Nafcillin and cefazolin are commonly used to treat infections with methicillin-susceptible Staphylococcus aureus. Nafcillin treatment was associated with lower rates of treatment completion and higher rates of drug-emergent events compared with cefazolin in our outpatient parenteral antimicrobial treatment program.
Keywords: nafcillin, cefazolin, MSSA, OPAT, tolerability
Abstract
Background. Nafcillin and cefazolin are considered first-line therapy for most infections with methicillin-susceptible Staphylococcus aureus (MSSA), and recent studies have suggested similar clinical efficacy. Limited data are available on the comparative tolerability of these agents.
Methods. In this retrospective cohort analysis of patients treated with either nafcillin or cefazolin for MSSA infection in the outpatient parenteral antimicrobial therapy clinic at Massachusetts General Hospital from 2007 to 2011, the frequency of premature antimicrobial discontinuation (PAD) and drug-emergent events (DEEs) was calculated.
Results. Three hundred sixty-six and 119 patients were treated with nafcillin or cefazolin, respectively. The median anticipated duration of therapy was comparable at 28 (interquartile range [IQR], 16–37) and 29 (IQR, 24–39) days, respectively, for those treated with nafcillin and cefazolin. Fewer patients completed the prespecified treatment course with nafcillin than with cefazolin (PAD rate, 33.8% vs 6.7%; P < .0001). The hazard ratio for PAD in the nafcillin vs cefazolin groups was 2.81 (95% confidence interval [CI], 1.26–3.68). More patients in the nafcillin group developed rash (13.9% vs 4.2%; P = .002), renal dysfunction (11.4% vs 3.3%; P = .006), and liver function abnormalities (8.1% vs 1.6%; P = .01). Overall rates of DEEs per 1000 patient-days were 16.9 (95% CI, 10.4–27.3) and 4.8 (95% CI, 1.1–10.2), respectively. In 9 cases of nafcillin discontinuation, treatment was changed to cefazolin; all 9 completed treatment with no further observed DEEs.
Conclusions. Nafcillin treatment was associated with higher rates of both PAD as well as DEEs compared with cefazolin treatment. This difference in tolerability, in addition to efficacy and cost, should be considered when decisions for outpatient parenteral MSSA treatment are made.
Despite the global emergence of methicillin-resistant strains, methicillin-susceptible Staphylococcus aureus (MSSA) remains an important pathogen in both community-acquired and healthcare-associated infections, accounting for a significant proportion of bone and joint infections, soft tissue infections, and bacteremia cases [1, 2]. Patients with MSSA infection often require prolonged administration of parenteral antimicrobial therapy. The administration of outpatient parenteral antimicrobial therapy (OPAT) has gained popularity, as experience has shown the practice to be safe, efficacious, practical, and cost-effective. In 2007, an estimated 400 000 patients in the United States alone were treated with OPAT [3–5]. Reportedly, approximately 20% of patients receiving OPAT are treated for infections caused by MSSA [5, 6]. The treatment of choice for MSSA infections usually involves a semisynthetic penicillin such as nafcillin or oxacillin, with first-generation cephalosporins offering an alternative since the 1970s [7]. Early reports raised concerns about the use of cefazolin due to the frequent production by MSSA of type A β-lactamase, which degrades cefazolin more than semisynthetic penicillins [8]. Recently, a significant inoculum effect for cefazolin has been demonstrated in up to 20% of MSSA isolates, reflecting reduced susceptibility in the presence of large numbers of MSSA, likely due to the effect of this β-lactamase [9, 10]. Nonetheless, cefazolin is widely used for treatment of MSSA infections, and several authors have reported clinical efficacy that is comparable to that of the semisynthetic penicillins, excluding central nervous system infections [1, 11–14]. The convenient dosing, favorable pharmacokinetics, and lower cost have all contributed to the increased use of cefazolin [1]. Cefazolin is considered a well-tolerated medication with low rates of drug-emergent events (DEEs), but there is a paucity of studies directly comparing the tolerability of nafcillin and cefazolin. Furthermore, OPAT patients constitute a distinctive population in that they experience less supervision and environmental control compared with the inpatient setting, and their treatment durations are often longer. The aim of this study was to assess the differential tolerability of these 2 commonly used antimicrobials in the OPAT population.
