Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer mortality in the United States. Several studies have shown that, in the past two decades, the survival rate of CRC patients has increased substantially due to the improvements in chemotherapy regimens, largely in clinical trial settings. However, it is unclear whether benefits from these improvements can be reproduced in population-based elderly patients. For example it is unclear whether newly developed chemotherapy regimens affect the changing patterns of the leading causes of death among elderly CRC patients. It is also unclear whether the impact on changing patterns of the leading causes of death differs by the presence of comorbidity or chemotherapy-related toxicity. In this study, we used the SEER–Medicare linked dataset of 13 registries (1992–2009) and examined the effects of newly developed chemotherapy regimens, comorbidities, and chemotherapy-related toxicities on the changing patterns of the leading causes of death in elderly patients with colorectal cancer. We hypothesized that the risk of CRC-specific death decreases over time when new and more effective chemotherapy regimens became available.
Keywords: colorectal cancer, cause-specific death, newly developed chemotherapy regimens, comorbidity, chemotherapy-related toxicity
Abstract
Background
Abundant evidences have shown that newly developed chemotherapy regimens improved 5-year survival rate of colorectal cancer (CRC) patients over the past two decades. However, their impact on risk of death from leading causes among elderly patients is still poorly understood.
Patients and methods
A retrospective cohort study of 69 718 elderly CRC patients with their first primary tumors in 1992–2009, identified from the 12 areas of Surveillance, Epidemiology, and End Results-Medicare linked database with their Medicare claims up to 2010. Multivariate Cox regression models were used to assess the effect of newly developed chemotherapy regimens, comorbidities, and chemotherapy related toxicities on cause-specific death and their temporal patterns among elderly CRC patients.
Results
The leading causes of death among CRC patients were CRC, circulation disorders, and secondary cancers, which accounted for 51.4%, 25%, and 4.6% of all-cause death, respectively. Patients diagnosed in more recent diagnostic time periods were significantly less likely to die of CRC [period 2: 5-year hazard ratio = 0.94, 95% confidence interval (CI) 0.90–0.97; period 3: 0.86, 0.83–0.90], circulation disorders (period 2: 0.94, 0.88–1.00; period 3: 0.80, 0.75–0.87), and more likely to die of secondary cancer (period 3: 1.42, 1.20–1.68) compared with those diagnosed in period 1. Charlson comorbidities index and the selected pre-existing comorbidities were significantly associated with increased 5-year risk of death from all three leading causes. Both hematological and gastric toxicity were associated with reduced risk of death from CRC and circulation disorders. The association between diagnostic time period and risk reduction in death from CRC depended on chemotherapy treatment (P < 0.0001). Subgroup analyses showed that the chemotherapy-dependent significant risk reduction was seen in patients with stage II–III CRC, patients without comorbidities, and patients without toxicities (P < 0.0001 for all).
Conclusion
The newly developed chemotherapy regimens were associated with the decreased risk of mortality from CRC.
introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer mortality in the United States [1]. In the past two decades, there has been a consistent increase in the 5-year survival rate of CRC patients due to remarkable advances in early detection and chemotherapy strategies. Major advances in chemotherapy for CRC patients include: novel cytotoxic agents: irinotecan, a topo-isomerase I inhibitor; Oxaliplatin, a DNA cross-linking agent; and a number of monoclonal antibodies such as bevacizumab and cetuximab. It was reported that 5-fluorouracil (5-FU)-based adjuvant chemotherapy increased 5-year survival by 3%–4% for patients with stage II colon cancer and 5%–12% for patients with stage III colon cancer when compared with surgery alone [2]. An international multicenter study (MOSAIC) comparing oxaliplatin in combination with 5-FU/Leucovorin (FOLFOX) versus 5-FU/Leucovorin alone showed that FOLFOX significantly improved 5-year cancer-free survival in patients with stage II and III colon cancer [3]. Of note, abundant evidences on survival benefit of chemotherapy among CRC patients were largely limited to younger patients or well-selected older patients with good performance status, while other elderly patients, especially those with poor overall health status, were under-represented in most randomized, controlled trials (RCT) [4, 5]. Ironically, it is known that CRC mainly affects the elderly and the median age of onset is about 69 years [6]. Nevertheless, the impact of advances in chemotherapy on the outcomes among elderly patients is still poorly understood and no study has been done to evaluate the impact of the newly developed chemotherapy regimens on the changing patterns of the leading causes of death among elderly CRC patients. In addition, elderly patients are very heterogeneous with regard to comorbidities status and chemotherapy-related toxicity. The presence of these conditions may affect disease progression, treatment decision, or life expectancy [7]. For example numerous studies showed a decreased use of chemotherapy among elderly with comorbidities compared with those without [7–10]. To date, few studies have assessed the effect of advances in chemotherapy on the outcomes of the disease among elderly patients with these conditions. Therefore, this study aimed to examine the effects of newly developed chemotherapy regimens, comorbidities, chemotherapy-related toxicities on the changing patterns of the leading causes of death in elderly patients with CRC. We hypothesized that the risk of CRC-specific death decreases over time when new and more effective chemotherapy regimens became available.
patients and methods
data sources
We used SEER–Medicare linked dataset in 1992–2009. The SEER data captures ∼13.4% of the US population and covers 12 areas of the United States, including 6 entire states (Connecticut, Hawaii, Iowa, New Mexico, Utah, and Alaska natives) and 6 metropolitan areas (Atlanta, Rural Georgia, Detroit, San Francisco-Oakland and San Jose-Monterey, Seattle-Puget Sound, Los Angeles) [11–13]. The SEER dataset includes information on patients' demographics, year of diagnosis, tumor characteristics, initial treatment, causes of death linked from center for vital statistics, and follow-up time [12]. Medicare data include treatment information, such as surgical, radiation, and chemotherapy, comorbidities, and subsequent follow-up for healthcare access in inpatient and outpatient services.
