined to extract cell cycle and ploidy sub-populations. Diploid fraction corresponds to a DNA normal 2C non-cycling DNA content. Diploid S fractions have DNA content between diploid and tetraploid values. The tetraploid sub-population contains both diploid G2/M cells and tetraploid G1 cells. The cells with DNA content between tetraploid and octaploid are S-phase tetraploid cells, Octaploid DNA content cells are the G2/M cells of the tetraploid population.
| Treatment | Dose (μl) | Diploid | Diploid S | Tetraploid | Tetraploid S | Octaploid |
| APF | 10 | 57.27 | 8.72 | 28.76 | 1.54 | 3.72 |
| APF | 10 | 58.87 | 3.88 | 32.26 | 1.18 | 3.81 |
| APF | 20 | 58.82 | 4.80 | 30.78 | 1.18 | 4.43 |
| APF | 20 | 58.17 | 8.44 | 30.02 | 1.07 | 2.31 |
| APF | 50 | 40.11 | 2.23 | 51.40 | 0.56 | 5.71 |
| APF | 50 | 34.89 | 4.68 | 54.24 | 1.08 | 5.11 |
| Mock | 10 | 87.72 | 1.64 | 9.60 | 0.50 | 0.55 |
| Mock | 10 | 90.14 | 1.83 | 7.13 | 0.25 | 0.64 |
| Mock | 20 | 90.60 | 2.05 | 6.77 | 0.34 | 0.24 |
| Mock | 20 | 91.14 | 1.47 | 6.80 | 0.15 | 0.44 |
| Mock | 50 | 73.08 | 5.98 | 20.75 | 0.00 | 0.19 |
| Mock | 50 | 72.45 | 5.85 | 20.22 | 0.40 | 1.09 |
Discussion
Interstitial cystitis is a chronic bladder disease that, although first described during the 19th century, today still has no certain etiologies, no specific pathology, and no predictably effective treatment or cure [14]. Hypotheses as to the causes of interstitial cystitis include infection, urinary toxins, increased epithelial permeability, mast cell involvement, neurogenic mechanisms, autoimmune or genetic abnormalities, or a combination of these [2-7]. However, no studies to date have effectively proven any of these mechanisms.
A recent breakthrough for interstitial cystitis has been the discovery of antiproliferative factor [8]. In addition to decreased tritiated thymidine incorporation by bladder epithelial cells treated with APF, explanted bladder epithelial cells from IC patients produce APF, have decreased proliferation, and produce significantly less HB-EGF than bladder cells from controls. Addition of HB-EGF to cell media can overcome the effects of APF on bladder epithelium and cell proliferation [10,11]. Finally, Chai et al showed that urine APF activity decreases while HB-EGF levels normalize in IC patients following two different IC therapies: bladder hydrodistension [14] and 3rd sacral nerve root stimulation [15]. These findings suggest the intriguing possibilities that bladder epithelial abnormalities in IC may be caused by APF, and that replacement of specific factor(s) altered by APF (such as HB-EGF) may result in normalization of bladder epithelial cell growth [16].
However, the mechanism for APF's action is unclear. It is hypothesized that IC symptoms exacerbate following bladder epithelial damage due to an acute sloughing of epithelial cells. The presence of APF in the urine inhibits appropriate epithelial cells regeneration by regulating autocrine HB-EGF and cell adhesion protein production, resulting in thinning or denudation of the bladder epithelium in IC patients [16].
The goal of the study was to help in the elucidation of APF's mechanism of action. Our primary conclusion from these data is that APF has a profound impact on cell cycle distributions. APF treated cells increase the proportion of cells in the G2M phase, which is likely due to a G2/M block.
