Abstract
Background
Variation in mate choice behaviour among females within a population may influence the strength and form of sexual selection, yet the basis for any such variation is still poorly understood. Condition-dependence may be an important source of variation in female sexual responsiveness and in the preference functions for male display traits that she expresses when choosing. We manipulated food intake of female guppies (Poecilia reticulata), and examined the effect on several measures of condition and various components of mate choice behaviour.
Results
Diet significantly influenced four measures of female condition: standard length, weight, reproductive status and somatic fat reserves. Diet also significantly affected female sexual responsiveness, but not preference functions: females in good and poor condition prefer the same males.
Conclusions
Variation in female condition within populations is therefore unlikely to influence the direction of sexual selection imposed by female choice. It may, however, influence the strength of sexual selection due to its effects on female responsiveness. The relative importance of female choice as a sexually selective force may also covary with female condition, however, because low responsiveness may result in sneak copulations being relatively more important as a determinant of the paternity of offspring. Differences among populations in mean condition may also influence geographic differences in the strength of sexual selection.
Background
Variation among individual females in mate choice behaviour may have important consequences for both sexual selection and mate choice evolution [1,2]. Until recently, however, few studies have addressed the possibility or importance of within-population differences in mate choice behaviour. Most studies have focussed on the modal or mean expression of female choice, and thus the net sexual selection operating on the male population as a whole. As a result, the extent of variation in mate choice and the underlying genetic and environmental basis of such variation remains poorly understood. Here we present the results of a laboratory study in which we manipulated diet and thus somatic condition of female guppies (Poecilia reticulata), and tested the effects that this had on various components of female choice behaviour.
In this paper, we consider two distinct components of female mate choice behaviour: responsiveness and preference functions. Responsiveness is the willingness of females to respond positively to males and thus to engage in active mate choice. It is an aspect of female choosiness [1-3]. Preference functions are measures of the male ornamental and display trait values that females favour [1-5]. The distinction between responsiveness and preference functions allows the question of how willing females are to respond to male courtship to be considered separately from which male trait values females prefer. It is conceivable that very different patterns of variation may be manifest at these two levels [3,4], and that the consequences may be quite distinct [1,4]. The responsiveness and preference functions of a female, together with any constraints on their expression, contribute to the eventual outcome of mate choice: which male/s the female mates with.
Female responsiveness may influence the strength of sexual selection in three possible ways. If females that are less responsive are more likely to mate at random than highly responsive females, then low responsiveness will be associated with weak sexual selection. If, however, low female responsiveness raises the threshold level of attractiveness required for a male to obtain a mating [6], then low female responsiveness will be associated with strong sexual selection. Which of these two possibilities pertain will depend on the nature of the mating system and the precise meaning of responsiveness in the species concerned. Last, in many species, including guppies, sneak copulations by males can circumvent female choice [7,8]. Reductions in responsiveness to male courtship are often associated with an increase in the importance of sneak copulations relative to courtship-choice copulations [7,9,10], and thus a reduction in the sexual selection imposed by mate choice.
Variation in preference functions might result in different females preferring different males [3,5,11]. This too will decrease the variance in male mating success and thus the opportunity for sexual selection. Variation in preference functions can also change the nature of sexual selection. For example, it may lead to frequency-dependent sexual selection on male traits [12] even if these traits are not preferred on average [3]. This makes variation in female mate choice a potential mechanism for generating and maintaining polymorphism in male secondary sexual traits within populations [1,2].
Several studies have shown that environmentally and socially imposed costs can affect female mate choice within populations. Female sticklebacks become less choosy close to spawning time or when forced to swim against a current when sampling males [13-15]. Female fiddler crabs (Uca annulipes) also become less choosy under time constraints [16]. Increased predation risk causes female crickets (Gryllus integer) to choose less attractive males than they choose in the absence of predators [17]. Parasite-infected females make fewer inspections of males before mating than uninfected females do in two fish species: bullies [18] and guppies [19].
The expression of costly mate choice may be dependent on female physiological condition [1,20]. If so, condition-dependence could account for some of the observed variation in female mate choice behaviour [1,20]. Variation in female (and male) condition within populations is likely to be common, due to stochastic environmental variation (e.g. seasonal and spatial variation in food availability) and heritable variation in resource acquisition [21,22]. Several studies support the idea that components of mate choice may be condition-dependent. Female sticklebacks (Gasterosteus aculeatus) from groups with a high average condition factor prefer redder males, whereas those reared under 'simulated winter conditions' prefer orange males [20]. Female stalk-eyed flies (Cyrtodiopsis dalmanii) under transient food stress have weaker preferences for males with large eye-spans than unstressed females [23]. Food supplementation results in a decrease in responsiveness of female fireflies (Photinus ignitus) to simulated male flashes [24], whereas food restriction results in lower responsiveness and reduced probability of mating in female cockroaches (Nauphoeta cinerea) [25].