study population
In this study, we included all Medicare beneficiaries at age 66 or older with pathologically confirmed primary CRC from 1 January 1992 through 31 December 2009. The study patients were restricted to those aged ≥66 to allow for a 1-year time period before cancer diagnosis during which pre-existing comorbidities from Medicare claims could be identified. The study patients were excluded if they (i) lacked continuous enrollment in Medicare Part A and B during the study period, or participated in health maintenance organizations (HMO) as their complete medical claim records may not be available in the SEER–Medicare linked dataset; (ii) received chemotherapy after 6 months of primary cancer diagnosis, or died within 6 months of primary cancer diagnosis as they less likely received the standard treatment; (iii) had unknown cause of death. A total of 69 718 patients aged 66 or older diagnosed with primary CRC between 1992 and 2009 were potentially eligible for inclusion in this study.
study variables
patient and tumor characteristics
Covariates considered in this study included age at diagnosis (66–74, 75–84, ≥85), gender (female, male), race (African American, Caucasian, and Others), tumor stage (AJCC stage I, II, III, and IV), site of tumor (colon and rectum), number of lymph nodes examined (categorized as 0, 1–5, 6–11, and ≥12 based on clinical guideline [14, 15]), and node positive cancers (yes, no, or unknown).
newly developed chemotherapy regimens
In this study, diagnostic time periods were categorized into four periods based on the timeline of major progresses in chemotherapy regimens: (i) in year 1992–1995, 5-FU was the mainstay of chemotherapy for CRC; (ii) in year 1996–2001, irinotecan was approved and used for advanced colon cancer (cancer spreader despite other treatment) [16]; (iii) in year 2002–2004, Oxaliplatin combined with 5-FU and leucovorin was approved and used for advanced colon cancer and stage III, consecutively [16]; and (iv) in year 2005–2009, a number of monoclonal antibodies were approved and used for advanced CRC [16].
use of chemotherapy
Chemotherapy use was defined if there was at least one claim for chemotherapy use from hospital, physician carrier, or outpatient files within 6 months of cancer diagnosis either as adjuvant therapy for those who received resection or as primary and palliative therapy for those with late stage without surgery. The methods of identifying chemotherapy use through the Medicare claims were discussed elsewhere [17–19]. In brief, patients were defined as having received chemotherapy if there was a claim for chemotherapy from any of the following Medicare codes that were made within 6 months of diagnosis: the ICD-9-CM procedure code of 9925 and V codes of V58.1, V66.2, or V67.2, the Common Procedure Terminology codes of 96400–96549, J8510, J8520, J8521, J8530-J8999, J9000-J9999 (J9035: bevacizumab, J9055: cetuximab, J9190: 5-FU, J9026: irinotecan, J9263: oxaliplatin), Q0083-Q0085, G code of G0921-G0932, and revenue center codes of 0331, 0332, and 0335.
chemotherapy-related toxicities
Chemotherapy-related toxicities assessed in this study were identified from inpatient, physician carrier, or outpatient files of study patients, which occurred within 3 months of the first course of chemotherapy [20]. Toxicities included: hematological toxicities (neutropenia: 288, thromocytopenia: 287.4); gastric toxicities (nausea/vomiting: 787.0×, diarrhea: 787.91, stomatitis: 528.0, gastroenteritis colitis: 558.2, 558.9); and neurotoxicity (neuropathy: 357.6, peripheral neuropathy: 356.9).
prior comorbidities
The Charlson comorbidity index was created using both physician and hospital claim files and calculated using the SAS macro developed and updated by National Cancer Institute [21–23]. In addition to this index, some common pre-existing comorbidities were assessed individually in this study including: heart disease (myocardial infarction: 410–4109, congestive heart failure: 428–4289), diabetes (250, 2500–2503, 2507), and chronic obstructive pulmonary disease (COPD) (490–495, 500–505, 5064). The time frame for both the index and the selected pre-existing comorbidities was 1 month before CRC diagnosis.
leading causes of death
This study focused on three leading causes of death including CRC (coding: 14 and 24), secondary cancers (1–13 and 27–86), and circulation disorders (154–169) that together accounted for more than 81% of all-cause of death. The causes of death were defined based on variable CODKM in Patient Entitlement and Diagnosis Summary File (PEDSF). Follow-up time was calculated from the date of CRC diagnosis to the recorded date of death or the end of follow-up (31 December 2009), whichever occurred first. The SEER registries used algorithms to process causes of death from death certificates across the country and, therefore, there was no loss of follow-up unless the person died overseas and was missed by the program.
statistical analysis
For all analyses using 5-year risk of cause-specific death as outcome, cases diagnosed during 2005–2009 were excluded to ensure all subjects were observed for at least 5 years.
Multivariate Cox proportional hazards regression models were used to examine the effect of diagnostic time periods, Charlson comorbidity index, common pre-existing comorbidities, and chemotherapy-related toxicities on the 5-year risks of cause-specific death. Covariates adjusted in the models included: age, gender, race, tumor stage, tumor site, number of lymph nodes examined, node positive cancers, and propensity scores. In calculation of cause-specific hazard ratios (HRs), censoring was defined as the group of individuals who did not experience the specific cause of death.
To examine whether the association between diagnostic time periods and 5-year risk of each cause-specific death depended on chemotherapy treatment, multivariate Cox regression models were used to examine the moderating effect of chemotherapy on the association. This model examined the main effects of diagnostic time periods and chemotherapy as well as the interaction effect between diagnostic periods and chemotherapy. The chemotherapy recipients and nonrecipients were matched based on propensity scores using the nearest available pair matching method. The predicted probability of receiving chemotherapy between matched individuals could vary by no >0.01 (1%) on a scale of 0–1.