METHODS
The study protocol was reviewed and approved by the Partners Human Research Committee at Massachusetts General Hospital (MGH).
Setting and Study Population
MGH is a 947-bed tertiary medical center in Boston, with approximately 50 000 admissions per year. The OPAT program of the Infectious Diseases Division of MGH oversees the antibiotic care of former inpatients with an anticipated remaining parenteral antimicrobial treatment course of >14 days at the time of discharge, and routinely cares for 70–90 OPAT patients at any given time. Most patients self-administer their antimicrobial therapy at home with the assistance of skilled home nursing care and infusion services, but close to 50% of patients enrolled in the OPAT program are discharged to skilled nursing or rehabilitation facilities. Surveillance laboratory monitoring is generally obtained weekly, as suggested by the practice standards that have been developed for the safe administration of such therapy in adults [14, 15]. A clinical database was developed in 2006 to allow tracking of patients and monitoring of complications. This database was queried retrospectively for the current study. Patients included in the analysis were defined as those having at least 1 culture growing MSSA and treated between January 2007 and December 2011 in the MGH OPAT program.
Data Collection and Definitions
The majority of patient data were collected prospectively in real time by a single clinic-based administrative assistant, and included the patient's name, medical record number, dates of treatment, site and/or type of infection, culture results, and antimicrobials administered, including subsequent medications if treatment was changed during therapy. At the start and end of therapy for each patient, the OPAT medical director, a board-certified practicing infectious diseases specialist, reviewed all medical charts and laboratory reports, verified the database entries, and noted whether development of potential DEEs had occurred. Data about potential selected DEEs were entered by a single infectious diseases physician (S. B. N.) as qualitative information (present/not present) as follows: rash, renal dysfunction (defined as an increase in serum creatinine of >0.5 mg/dL or 50% increase from baseline), liver abnormalities (alanine transaminase >100 µ/L), neutropenia (neutrophil count <1000/µL), thrombocytopenia (platelet count <100 000/µL), eosinophilia (eosinophil count >500/µL), and Clostridium difficile colitis.
To compare select demographics and baseline comorbidities that were not originally included in the prospectively maintained database, we retrospectively queried the Partners’ Research Patient Data Registry, a centralized clinical data registry maintained by Partners Healthcare (of which MGH is a founding member) that gathers data from various affiliated hospital legacy systems and stores it on a single server. Specifically, we searched for International Classification of Diseases, Ninth Revision codes, including the following group headings and all branching terms: chronic renal failure (585.9), chronic heart failure (428), chronic liver disease (571), diabetes mellitus (648, 250), and malignancies (V67.2, V76.8, 338.3, 200–208).
Outcomes
The primary outcome was all-cause premature antimicrobial discontinuation (PAD) of either study medication, calculated per 1000 patient-days on the medication. For the purposes of this study, therapy discontinuation was considered premature if the patient completed <80% of the planned treatment course with the initial antimicrobial. Secondary outcomes included rates of specific DEEs as listed above, expressed as absolute number of events per patient population and rates of events per 1000 patient-days on each medication.
Data Analysis
Continuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR) and were compared between groups using the independent t test or Mann–Whitney test. Categorical variables are presented as number and percentage of patients within each treatment group and compared using the χ2 or the Fisher exact test. Normality of distribution was assessed using the Kolmogorov-Smirnov test.
Kaplan-Meier survival analysis was performed for the nafcillin and cefazolin treatment groups with premature discontinuation of the medication as the primary event, and significance was determined with the log-rank (Mantel-Cox) test. Patients were right-censored after completion of planned treatment course. The hazard ratio for PAD was calculated using a Cox regression model. Potential confounders, including age, sex, planned treatment course, number of underlying chronic disorders, and length of hospital stay, were analyzed individually. All confounders with a P value <.2 were included in a multivariate regression model.
Rates of DEEs per 1000 patient-days were calculated for the nafcillin and cefazolin treatment groups, with confidence intervals constructed using exact Poisson methods. If a DEE occurred and the medication was not discontinued, patients continued to contribute patient-days to their medication group as they were still at risk for additional DEEs.