Studies of mate choice in female guppies have demonstrated significant levels of repeatable variation among females within populations in the extent to which they prefer particular male traits [3,26-28]. The only study to dissect the components of mate choice [3] found some differences among females in preference functions, and that these differences lead individual females to prefer different males. However, most of the phenotypic variation, and the only significant levels of heritable variation were in responsiveness [3]. Here we experimentally manipulate the condition of female guppies by controlling food intake over four weeks. We then test the hypotheses that: 1) females in good condition are more sexually responsive and 2) express stronger preference functions than females in poor condition. We also test whether females in good and poor condition prefer the same individual males. Our approach allows us not only to estimate whether female condition influences mate choice behaviour, but also to resolve which components of choice it influences.
Results
Female condition
Feeding treatment had significant effects on all growth measures, reproductive status and somatic fat reserves, but not on critical swimming speed (Table 1). Females fed on the high food diet were, on average, 19% longer, 69% heavier and had 3% more body fat and 3.5 times as many ova as females on the low diet. All measures were significantly different among blocks, except for number of ova which was marginal. This indicates that either the timing of the application of the treatment or the stocks from which each block were sampled were different in how they acquired condition. The paired nature of our design, however, means that these block effects are unlikely to bias the outcome of our tests of treatment effects on mate choice.
Table 1.
Two-way ANOVA to test for a difference in measures of female condition after four weeks in 'high' or 'low' food treatments.
| Mean (SD) | treatment | Block | treatment × block | ||||||||
| Condition measure | High | Low | df | F | P | df | F | P | df | F | P |
| Standard length | 24.18 mm (1.72) | 20.30 mm (1.72) | 1,98 | 294.16 | 0.001 | 9,9 | 19.48 | 0.000 | 9,98 | 1.22 | 0.294 |
| Total length | 31.73 mm (2.34) | 26.95 mm (2.34) | 1,98 | 534.89 | 0.001 | 9,9 | 20.97 | 0.000 | 9,98 | 0.57 | 0.816 |
| Weight | 0.314 g (0.060) | 0.186 g (0.047) | 1,98 | 258.80 | 0.001 | 9,9 | 16.14 | 0.000 | 9,98 | 1.40 | 0.199 |
| Somatic fat reserves | 0.182% (0.043) | 0.152% (0.042) | 1,97 | 17.82 | 0.002 | 9,9 | 17.58 | 0.000 | 9,97 | 2.04 | 0.043 |
| Number of ova | 15.18 (5.50) | 4.28 (0.298) | 1,97 | 157.81 | 0.001 | 9,9 | 1.19 | 0.054 | 9,97 | 1.22 | 0.292 |
| Critical swimming speed | 17.4 cm.s-1 2.5 | 16.1 cm.s-1 (2.7) | 1,50 | 4.07 | 0.144 | 4,4 | 3.04 | 0.025 | 4,50 | 0.70 | 0.597 |
Female mate choice
Female responsiveness (proportion of overall male displays obtaining a positive "glide" response) was significantly higher in the good condition treatment than in the poor condition treatment (good condition mean = 0.218 ± 0.059 S.D., poor condition mean = 0.127 ± 0.076; paired-t = 6.45; d.f. = 9; P = 0.000). In total, we observed the responses of high and poor condition females to male courtship for 1400 minutes each. Within this time we observed good condition females mating 25 times and poor condition females 19 times. There were no differences between treatments in the frequency of such courtship matings (χ2 = 0.81, df = 1, P = 0.368).
Individual males' attractiveness (proportion positive response) to the two treatment groups of females was significantly positively correlated (r = 0.405, P = 0.002, N = 58) (Figure 2). This indicates that females from the two treatments generally preferred the same males.
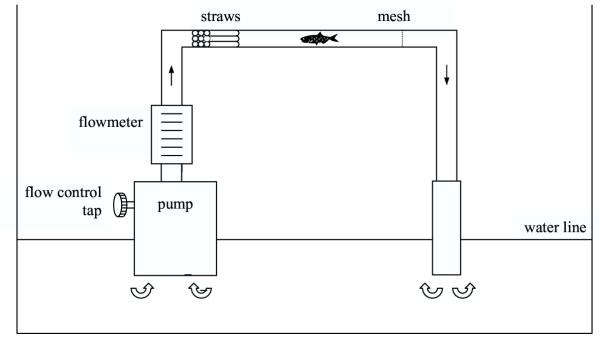
Figure 2.