A two-sided significance level of a = 0.05 was used for all statistical analyses. All analyses were carried out using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
results
Table 1 presents the characteristics of 69 718 elderly patients with CRC by four diagnostic time periods. The proportion of cases in stage II increased from 34% in 1992–1995 to 39% in 1996–2001, and to 41% in 2002–2004. Interestingly, the proportion dropped to 31% in period 2005–2009. There was a consecutive increase in extent of lymph nodes evaluation over time. Specifically, the proportion of ≥12 lymph nodes being evaluated almost doubled in period 2005–2009 compared with period 1992–1995.
Table 1.
Patient characteristics by diagnostic time periods among elderly patients with colorectal cancer
| Demo/clinical characteristics | Total | Diagnostic time periods, N (%) |
|||
|---|---|---|---|---|---|
| 1992–1995 | 1996–2001 | 2002–2004 | 2005–2009 | ||
| Total, N | 69 718 | 13 018 (18.7) | 20 818 (29.9) | 12 668 (18.2) | 23 214 (33.3) |
| Age (years) | |||||
| 66–74 | 26 158 (37.5) | 9896 (38.9) | 6088 (35.8) | 4624 (36.5) | 8698 (37.5) |
| 75–84 | 30 655 (44.0) | 11 185 (44.0) | 7760 (45.6) | 5779 (45.6) | 9874 (42.5) |
| ≥85 | 12 905 (18.5) | 4360 (17.1) | 3166 (18.6) | 2265 (17.9) | 4642 (20.0) |
| Gender | |||||
| Male | 30 626 (43.9) | 10 996 (43.2) | 7396 (43.5) | 5648 (44.6) | 10 375 (44.7) |
| Female | 39 092 (56.1) | 14 445 (56.8) | 9618 (56.5) | 7020 (55.4) | 12 839 (55.3) |
| Race | |||||
| Caucasian | 58 836 (84.4) | 21 930 (86.2) | 14 382 (84.5) | 10 622 (83.8) | 19 142 (82.5) |
| African American | 4501 (6.5) | 1621 (6.4) | 937 (5.5) | 713 (5.6) | 1694 (7.3) |
| Other | 6381 (9.2) | 1890 (7.4) | 1695 (10.0) | 1333 (10.5) | 2378 (10.2) |
| Tumor grade | |||||
| Well differentiated | 5596 (8.0) | 1979 (7.8) | 1287 (7.6) | 1041 (8.2) | 1959 (8.4) |
| Moderately differentiated | 43 144 (61.9) | 15 727 (61.8) | 10 645 (62.6) | 7838 (61.9) | 14 306 (61.6) |
| Poorly differentiated | 15 375 (22.1) | 5623 (22.1) | 3800 (22.3) | 2824 (22.3) | 5043 (21.7) |
| Unknown/missing | 5603 (8.0) | 2112 (8.3) | 1282 (7.5) | 965 (7.6) | 1906 (8.2) |
| AJCC stage | |||||
| In situ/stage I | 14 043 (20.1) | 4657 (18.3) | 3607 (21.2) | 2691 (21.2) | 4918 (21.2) |
| Stage II | 23 530 (33.8) | 8529 (33.5) | 6690 (39.3) | 5183 (40.9) | 7093 (30.6) |
| Stage III | 17 907 (25.7) | 6763 (26.6) | 3557 (20.9) | 2301 (18.2) | 6501 (28.0) |
| Stage IV | 11 936 (17.1) | 4660 (18.3) | 2729 (16.0) | 2114 (16.7) | 3834 (16.5) |
| Unknown/missing | 2302 (3.3) | 832 (3.3) | 431 (2.5) | 379 (3.0) | 868 (3.7) |
| Tumor site | |||||
| Colon | 58 517 (83.9) | 21 124 (83.0) | 14 365 (84.4) | 10 730 (84.7) | 19 587 (84.4) |
| Rectum | 11 201 (16.1) | 4317 (17.0) | 2649 (15.6) | 1938 (15.3) | 3627 (15.6) |
| No. of lymph nodes examined | |||||
| 0 | 7617 (10.9) | 3199 (12.6) | 1715 (10.1) | 1232 (9.7) | 2304 (9.9) |
| 1–5 | 10 598 (15.2) | 5212 (20.5) | 2961 (17.4) | 2033 (16.0) | 1830 (7.9) |
| 6–11 | 18 857 (27.0) | 7542 (29.6) | 5258 (30.9) | 3760 (29.7) | 4895 (21.1) |
| ≥12 | 29 393 (42.2) | 7550 (29.7) | 6375 (37.5) | 5258 (41.5) | 13 680 (58.9) |
| Unknown/missing | 3253 (4.7) | 1938 (7.6) | 705 (4.1) | 385 (3.0) | 505 (2.2) |
| No. of positive lymph nodes | |||||
| 0 | 35 094 (50.3) | 12 322 (48.4) | 8714 (51.2) | 6521 (51.5) | 11 944 (51.5) |
| 1–9 | 34 624 (49.7) | 13 119 (51.6) | 8300 (48.8) | 6147 (48.5) | 11 270 (48.5) |
| SEER registry by area | |||||
| West | 35 285 (50.6) | 5188 (39.9) | 9525 (45.8) | 7462 (58.9) | 13 110 (56.5) |
| South | 4227 (6.1) | 296 (2.3) | 517 (2.5) | 274 (2.2) | 3140 (13.5) |
| North Central | 20 755 (29.8) | 5139 (39.5) | 7531 (36.2) | 3289 (26.0) | 4796 (20.7) |
| Northeast | 9451 (13.6) | 2395 (18.4) | 3245 (15.6) | 1643 (13.0) | 2168 (9.3) |
Table 2 presents the proportion of death and the 5-year risk of death due to each leading cause by diagnostic time periods, chemotherapy treatment, comorbidities, and chemotherapy-related toxicities. The leading causes of death were CRC, circulation disorders, and secondary cancer that accounted for 51.5%, 25%, and 4.6% of all-cause death, respectively. Multivariate Cox regression models showed that patients diagnosed in periods 2 and 3 were significantly less likely to die of CRC [period 2: HR = 0.94, 95% confidence interval (CI) 0.90–0.97; period 3: 0.86, 0.83–0.90] and circulation disorders (period 2: 0.94, 0.88–1.00; period 3: 0.80, 0.75–0.87) than patients diagnosed in period 1; and patients diagnosed in period 3 were significantly more likely to die of secondary cancer (1.42, 1.20–1.68) than patients diagnosed in period 1. Patients with Charlson comorbidity index score ≥1 were significantly more likely to die of all three leading causes than those without comorbidities. Patients that experienced hematological toxicity were less likely to die of CRC (0.79, 0.70–0.88) and circulation disorders (0.44, 0.30–0.65) than those without; and patients that experienced gastric toxicity were less likely to die of CRC (0.80, 0.77–0.84) and circulation disorder (0.67, 0.60–0.75) than those without.