Another possible effect of APF that might lead to changes in the bladder epithelia is the production of polyploidy. The appearance of polyploidy is not uncommon in tissue culture and even normal tissue [18,19]. Polyploidy may result from cellular and then nuclear fusion, or conceivably through mitosis in which cellular DNA is replicated but mitosis and/or cytokinesis fail to occur. Cellular fusion is not uncommon and is even part of normal function in some cell types. After cellular fusion, nuclear fusion probably requires synchronous mitosis, fusion of the mitotic spindle producing two tetraploid daughter cells. This could in turn affect epithelial regeneration as is hypothesized to occur in IC patients. Future studies are needed to help address this issue and to determine if this polyploidy has any significance in APF's mechanism or IC development, or whether it is an unrelated in vitro phenomenon.
The primary bladder epithelial cells used in these experiments were treated with APF or Mock preparations in serum free media. Unlike many other cell types, the lack of exogenous growth factors does not produce a cell cycle blockade. The cells produce sufficient autocrine signaling to maintain a population in a proliferative state, and therefore serum starvation is insufficient to produce cell cycle blockade. Study of synchronized cell populations will greatly facilitate investigation of the mechanism of APF mediated cell cycle changes.
There was an unexpected increase in the proportion of cells with tetraploid DNA content at the highest mock dose. This may be due to the acetonitrile and/or TFA used in the high performance liquid chromatography (HPLC) preparation. There may be a low level of APF in the mock preparation, but no decrease in tritiated thymidine has been observed in similarly controlled experiments, nor can any APF be detected by UV absorbance in the HPLC. Both preparations had equivalent amounts of acetonitrile/TFA so as to control for any affect that these two substances might have on the cells.
Conclusions
Antiproliferative factor has been shown to be a specific protein associated with interstitial cystitis, although its role in the pathogenesis of IC remains unknown. This study shows that APF modulates the cell cycle and in fact might lead to G2 blockade or the production of polyploidy. This would in effect prevent regeneration of bladder epithelial cells, therefore possibly causing the epithelial thinning and/or ulceration associated with IC. Greater understanding of APF's mechanism of action could aid in the diagnoses and treatment of interstitial cystitis.
Competing interests
None declared.
Authors' contributions
All authors participated in the design of the experiment. HR drafted the manuscript and assisted with DNA cytometry and statistical analysis. JR, EM, and SK conceived the study. JR performed the statistical analysis, MO performed the DNA cytometry, CZ performed the tissue culture, and SK prepared the APF. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Toby Chai of the University of Maryland provided the patient biopsies from which the explant cultures were generated. Supported by National Institutes of Health, NIDDK U01 DK54133, R01 DK52596, R21 DK066123, and Veteran's Administration Merit Review Funding.
Contributor Information
Hani H Rashid, Email: hani_rashid@urmc.rochester.edu.
Jay E Reeder, Email: jay_reeder@urmc.rochester.edu.
Mary J O'Connell, Email: mary_o'connell@urmc.rochester.edu.
Chen-Ou Zhang, Email: czhan001@umaryland.edu.
Edward M Messing, Email: edward_messing@urmc.rochester.edu.
Susan K Keay, Email: skeay@medicine.umaryland.edu.