Schematic illustration of the flow-chamber apparatus used to measure critical swimming speed. Water, pump and outflow end were in a 300 litre plastic tub.
There were no significant differences between the two treatments in preference slopes (Table 2). This indicates that there are no detectable differences in linear preference functions between good condition and poor condition females. Both good and poor condition females in the free swimming trials preferred males that have large proportions of their body covered in orange and iridescent colouration (Table 2).
Table 2.
Mean preference function slopes expressed by high and low food females, and overall preference function slopes expressed by all females. Differences among treatments are assessed using a paired-sample t-test. Deviations of common preference functions from zero are assessed using a one-sample t-test.
| Comparison of treatment preference functions | Common preference function | ||||||
| Mean slopes* | Paired t-test | Mean slope* (SE) | One-sample t-test | ||||
| High | Low |
t9 |
P | t19 | P | ||
| Body area | 0.6 (0.4) | 0.3 (0.4) | 0.506 | 0.625 | 0.4 (0.3) | 1.443 | 0.083 |
| Tail area | 0.8 (0.9) | 0.7 (0.8) | 0.092 | 0.928 | 0.6 (0.6) | 1.252 | 0.113 |
| Black area | 52.3 (60.1) | 60.8 (52.1) | -0.152 | 0.882 | 56.5 (38.7) | 1.460 | 0.081 |
| Fuzzy black area | 13.2 (53.3) | 63.5 (48.2) | -0.848 | 0.418 | 38.3 (35.5) | 1.082 | 0.147 |
| Orange area | 41.3 (32.4) | 64.6 (28.8) | -1.269 | 0.236 | 52.9 (21.3) | 2.488 | 0.011 |
| Iridescent area | 60.8 (46.5) | 75.5 (55.7) | -0.273 | 0.791 | 68.1 (35.4) | 1.928 | 0.035 |
* all mean slopes are × 100
The number of male sigmoid displays per minute was not affected by female condition (high condition mean = 12.7 ± 4.5 S.D., low condition mean = 15.9 ± 5.8; paired-t = -2.21; d.f. = 9; P = 0.054). There is thus no evidence from our experiment that males exert a mating bias due to differentially displaying to females that were larger or in better condition. Such a bias may have been underestimated, however, because males only ever saw females from one treatment at a time.
Discussion and conclusions
Variation in traits may arise if they are costly and condition-dependent [22,29,30]. Thus, if female choice is costly and if individuals in good condition can sustain higher costs than those in poor condition, then condition-dependent choice may occur [1,20]. Here we have presented evidence that in female guppies, one aspect of mate choice behaviour, responsiveness, is influenced by environmentally induced variation. We found no evidence, however, of condition-dependent effects on preference functions. In fact, there were significant common preference functions for several components of male display, and females from the two treatments preferred the same males. Our findings are consistent with those of Brooks and Endler (2001) that variation in responsiveness is more prevalent than variation in preference functions in guppies.
Paying attention to and possibly responding to male sigmoid displays in guppies takes time, and is a diversion from foraging and vigilance for predators. Females with depleted energy reserves may therefore allocate more time and effort to foraging and rebuilding condition, at the expense of assessing and responding to male courtship. Alternatively, decreased responsiveness in poor condition females may occur if these females are avoiding or trying to delay impregnation and thus the costs of producing offspring. This would be likely if mating and/or pregnancy were too costly for poor condition females or if poor condition females obtain a fecundity benefit by waiting until they have more reserves to invest in offspring. In our study, however, poor condition females were still capable of reproduction, and there was no significant difference in the number of successful matings between the two treatments. Moreover, females expressed similar preferences regardless of their condition. It is therefore unlikely that poor condition females were avoiding mating altogether.
Our finding that there were no differences in preference functions between treatments suggests that even though poor condition females are less responsive, they are just as capable of choosing attractive males when they do respond. Not only do female guppies in good and poor condition prefer the same male ornaments, but they prefer the same individual males. Thus females in poor condition still appear to be capable of choosing between males, and applying the same criteria as females in good condition. We predict that poor condition females would be less likely to mate with attractive males because they are less likely to pay attention to their courtship, but not because they do not like them or because they are less likely to mate per se. This prediction remains to be tested directly.