Table 2.
Effect of diagnostic time periods, comorbidities, and chemotherapy-related toxicities on 5-year risks of cause-specific death
| Five-year risk of death | Censored | Causes of death |
||||||
|---|---|---|---|---|---|---|---|---|
| CRC |
Circulatory disorders |
Secondary cancer |
Other causes | |||||
| N = 11 322 (24.4%) | N = 18 091 (38.9%) | HR† (95% CI) | N = 8793 (18.9%) | HR† (95% CI) | N = 1607 (3.5%) | HR† (95% CI) | N = 6691 (14.4%) | |
| Diagnostic periods | ||||||||
| 1992–1995 | 1323 (10.2) | 5686 (43.7) | Ref | 3340 (25.7) | Ref | 466 (3.6) | Ref | 2203 (16.9) |
| 1996–2001 | 4954 (23.8) | 8047 (38.7) | 0.94 (0.90–0.97) | 3911 (18.8) | 0.94 (0.88–1.00) | 720 (3.5) | 1.09 (0.93–1.27) | 3186 (15.3) |
| 2002–2004 | 5045 (39.8) | 4358 (34.4) | 0.86 (0.83–0.90) | 1542 (12.2) | 0.80 (0.75–0.87) | 421 (3.3) | 1.42 (1.20–1.68) | 1302 (10.3) |
| Chemotherapy | ||||||||
| No | 6524 (24.3) | 7737 (28.8) | Ref | 6722 (25.0) | Ref | 861 (3.2) | Ref | 5020 (18.7) |
| Yes | 4798 (24.4) | 10 354 (52.7) | 0.78 (0.76–0.82) | 2071 (10.5) | 0.39 (0.36–0.42) | 746 (3.8) | 0.72 (0.61–0.84) | 1671 (8.5) |
| Charlson Comorbidity Index | ||||||||
| 0 | 7999 (28.5) | 11 644 (41.5) | Ref | 4281 (15.3) | Ref | 970 (3.5) | Ref | 3153 (11.2) |
| 1 | 2440 (20.7) | 4327 (36.8) | 1.06 (1.02–1.10) | 2533 (21.5) | 1.69 (1.58–1.80) | 429 (3.6) | 1.35 (1.17–1.55) | 2034 (17.3) |
| 2 | 883 (13.2) | 2120 (31.7) | 1.09 (1.04–1.14) | 1979 (29.6) | 3.04 (2.81–3.29) | 208 (3.1) | 1.60 (1.34–1.90) | 1504 (22.5) |
| Comorbidities | ||||||||
| Heart disease | ||||||||
| No | 10 275 (27.0) | 15 327 (40.3) | Ref | 6021 (15.8) | Ref | 1342 (3.5) | Ref | 5075 (13.3) |
| Yes | 1047 (12.4) | 2764 (32.7) | 1.12 (1.08–1.17) | 2772 (32.8) | 2.55 (2.40–2.71) | 265 (3.1) | 1.37 (1.17–1.61) | 1616 (19.1) |
| COPD | ||||||||
| No | 10 437 (25.3) | 16 282 (39.5) | Ref | 7690 (18.7) | Ref | 1403 (3.4) | Ref | 5361 (13.0) |
| Yes | 885 (16.6) | 1809 (33.9) | 1.10 (1.04–1.15) | 1103 (20.7) | 1.20 (1.11–1.30) | 204 (3.8) | 1.56 (1.31–1.86) | 1330 (24.9) |
| Diabetes | ||||||||
| No | 9601 (25.0) | 15 244 (39.7) | Ref | 6970 (18.2) | Ref | 1332 (3.5) | Ref | 5226 (13.6) |
| Yes | 1721 (21.2) | 2847 (35.0) | 0.97 (0.93–1.01) | 1823 (22.4) | 1.36 (1.27–1.46) | 275 (3.4) | 1.19 (1.02–1.40) | 1465 (18.0) |
| Chemo-toxicities | ||||||||
| Hematologicala | ||||||||
| No | 11 099 (24.2) | 17 740 (38.7) | Ref | 8743 (19.1) | Ref | 1577 (3.4) | Ref | 6627 (14.5) |
| Yes | 223 (31.1) | 351 (48.9) | 0.79 (0.70–0.88) | 50 (7.0) | 0.44 (0.30–0.65) | 30 (4.2) | 1.16 (0.78–1.72) | 64 (8.9) |
| Gastricb | ||||||||
| No | 10 562 (24.1) | 16 785 (38.3) | Ref | 8498 (19.4) | Ref | 1510 (3.4) | Ref | 6469 (14.8) |
| Yes | 760 (28.4) | 1306 (48.7) | 0.80 (0.77–0.84) | 295 (11.0) | 0.67 (0.60–0.75) | 97 (3.6) | 1.04 (0.88–1.23) | 222 (8.3) |
| Neurologicc | ||||||||
| No | 11 308 (24.3) | 18 078 (38.9) | Ref | 8786 (18.9) | Ref | 1606 (3.5) | Ref | 6683 (14.4) |
| Yes | 14 (32.6) | 13 (30.2) | 0.91 (0.50–1.64) | 7 (16.3) | 0.90 (0.38–2.17) | 1 (2.3) | 1.20 (0.17–8.53) | 8 (18.6) |
HR adjusted for demographics (age, gender, race, SEER regions), tumor characteristics (tumor grade, tumor stage, tumor site, no. of lymph nodes examined, no. of positive lymph nodes), and propensity score of receiving chemotherapy.