All statistical tests were 2-sided; a P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical software, version 18 (SPSS Inc, Chicago, Illinois).
RESULTS
The database included 2372 unique patients enrolled in the OPAT program between January 2007 and December 2011, with a total of 509 patients having at least 1 culture growing MSSA. Of those patients, 366 were treated with nafcillin, and 119 patients were treated with cefazolin. The most common infection site was bone (31%) and joint (18%), followed by skin and soft tissue (11%), bloodstream (9%), and heart valve (endocarditis, 7%). The standard adult dosing used in the majority of patients was 2 g every 4 hours for nafcillin and 2 g every 8 hours for cefazolin. No patients were treated with continuous infusions. Approximately one-third of patients received additional antimicrobials while on nafcillin or cefazolin. As shown in Table 1, baseline characteristics in the 2 groups were comparable, as was the planned total treatment length, with a median of 28 (IQR, 16–37) and 29 (IQR, 24–39) days, respectively. Five patients died during OPAT care: 4 of 366 (1.09%) in the nafcillin group and 1 of 119 (0.84%) in the cefazolin group. These events were included in counts of all-cause PAD.
Table 1.
Select Characteristics of Study Population Stratified by Treatment Group
| Characteristic | Nafcillin (n = 366) | Cefazolin (n = 119) | P Value |
|---|---|---|---|
| Age, ya | 57 ± 14 | 56 ± 18 | .52 |
| Male sexb | 223 (60.9) | 70 (58.8) | .74 |
| Primary infection siteb | |||
| Osteomyelitis | 112 (30.6) | 39 (32.7) | .82 |
| Septic arthritis | 65 (17.7) | 22 (18.4) | .89 |
| SSTI | 43 (11.7) | 11 (9.2) | .50 |
| Bacteremia | 33 (9.0) | 10 (8.4) | 1.00 |
| Endocarditis | 28 (7.6) | 7 (5.8) | .68 |
| Underlying diseaseb | |||
| Heart failure | 73 (19.9) | 26 (21.8) | .69 |
| Diabetes | 43 (11.7) | 9 (7.5) | .23 |
| Chronic renal failure | 29 (7.1) | 12 (10.0) | .45 |
| Hepatic dysfunction | 21 (5.7) | 7 (5.8) | 1.00 |
| Malignancy | 70 (19.1) | 26 (21.8) | .51 |
| Coadministration of other antimicrobialsb | |||
| All antimicrobials | 138 (37.7) | 39 (32.7) | .38 |
| β-lactams | 14 (3.8) | 3 (2.5) | .77 |
| Prior history of β-lactam allergyb | 19 (5.1) | 7 (5.8) | .81 |
| Location of care after dischargeb | |||
| Home | 186 (50.8) | 65 (54.6) | .52 |
| Skilled nursing facility | 145 (39.6) | 42 (35.2) | .44 |
| Rehabilitation facility | 35 (9.5) | 12 (10.0) | .85 |
| Initial treatment plan, dc | 28 (16–37) | 29 (24–39) | … |
| Duration of hospitalization prior to OPAT initiationc | 4.7 (1–11) | 5.0 (1–11) | .46 |
| Duration of treatment with nafcillin or cefazolin prior to OPAT initiationc | 1.9 (0.8–6.0) | 2.2 (0.8–7.5) | .84 |
Abbreviations: OPAT, outpatient antimicrobial therapy; SSTI, skin and soft tissue infection.
a Mean ± Standard Deviation.
b No. (%).
c Median (interquartile range).