References
- Ratliff TL, Klutke Cg, McDougall EM. The etiology of interstitial cystitis. Urol Clin North Am. 1994;21:21–30. [PubMed] [Google Scholar]
- Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163–176. [PubMed] [Google Scholar]
- Warren JW. Interstitial cystitis as an infectious disease. Urol Clin North Am. 1994;21:31–40. [PubMed] [Google Scholar]
- Parsons CL, Zupkas P, Parsons JK. Intravesical potassium sensitivity in patients with interstitial cystitis and urethral syndrome. Urology. 2001;57:428–432. doi: 10.1016/S0090-4295(00)01110-9. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57:47–55. doi: 10.1016/S0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- Wesselmann U. Interstitial cystitis: a chronic visceral pain syndrome. Urology. 2001;57:32–39. doi: 10.1016/S0090-4295(01)01123-2. [DOI] [PubMed] [Google Scholar]
- Warren JW, Keay SK, Meyers D, Xu J. Concordance of interstitial cystitis in monozygotic and dizygotic twin pairs. Urology. 2001;57:22–25. doi: 10.1016/S0090-4295(01)01120-7. [DOI] [PubMed] [Google Scholar]
- Keay SK, Zhang CO, Trifillis AL, Hise MK, Hebel JR, Jacobs SC, Warren JW. Decreased 3H-thymidine incorporation by human bladder epithelial cells following exposure to urine from interstitial cystitis patients. Journal of Urology. 1996;156:2073–2078. doi: 10.1097/00005392-199612000-00052. [DOI] [PubMed] [Google Scholar]
- Keay SK, Zhang CO, Kagen DI, Hise MK, Jacobs SC, Hebel JR, Gordon D, Whitmore K, Bodison S, Warren JW. Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol. 1997;158:1983–1988. doi: 10.1016/s0022-5347(01)64198-3. [DOI] [PubMed] [Google Scholar]
- Keay SK, Kleinberg M, Zhang CO, Hise MK, Warren JW. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J Urol. 2000;164:2112–2118. doi: 10.1097/00005392-200012000-00074. [DOI] [PubMed] [Google Scholar]
- Keay SK, Zhang CO, Shoenfelt J, Erickson DR, Whitmore K, Warren JW, Marvel R, Chai T. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57:9–14. doi: 10.1016/S0090-4295(01)01127-X. [DOI] [PubMed] [Google Scholar]
- Keay SK, Zhang CO, Hise MK, Hebel JR, Jacobs SC, Gordon D, Whitmore K, Bodison S, Gordon N, Warren JW. A diagnostic in vitro urine assay for interstitial cystitis. Urology. 1998;52:974–978. doi: 10.1016/S0090-4295(98)00488-9. [DOI] [PubMed] [Google Scholar]
- Trifillis AL, Cui X, Jacobs S, Warren JW. Culture and characterization of normal epithelium from cystoscopic biopsies of human bladder. In vitro Cell Dev Biol Anim. 1993;29A:908–911. doi: 10.1007/BF02634226. [DOI] [PubMed] [Google Scholar]
- Chai TC, Zhang C-O, Shoenfelt JL, Johnson HW, Warren JW, Keay S. Bladder stretch alters urinary heparin-binding epidermal growth factor and antiproliferative factor in patients with interstitial cystitis. J Urol. 2000;163:1440–1444. doi: 10.1097/00005392-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Chai TC, Zhang C-O, Warren JW, Keay SK. Percutaneous sacral third nerve root neurostimulation improves symptoms and normalizes urinary HB-EGF levels and antiproliferative activity in patients with interstitial cystitis. Urology. 2000;55:643–646. doi: 10.1016/S0090-4295(00)00476-3. [DOI] [PubMed] [Google Scholar]
- Keay S, Warren JW. A hypothesis for the etiology of interstitial cystitis based upon inhibition of bladder epithelial repair. Med Hypotheses. 1998;51:79–83. doi: 10.1016/s0306-9877(98)90260-2. [DOI] [PubMed] [Google Scholar]
- Keay SK, Zhang CO, Schoenfelt JL, Chai TC. Decreased in vitro proliferation of bladder epithelial cells from patients with interstitial cystitis. Urology. 2003;61:1278–1284. doi: 10.1016/S0090-4295(03)00005-0. [DOI] [PubMed] [Google Scholar]
- Sharief Y, Reich CF, 3rd, Bonar RA. Polyploidy in mammalian urothelial cells. Urol Res. 1980;8:153–161. doi: 10.1007/BF00256410. [DOI] [PubMed] [Google Scholar]
- Kline MJ, Wilkinson EJ, Askeland R, Given RW, Stephen C, Hendricks JB. DNA tetraploidy in Feulgen-stained bladder washings assessed by image cytometry. Anal Quant Cytol Histol. 1995;17:129–134. [PubMed] [Google Scholar]