†HR (95% CI): 5-year hazards ratio with 95% confidence interval.
aHematological toxicity includes: neutropenia and thrombocytopenia.
bGastric toxicity includes: nausea/vomiting, diarrhea, stomatitis, gastroenteritis colitis.
cNeurologic toxicity includes: neuropathy and peripheral neuropathy.
Table 3 presents the moderating effects of chemotherapy on the association between diagnostic time periods and 5-year cause-specific risk of death. Interaction analysis showed that the magnitude of risk reduction in death from CRC (P < 0.0001, supplementary Table S1, available at Annals of Oncology online) significantly differed between chemotherapy recipients and nonrecipients. Stratified analyses by chemotherapy showed that significant risk reduction in CRC-specific death was only seen among chemotherapy recipients (period 2 versus period 1: 0.92, 0.86–0.97; period 3 versus period 1: 0.76, 0.71–0.81), but not among nonrecipients. In addition, subgroup analyses showed that the chemotherapy-dependent significant risk reduction in death from CRC was seen in patients with stage II–III CRC; patients without comorbidities, and patients without toxicities (P < 0.0001 for all). Figure 1 showed the 5-year cause-specific HRs (95% CI) by diagnostic time periods and chemotherapy. Figure 2 showed the 5-year cause-specific HRs (95% CI) by diagnostic time periods, chemotherapy, and the presence of complications.
Table 3.
Associations between diagnostic time periods and 5-year risk of cause-specific death stratified by chemotherapy treatment
| Five-year risk of cause-specific death, HR (95% CI)a |
||||||
|---|---|---|---|---|---|---|
| Colorectal cancer |
Circulation disorders |
Secondary cancer |
||||
| No chemo | Chemo | No chemo | Chemo | No chemo | Chemo | |
| All stages | ||||||
| 1996–2001 versus 1992–1995 | 1.04 (0.98–1.12) | 0.92 (0.86–0.97) | 0.73 (0.65–0.82) | 0.75 (0.64–0.88) | 1.58 (1.22–2.06) | 0.96 (0.74–1.25) |
| 2002–2004 versus 1992–1995 | 0.93 (0.87–1.02) | 0.76 (0.71–0.81) | 0.56 (0.50–0.63) | 0.60 (0.50–0.72) | 1.68 (1.29–2.18) | 1.46 (1.15–1.85) |
| Subgroups | Stratified analysis | |||||
| Stage II–III | ||||||
| 1996–2001 versus 1992–1995 | 1.03 (0.93–1.15) | 0.80 (0.73–0.88) | 0.81 (0.71–0.93) | 0.72 (0.59–0.89) | 1.47 (1.00–2.16) | 0.70 (0.48–1.03) |
| 2002–2004 versus 1992–1995 | 0.87 (0.79–0.96) | 0.73 (0.66–0.81) | 0.55 (0.47–0.64) | 0.65 (0.52–0.81) | 1.78 (1.24–2.56) | 1.15 (0.80–1.66) |
| Stage IV | ||||||
| 1996–2001 versus 1992–1995 | 1.13 (1.01–1.25) | 0.97 (0.88–1.08) | 1.06 (0.73–1.54) | 0.84 (0.52–1.35) | 1.03 (0.65–1.63) | 1.43 (0.84–2.44) |
| 2002–2004 versus 1992–1995 | 1.03 (0.91–1.16) | 0.86 (0.76–0.97) | 0.75 (0.48–1.17) | 0.62 (0.34–1.13) | 1.40 (0.87–2.26) | 1.08 (0.57–2.08) |
| No comorbidity, all stages | ||||||
| 1996–2001 versus 1992–1995 | 1.06 (0.98–1.16) | 0.87 (0.80–0.94) | 0.83 (0.69–0.99) | 0.52 (0.40–0.68) | 1.47 (1.03–2.11) | 1.21 (0.86–1.69) |
| 2002–2004 versus 1992–1995 | 0.90 (0.82–0.98) | 0.75 (0.68–0.82) | 0.52 (0.42–0.65) | 0.53 (0.40–0.71) | 1.91 (1.33–2.74) | 1.60 (1.14–2.23) |
| Comorbidity, all stages | ||||||
| 1996–2001 versus 1992–1995 | 1.02 (0.91–1.14) | 0.95 (0.86–1.05) | 0.69 (0.59–0.80) | 0.94 (0.76–1.15) | 1.41 (0.94–2.13) | 0.73 (0.48–1.13) |
| 2002–2004 versus 1992–1995 | 1.01 (0.91–1.12) | 0.80 (0.72–0.88) | 0.56 (0.48–0.65) | 0.62 (0.50–0.78) | 1.67 (1.14–2.44) | 1.18 (0.82–1.68) |
| No toxicity, all stages | ||||||
| 1996–2001 versus 1992–1995 | 1.06 (0.99–1.13) | 0.89 (0.84–0.95) | 0.72 (0.64–0.82) | 0.76 (0.64–0.90) | 1.41 (1.07–1.86) | 1.04 (0.79–1.37) |
| 2002–2004 versus 1992–1995 | 0.94 (0.88–1.01) | 0.77 (0.71–0.82) | 0.54 (0.48–0.62) | 0.64 (0.53–0.78) | 1.53 (1.16–2.01) | 1.46 (1.12–1.89) |
| Toxicity, all stages | ||||||
| 1996–2001 versus 1992–1995 | 0.87 (0.65–1.17) | 0.96 (0.73–1.27) | 0.89 (0.63–1.25) | 0.88 (0.50–1.55) | 0.84 (0.26–2.69) | 1.49 (0.56–3.93) |
| 2002–2004 versus 1992–1995 | 1.10 (0.85–1.44) | 0.83 (0.64–1.06) | 0.81 (0.57–1.14) | 0.68 (0.38–1.21) | 3.51 (1.43–8.59) | 1.86 (0.80–4.35) |
aChemotherapy recipients were matched with nonrecipients bases on propensity score, HR adjusted for demographics (age, gender, race, SEER regions), tumor characteristics (tumor grade, tumor stage, tumor site, no. of lymph nodes examined, no. of positive lymph nodes).