Premature Antimicrobial Discontinuation
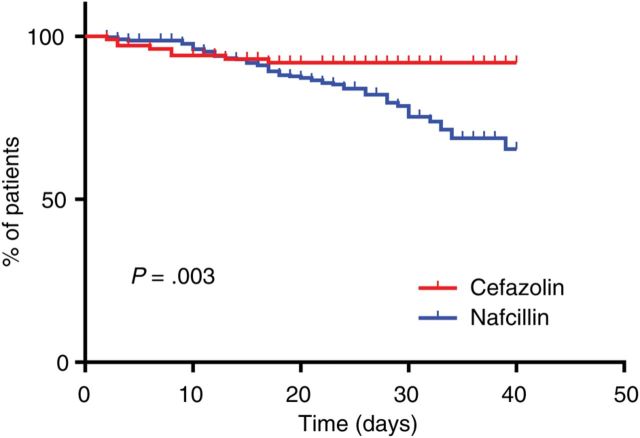
Significantly fewer patients completed the prespecified treatment course with nafcillin (33.8% all-cause discontinuation rate for nafcillin vs 6.7% for cefazolin, P < .001; Table 2). Kaplan-Meier survival analysis showed significantly different curves (P = .003; Figure 1). The mean difference in actual vs planned treatment length was −8.1 ± 2.1 days in the nafcillin group (n = 366) compared with −2.9 ± 11.1 days in the cefazolin group (n = 119; P < .001). Analyzing only the subgroup of patients who had PAD (n = 132), the difference between planned and actual treatment length was −13.0 ± 4.8 days in the nafcillin group (n = 124) and −21.1 ± 6.3 for the cefazolin group (n = 8). In 7 of 8 (87.5%) patients with PAD in the cefazolin group, the antimicrobial was discontinued within the first 10 days of treatment, compared with only 19 of 124 (15.3%) in the nafcillin group (P < .001).
Table 2.
Premature Antimicrobial Discontinuation and Drug-Emergent Events Stratified by Treatment Groupa
| Event | Nafcillin (n = 366) | Cefazolin (n = 119) | P Value |
|---|---|---|---|
| PAD | 124 (33.8) | 8 (6.7) | <.001 |
| DEEsa | 114 (31.1) | 14 (11.7) | <.001 |
| Rash | 51 (13.9) | 5 (4.2) | .002 |
| Renal impairment | 42 (11.4) | 4 (3.3) | .006 |
| Liver abnormalities | 30 (8.1) | 2 (1.6) | .01 |
| Neutropenia | 31 (8.4) | 4 (3.3) | .06 |
| Clostridium difficile colitis | 9 (2.4) | 1 (0.8) | .46 |
Data are presented as No. (%).
Abbreviations: DEEs, drug-emergent events; PAD, premature antimicrobial discontinuation.
a Represents unique patients experiencing drug-related adverse events (DRAEs); each patient could experience >1 DRAE. DRAEs were defined as follows: renal impairment, an increase in serum creatinine of >0.5 mg/dL or 50% increase from baseline; liver abnormalities, alanine aminotransferase >100µ/L; neutropenia, neutrophil count <1000/µL.
Figure 1.
Kaplan-Meier estimates of premature antimicrobial discontinuation over time. The adjusted hazard ratio for premature antimicrobial discontinuation in patients treated with nafcillin was 2.81 (95% confidence interval, 1.26–3.68).
The unadjusted hazard ratio for PAD in the nafcillin group was 2.85 (95% confidence interval [CI], 1.3–3.70). Only age (P = .156) and duration of hospitalization (P = .11) were included in the final regression model, resulting in an adjusted hazard ratio of 2.81 (95% CI, 1.26–3.68).
The majority of patients with PAD were switched to alternative parenteral treatment (87.5% in the cefazolin group and 81.4% in the nafcillin group; P = .99). The most commonly used alternative was vancomycin in 71 of 108 (65.7%) patients switched to alternative antimicrobials. Other alternative treatments included daptomycin (n = 6), clindamycin (n = 5), linezolid (n = 4), and cephalosporins (n = 13).
Drug-Emergent Events
One hundred sixty-three DEEs were reported with nafcillin treatment, occurring in 114 of 336 (31.1%) unique patients. In contrast, 16 DEEs were reported with cefazolin treatment, occurring in 14 of 119 (11.7%) patients (P < .001). Rash, renal impairment, and liver function abnormalities occurred significantly more frequently in the nafcillin-treated patients (Table 2). Neutropenia and C. difficile colitis were also observed more frequently in the nafcillin group, but this difference was not statistically significant. The overall DEE rate was significantly higher for the nafcillin-treated group compared with the cefazolin-treated group (16.9 per 1000 patient-days on nafcillin vs 4.8 per 1000 patient-days on cefazolin, P < .001; Table 3).