Figure 1.
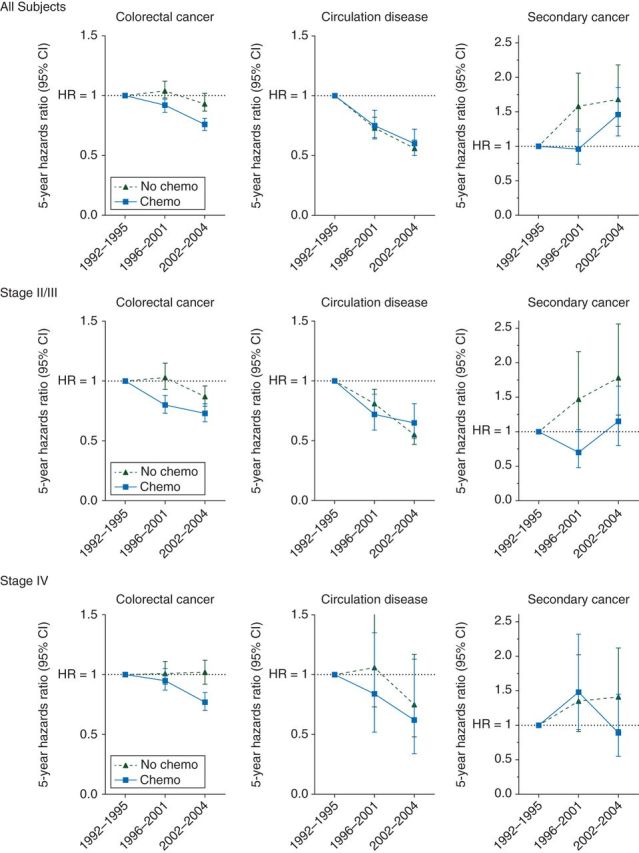
Five-year cause-specific hazard ratio, stratified by chemotherapy and tumor stage. Chemotherapy recipients and nonrecipients were matched by propensity score of receiving chemotherapy.
Figure 2.
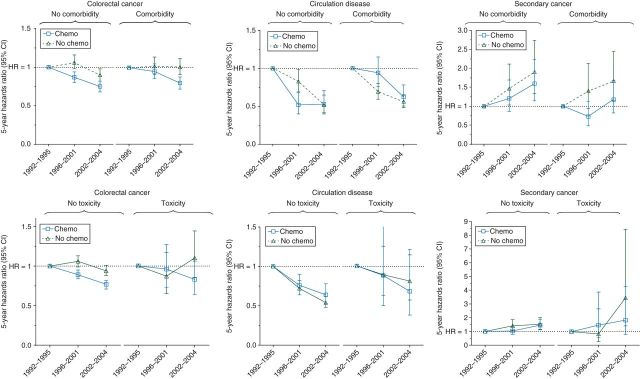
Five-year cause-specific hazard ratio, stratified by chemotherapy and by presence of complications. Chemotherapy recipients and nonrecipients were matched by propensity score of receiving chemotherapy.
discussion
To our best knowledge, this study is the first to evaluate the impact of newly developed chemotherapy regimens on the temporal trends of leading causes of death among population-based elderly CRC patients with comorbidities and chemotherapy-related toxicities and among those without such complications.
In this study, we found that risk of death from CRC significantly decreased with diagnostic time periods among elderly CRC patients. It is not clear whether the effect of a diagnostic time periods on the risk of death dependent on advances in chemotherapy. We found that risk of CRC-specific death decreased with diagnostic time periods only in chemotherapy recipients but not in nonrecipients, suggesting the risk reduction was mainly due to newly developed chemotherapy regimens. It is likely that improved specimen assessment and tumor staging have accounted for some degrees of the risk reduction. Several studies have reported that a more extensive lymph node evaluation may reduce the risk of under staging, enhance appropriate treatment, and improve survival [24, 25]. In this study, we found the proportion of patients with 12 or more lymph nodes examined increased from 30% in 1992–1995 to 42% in 2002–2004, which may lead to improved survival.
In subgroup analyses, we found that the chemotherapy-dependent risk reduction in death from CRC was significant and apparent among patients with stage II–III CRC but less apparent among patients with stage IV CRC. This discrepancy may be expected as the median survival of patients with stage IV CRC is short; hence it is difficult to identify changes in 5-year risk of death among these patients. Further research is needed to clarify the palliative benefit of chemotherapy in elderly patients via assessing symptom control, quality of life, and survival [26, 27]. In addition, it is debated that whether elderly CRC patients with comorbidities or chemotherapy-related toxicities would benefit from the newly developed chemotherapy regimens. We found that elderly patients with such complications did not gain expected benefit from newly developed chemotherapy regimens. Existing studies examining the influence of comorbidities on chemotherapy use and outcomes also showed inferior survival among CRC patients with comorbidities [7, 28, 29]. The lack of benefits could be due to the following possibilities: first, the small net benefit from the newly developed chemotherapy regimens may be offset by the increased risk of mortality due to the complications; secondly, patients with complications may be less likely to receive a standard treatment in terms of dose and length of treatment or their tolerance and compliance to the treatment may be poorer than those fit elderly patients. Given that the median age at diagnosis for CRC is 69 in the United States [6], and older adults are disproportionately affected by comorbidities, chemotherapy-related toxicities, or suboptimal care when compared with their younger counterparts, we further stratified our study cohort by age groups (supplementary Table S2, available at Annals of Oncology online). We found that chemotherapy-dependent risk reduction of death from CRC was seen in both patients aged 66–74 and patients aged 75 or older.
In this study, we found that the risk of death from circulation disorders was significantly decreased with diagnostic time periods among both chemotherapy recipients and nonrecipients. The decreasing trend of death from circulation disorders may reflect the progress in preventing and treating this disease over the same time period among general population [30–32]. Studies showed that there was an over 30% decline in death rates from stroke and heart disease among US population age 65 or older from 1980 to 2004 [32]. Numerous evidences showed that the reduction in mortality from circulation disorders was associated with advances in medical treatments as well as improvements in cardiovascular risk factors, especially total cholesterol and systolic blood pressure [33, 34]. In addition, we found that risk of death from secondary cancer was significantly increased during the diagnostic time period 2002–2004 among elderly CRC patients. It is possible that, with enhanced long-term survival of elderly CRC patients, their risk of developing and dying from secondary cancer is also increased. Further, with increased use of cancer screening tests over time, more previously unrecognized malignancies may be detected. Several studies showed that in the past two decades there was at least 15% increase in rate of screening for CRC, breast cancer, and prostate cancer [35–37].
The primary strengths of this study include the consistencies in SEER variables over time, long-term of follow-up, broad coverage of US population, and the validity of the data [38, 39]. This study has several limitations. First, although SEER cancer registries used algorithms to process the COD from death certificates, assigning a single COD may not be easy in some cases [40]. These inaccuracies likely introduced misclassification bias in COD classification. In particular, when dealing with specific cardiovascular causes of death there is some degree of inaccuracy. Therefore, we decided to use all circulation system disease as a unique group of cause of death, in which heart disease and cerebrovascular diseases accounted for over 90% of the deaths. Secondly, there may be some systematic bias between the chemotherapy recipients and nonrecipients. Although, in this study, we have matched the chemotherapy recipients and nonrecipients by demographic, tumor characteristics, and burden of comorbidities, it might not be sufficient to offset the potential difference between them. The potential bias may exaggerate the true difference in risk of death between the two groups but it will not affect the moderating effect of chemotherapy on the temporal trend of death. Thirdly, due to the retrospective nature of the study, we cannot detail the delivery of chemotherapy, compliance to the treatment, and severity of comorbidities and chemotherapy-related toxicities, which may confound the impact of newly developed chemotherapy regimens on temporal patterns of deaths.
In conclusion, this study showed that the risks of death were significantly decreased with diagnostic time periods for causes from CRC and circulation disorders but increased for cause from secondary cancer. The risk reduction of death form CRC significantly depended on newly developed chemotherapy regimens. The survival benefit of newly developed chemotherapy regimens was more apparent to patient without comorbidities or chemotherapy-related toxicities than to those with such complications. The findings suggest that use of newly developed regimens irinotecan or oxaliplatin, especially oxaliplatin-based regimen may be appropriate for treating elderly patients. Consideration should be given to the patient's overall health performance and potential for tolerating chemotherapy-related toxicities.
funding
This study was supported in part by a grant from the Agency for Healthcare Research and Quality (R01-HS018956).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We acknowledge the efforts of the National Cancer Institute in the creation of this database. The interpretation and reporting of these data are the sole responsibilities of the authors.