Table 3.
Rate of Drug-Emergent Events per 1000 Patient-days on Nafcillin or Cefazolin as Part of Monotherapy or Polytherapy
| Type of Therapy | Nafcillin, Rate (95% CI) | Cefazolin, Rate (95% CI) |
|---|---|---|
| Monotherapy | 16.8 (10.2–27.3) | 4.1 (.8–9.8) |
| As part of polytherapy | 17.4 (10.2–27.6) | 5.0 (1.6–12.0) |
| All patients | 16.9 (10.4–27.3) | 4.8 (1.1–10.2) |
Abbreviation: CI, confidence interval.
Treatment Change From Nafcillin to Cefazolin
Nine of 124 (7.25%) patients with PAD from the nafcillin group were switched to cefazolin. Reasons for discontinuation of nafcillin included rash (n = 3), renal impairment (n = 1), neutropenia (n = 1), and undocumented (n = 4). All 9 patients completed cefazolin therapy with no further DEEs reported. There were no cases of patients switched from cefazolin to nafcillin.
DISCUSSION
In the current study, we evaluated the differential tolerability of nafcillin and cefazolin in the OPAT population. We found nafcillin therapy to be associated with significantly higher rates of DEEs, with 16.9 per 1000 patient-days on nafcillin therapy vs 4.8 per 1000 patient-days on cefazolin. Most notably, we discovered that only two-thirds of patients treated with nafcillin successfully completed >80% of their treatment plan. This observed differential tolerability is consistent with what has been suggested, although not quantified, in prior small retrospective efficacy studies [11, 13].
The issue of medication tolerability is especially significant in the OPAT population, as much less supervision and environmental control are available in patients’ homes than in the hospital environment, and patients are potentially at greater risk of severe reactions to medication or rapid deterioration of their conditions given diminished oversight [16–18]. The main factors contributing to the expanding use of OPAT are cost containment, more efficient use of hospital resources, and an effort to improve the patient experience by offering a more convenient alternative site of treatment that allows many patients to return to daily activities during treatment. To realize all 3 goals, it is important to optimize the likelihood of treatment success by choosing a medication regimen that is not only associated with high microbiological and clinical efficacy, but also has a high rate of treatment completion and low rate of important complications.
PAD and DEEs may have additional detrimental consequences beyond their obvious clinical impact on the patient. When DEEs are identified, patients require evaluation and treatment, either by the OPAT team or, if severe, as an inpatient. These evaluations impose additional costs to both providers and patients. Furthermore, inpatient interventions may result in exposures to potential complications such as hospital-associated infections. Some patients may be less likely to continue their treatment regimen after developing a DEE, resulting in a partially treated infection. Finally, when patients develop adverse effects to a first-line antistaphylococcal medication, healthcare providers may be reluctant to treat the patient with another β-lactam antimicrobial and instead expose the patient to a non–β-lactam antibiotic, such as vancomycin, with the attendant potential for higher toxicities, development of resistance, and inferior efficacy. Indeed, in our study population, 65% of patients with PAD were switched to vancomycin, most likely driven by providers’ fear of cross-reactivity between β-lactams. This practice might change in the future, as recently published data suggest that patients with non-IgE-mediated reactions to nafcillin can safely be transitioned to cefazolin [19].
An interesting finding in our study was the observation that although PAD was less frequent in the cefazolin group, when it occurred it was earlier in the treatment course. In fact, in 7 of 8 patients with PAD, treatment was changed within 10 days of initiation (in 2 cases due to widespread rash; 1 case each of renal impairment, liver function abnormalities, and neutropenia; and 2 cases due to late culture results with pathogens other than MSSA). This timing is in clear contrast to the nafcillin group, in which early discontinuation occurred throughout therapy but most notably after the second week of treatment.
Another important consideration to keep in mind is the differential financial burden associated with each medication. Based on standard adult dosing, the average wholesale price of treatment with nafcillin (2 g, 6 times daily) in July 2013 was $169.92 per day, whereas the daily cost of treatment with cefazolin (2 g, 3 times daily) was $26.28. In an outpatient clinic, such as ours, that treats approximately 100 patients a year with either of these antistaphylococcal medications for a median of 28 days, these costs amount to a total wholesale expenditure of $475 776 if the 100 patients are treated with nafcillin vs $73 584 for 100 patients treated with cefazolin—a potential savings of $402 192 per year. This amount is likely much higher when considering the cost of additional medical attention associated with responding to DEEs or PAD, including the possibility of readmission.
Many other important considerations inform treatment decisions for patients with MSSA infections. Early case reports of treatment failures in patients given cefazolin for infective endocarditis [20], as well as concerns about β-lactamase production by MSSA, have led some authors to recommend avoiding cefazolin in patients with a deep focus of infection and a high bacterial load [9, 10, 21]. Although cefazolin has been shown to have similar efficacy to nafcillin in the treatment of patients with MSSA bacteremia and bone and joint infections [11–14, 22, 23] and is now considered first-line therapy for these indications [24–27], cefazolin is currently not recommended for treatment of central nervous system infections due to poor penetration of the blood-brain barrier, and remains a second-line medication for endocarditis in treatment guidelines. Other clinical considerations, such as compatibility of administration with renal replacement therapy, should also play a role in the choice of antimicrobial in individual patients. In our study, only 2 patients in each group were on hemodialysis at the time of OPAT.
Our study is subject to some limitations. First, it was a single-center retrospective review of laboratory and clinical data that were collected prospectively as part of routine OPAT clinic policy. Information bias is always a concern, especially as this information was collected for clinical care and strict research definitions for DEEs were not formulated at implementation of data collection. However, whereas clinical care was led by a multitude of providers, data were entered or verified by a single infectious diseases clinician, thus standardizing data entries. Furthermore, our primary study outcome, PAD, was easily validated and not subject to interpretation. A second concern is treatment selection bias, in which some clinicians might preferentially treat sicker patients with nafcillin over cefazolin, potentially leading to greater susceptibility to complications that could be interpreted as DEEs. We have addressed this concern in part by demonstrating that the groups were comparable with regard to presence of most chronic illnesses at baseline, length of admission prior to OPAT initiation, and rate of coadministered medications. Even so, bias by indication through unmeasured confounders remains a concern. Third, differences in medication compliance related to administration schedules may influence the findings, especially in settings of minimal supervision. Although we have no measure of compliance in our study, approximately 50% of patients in both groups were treated in a supervised nursing facility, minimizing the variability in adherence. Even in this subset of the study population, there was a higher frequency of PAD in patients receiving nafcillin compared with cefazolin (52/180 [29.6%] vs 7/54 [5.8%], respectively; P = .02). Last, the rationale for early discontinuation or change in antimicrobial treatment in a minority of subjects is unknown. Although we suspect the high rate of DEEs in the nafcillin group accounts for the majority of discontinuations, providers may have been biased toward earlier intervention and change in treatment plans in patients treated with nafcillin. However, our primary measure was all-cause premature discontinuation. Even if early discontinuation was driven in part by provider bias, the detrimental consequences of PAD remain valid, and the fact that one-third of patients treated with nafcillin were unable to complete their planned therapy is of concern. In addition, despite the difference in timing of PAD, a similar proportion of patients were switched to alternative parenteral antimicrobial treatment after discontinuation of the originally prescribed medication. This similarity implies that the increased rate of PAD observed in the nafcillin group is not explained by better clinical efficacy and earlier cure.
In conclusion, we suggest that nafcillin therapy for invasive MSSA infections is associated with higher rates of PAD and DEEs than cefazolin. This difference in tolerability, in addition to efficacy and cost, should be taken into account when deciding on long-term parenteral treatment for MSSA in the OPAT setting.
Notes
Acknowledgments. The authors thank Karen Manning for her help with data management and Tomer Ziv-Baran for his help with data analysis and statistical review.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, the National Institutes of Health, Harvard University, its affiliated academic healthcare centers, or its corporate contributors.