references
- 1.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64: 104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Comparison of flourouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: a randomised trial. QUASAR Collaborative Group. Lancet. 2000;355(9215):1588–1596. [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 4.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 5.Aparicio T, Navazesh A, Boutron I, et al. Half of elderly patients routinely treated for colorectal cancer receive a sub-standard treatment. Crit Rev Oncol Hematol. 2009;71(3):249–257. doi: 10.1016/j.critrevonc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 6. SEER Stat Fact Sheets: colon and rectum cancer http://seer.cancer.gov/statfacts/html/colorect.html. (11 April 2014, date last accessed)
- 7.Lee L, Cheung WY, Atkinson E, et al. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29(1):106–117. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 8.Papamichael D, Audisio R, Horiot JC, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol. 2009;20(1):5–16. doi: 10.1093/annonc/mdn532. [DOI] [PubMed] [Google Scholar]
- 9.Audisio RA, Papamichael D. Treatment of colorectal cancer in older patients. Nat Rev Gastroenterol Hepatol. 2012;9(12):716–725. doi: 10.1038/nrgastro.2012.196. [DOI] [PubMed] [Google Scholar]
- 10.Bakogeorgos M, Mountzios G, Kotsantis G, et al. Chemotherapy compliance, tolerance and efficacy in elderly and non-elderly patients with metastatic colorectal cancer: a single institution comparative study. J BUON. 2013;18(3):629–634. [PubMed] [Google Scholar]
- 11.Rueth NM, Parsons HM, Habermann EB, et al. The long-term impact of surgical complications after resection of stage I nonsmall cell lung cancer: a population-based survival analysis. Ann Surg. 2011;254(2):368–374. doi: 10.1097/SLA.0b013e31822150fe. [DOI] [PubMed] [Google Scholar]
- 12.Abraham A, Al-Refaie WB, Parsons HM, et al. Disparities in pancreas cancer care. Ann Surg Oncol. 2013;20(6):2078–2087. doi: 10.1245/s10434-012-2843-z. [DOI] [PubMed] [Google Scholar]
- 13.White A, Vernon SW, Franzini L, et al. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116(19):4622–4631. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright FC, Law CH, Berry S, et al. Clinically important aspects of lymph node assessment in colon cancer. J Surg Oncol. 2009;99(4):248–255. doi: 10.1002/jso.21226. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Greene FL. TNM classification: clarification of number of regional lymph nodes for pNo. Cancer. 2001;92(2):452. doi: 10.1002/1097-0142(20010715)92:2<452::aid-cncr1342>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16. Cancer advances in focus: colorectal cancer http://www.cancer.gov/cancertopics/factsheet/cancer-advances-in-focus/colorectal. (11 April 2014, date last accessed)
- 17.6th edition. ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification. [Google Scholar]
- 18.American Medical Association: Physician Current Procedural Terminology: CPT 94. Chicago, IL: American Medical Association; 1993. [Google Scholar]
- 19.Health Care Financing Administration: HCFA Common Procedure Coding System (HCPCS): National Level II Medicare Codes. Losangeles, CA: Practice Management Information Corporation; 1994. [Google Scholar]
- 20.Cen P, Liu C, Du XL. Comparison of toxicity profiles of fluorouracil versus oxaliplatin regimens in a large population-based cohort of elderly patients with colorectal cancer. Ann Oncol. 2012;23(6):1503–1511. doi: 10.1093/annonc/mdr449. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22. Charlson Comorbidity Index. http://appliedresearch.cancer.gov/seermedicare/program/comorbidity.html. (11 April 2014, date last accessed)
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Parsons HM, Tuttle TM, Kuntz KM, et al. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA. 2011;306(10):1089–1097. doi: 10.1001/jama.2011.1285. [DOI] [PubMed] [Google Scholar]
- 25.Parsons HM, Begun JW, Kuntz KM, et al. Lymph node evaluation for colon cancer in an era of quality guidelines: who improves? J Oncol Pract. 2013;9(4):e164–e171. doi: 10.1200/JOP.2012.000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leo S, Accettura C, Gnoni A, et al. Systemic treatment of gastrointestinal cancer in elderly patients. J Gastrointest Cancer. 2013;44(1):22–32. doi: 10.1007/s12029-012-9447-5. [DOI] [PubMed] [Google Scholar]
- 27.Pentheroudakis G, Fountzilas G, Kalofonos HP, et al. Palliative chemotherapy in elderly patients with common metastatic malignancies: a Hellenic Cooperative Oncology Group registry analysis of management, outcome and clinical benefit predictors. Crit Rev Oncol Hematol. 2008;66(3):237–247. doi: 10.1016/j.critrevonc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Hung A, Mullins CD. Relative effectiveness and safety of chemotherapy in elderly and nonelderly patients with stage III colon cancer: a systematic review. Oncologist. 2013;18(1):54–63. doi: 10.1634/theoncologist.2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemmens VE, Janssen-Heijnen ML, Verheij CD, et al. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92(5):615–623. doi: 10.1002/bjs.4913. [DOI] [PubMed] [Google Scholar]
- 30.Remington PL, Brownson RC. Fifty years of progress in chronic disease epidemiology and control. MMWR Surveill Summ. 2011;60(Suppl 4):70–77. [PubMed] [Google Scholar]
- 31.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 32.Wijeysundera HC, Machado M, Farahati F, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303(18):1841–1847. doi: 10.1001/jama.2010.580. [DOI] [PubMed] [Google Scholar]
- 33.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 34.O'Flaherty M, Buchan I, Capewell S. Contributions of treatment and lifestyle to declining CVD mortality: why have CVD mortality rates declined so much since the 1960s? Heart. 2013;99(3):159–162. doi: 10.1136/heartjnl-2012-302300. [DOI] [PubMed] [Google Scholar]
- 35.Myer PA, Mannalithara A, Singh G, et al. Proximal and distal colorectal cancer resection rates in the United States since widespread screening by colonoscopy. Gastroenterology. 2012;143(5):1227–1236. doi: 10.1053/j.gastro.2012.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 37.NCI. Cancer progress report: early detection of breast cancer http://progressreport.cancer.gov/summary-tables_female.asp. (11 April 2014, date last accessed)
- 38. SEER: quality improvement process http://seer.cancer.gov/qi/process.html. (11 April 2014, date last accessed)
- 39.Ambs A, Warren JL, Bellizzi KM, et al. Overview of the SEER—Medicare Health Outcomes Survey linked dataset. Health Care Financ Rev. 2008;29(4):5–21. [PMC free article] [PubMed] [Google Scholar]
- 40.Surveillance E End Results Program. 2008. SEER cause-specific death classification http://seercancergov/causespecific/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