Financial support. I. Y. received career support from Harvard Catalyst, The Harvard Clinical and Translational Science Center, funded by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (award number 8UL1TR000170-05), and financial contributions from Harvard University and its affiliated academic healthcare centers.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11:208–22. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 2.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–8. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 3.Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51(suppl 2):S198–208. doi: 10.1086/653520. [DOI] [PubMed] [Google Scholar]
- 4.Chapman AL, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother. 2009;64:1316–24. doi: 10.1093/jac/dkp343. [DOI] [PubMed] [Google Scholar]
- 5.Barr DA, Semple L, Seaton RA. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents. 2012;39:407–13. doi: 10.1016/j.ijantimicag.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Cox AM, Malani PN, Wiseman SW, Kauffman CA. Home intravenous antimicrobial infusion therapy: a viable option in older adults. J Am Geriatr Soc. 2007;55:645–50. doi: 10.1111/j.1532-5415.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 7.Madhavan T, Quinn EL, Freimer E, et al. Clinical studies of cefazolin and comparison with other cephalosporins. Antimicrob Agents Chemother. 1973;4:525–31. doi: 10.1128/aac.4.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J Infect Dis. 1973;128(suppl):S386–9. doi: 10.1093/infdis/128.supplement_2.s386. [DOI] [PubMed] [Google Scholar]
- 9.Nannini EC, Stryjewski ME, Singh KV, et al. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother. 2009;53:3437–41. doi: 10.1128/AAC.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nannini EC, Stryjewski ME, Singh KV, et al. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:2206–8. doi: 10.1128/AAC.01325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Choe PG, Song K-H, et al. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother. 2011;55:5122–6. doi: 10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer ML, Furuno JP, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul M, Zemer-Wassercug N, Talker O, et al. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect. 2011;17:1581–6. doi: 10.1111/j.1469-0691.2010.03425.x. [DOI] [PubMed] [Google Scholar]
- 14.Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J. 2005;98:590–5. doi: 10.1097/01.SMJ.0000145300.28736.BB. [DOI] [PubMed] [Google Scholar]
- 15.Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–72. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 16.Nathwani D, Zambrowski JJ. Advisory group on Home-based and Outpatient Care (AdHOC): an international consensus statement on non-inpatient parenteral therapy. Clin Microbiol Infect. 2000;6:464–76. doi: 10.1046/j.1469-0691.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapman ALN. Outpatient parenteral antimicrobial therapy. BMJ. 2013;346:f1585. doi: 10.1136/bmj.f1585. [DOI] [PubMed] [Google Scholar]
- 18.Török ME, Chapman ALN, Lessing MPA, Sanderson F, Seaton RA. Outpatient parenteral antimicrobial therapy: recent developments and future prospects. Curr Opin Investig Drugs. 2010;11:929–39. [PubMed] [Google Scholar]
- 19.Blumenthal KG, Youngster I, Shenoy ES, Banerji A, Nelson SB. Tolerability of cefazolin after immune-mediated hypersensitivity reactions to nafcillin in the outpatient setting. Antimicrob Agents Chemother. 2014 doi: 10.1128/AAC.02504-13. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant RE, Alford RH. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA. 1977;237:569–70. [PubMed] [Google Scholar]
- 21.Fernández-Guerrero ML, de Górgolas M. Cefazolin therapy for Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;41:127. doi: 10.1086/430833. [DOI] [PubMed] [Google Scholar]
- 22.Shuford JA, Piper KE, Hein M, Trampuz A, Steckelberg JM, Patel R. Lack of association of Staphylococcus aureus type A beta-lactamase with cefazolin combined with antimicrobial spacer placement prosthetic joint infection treatment failure. Diagn Microbiol Infect Dis. 2006;54:189–92. doi: 10.1016/j.diagmicrobio.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Carrizosa J, Santoro J, Kaye D. Treatment of experimental Staphylococcus aureus endocarditis: comparison of cephalothin, cefazolin, and methicillin. Antimicrob Agents Chemother. 1978;13:74–7. doi: 10.1128/aac.13.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 25.Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 26.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 27.Naber CK, Baddour LM, Giamarellos-Bourboulis EJ, et al. Clinical consensus conference: survey on gram-positive bloodstream infections with a focus on Staphylococcus aureus. Clin Infect Dis. 2009;48(suppl 4):S260–70. doi: 10.1086/598185. [DOI] [PubMed] [Google Scholar]