Feeding regime had a direct effect on all measured aspects of female condition except for critical swimming speed. This suggests that critical swimming speed may not be a good indicator of energetic stores or dietary condition. Despite the differences in condition, females from the poor condition lines still had mature ova and were capable of mating and bearing young.
Implications for sexual selection
Our findings may have important implications for the way sexual selection on attractive male traits occurs in guppy populations. Condition-dependent variation in female responsiveness may significantly influence the strength of sexual selection within populations, and may also lead to differences among populations in the importance of mate choice as an evolutionary force [1,2]. This may happen in either or both of two ways: by changing the importance of courtship mating relative to sneak copulation as a determinant of sexual selection, and by changing the strength of sexual selection imposed by choice.
In guppies, females exercise choice by responding differentially to the courtship of preferred and non-preferred males. Sneak copulations by males are also possible, however, and they appear to bypass female choice [31]. The relative importance of courtship-choice and sneak copulation varies among populations and covaries with environmental conditions within populations [7,9,10]. High relative rates of sneak copulation reduce the importance of mate choice as a determinant of male mating success [8,32], and thus probably as an agent of sexual selection. Our finding that females in poor condition were less responsive to courting males suggests that females in poor condition are more likely to be mated by sneak copulation and less likely to mate with chosen males than are females in good condition. Thus the sexual selection that females in poor condition impose on males is likely to be weaker than that imposed by females in good condition even though both types of female express the same preference function.
Aside from the relative importance of choice and sneak copulation, condition-dependence may influence the distribution of courtship-related matings among males in two further, opposite, ways. First, because females in poor condition appear to invest less in assessing males, the males they mate with may represent a wider range of quality, and thus the difference in mating success between attractive and unattractive males may be small relative to that imposed by females in good condition. Variation in female condition within a population would then weaken sexual selection on attractive male traits. By contrast, reluctance of poor-condition females to respond to displaying males might "raise the bar" such that a smaller number of extremely attractive males get to copulate with females (as per the antagonistic "chase-away" model of mate choice evolution – [6]). This would result in stronger sexual selection on attractive male phenotypes.
In the firefly Photinus ignitus, food supplementation results in a reduction in female responsiveness, and probably an increase in sexual selection on male flash duration as only the longest-flashing males are likely to attract a mate [24]. In the cockroach Nauphoeta cinerea, however, females reared on a restricted diet are less likely to respond to male courtship and less likely to mate at all, thus increasing the strength of sexual selection on male attractiveness [25]. Similarly, hunger resulted in greater reluctance to mate among female water striders (Gerris buenoi), and under these circumstances larger males experienced elevated mating success [33] as they were best able to overcome female reluctance to mate. These three examples illustrate the fact that the effect of condition on mate choice (or, more broadly, mating biases sensu [34]) and thus sexual selection is likely to differ depending on the natural history of the species concerned. Photinus ignitus females feed on the spermatophore offered by males, and thus poor condition females may be expected to be more willing to mate and less likely to choose. There is no such direct benefit of mating in Nauphoeta cinerea, and the reduction in willingness to mate and thus the likely increase in the strength of sexual selection may be due to attempts to reduce the costs of mating. Which, if either, of these dynamics arising from condition-dependence influences the strength of sexual selection most profoundly in guppies and other livebearing fishes is an important topic for future empirical study.
The fewer ova, and thus the lower reproductive value of females in poor condition may mean that condition-dependent variation within populations does not influence the net intensity of sexual selection very much because the most responsive females are also the ones that leave the most offspring. We do predict, however, that condition-dependence of responsiveness may generate differences among populations in the intensity of sexual selection if the mean condition of females influences mean responsiveness of females within each population.
Methods
Fish were second- and third-generation laboratory-reared individuals descended from approximately 300 adults collected from Alligator Creek, 30 km southwest of Townsville, Australia. Males were randomly collected from the stock tank and therefore had sexual experience prior to being used in this experiment. Sexually mature females were drawn from a virgin culture in a 200 litre aquarium. In both the stock and the virgin culture fish were kept at densities of less than 100 per 300 litre aquarium, under natural day length regimes and fed brine shrimp six days a week.
Experimental feeding regime
We applied two feeding treatments: a "high-food" treatment designed to maximise normal growth and a "low-food" treatment designed to minimise growth but not suppress reproduction. Females on this low food diet did not show any signs of distress or ill health. The treatments were applied in ten blocks because of time constraints on the number of females that could be tested in behavioural trials at one time. In each block, 16 virgin females were separated into individual clear plastic food containers, each containing 500 ml of water. Eight females were randomly assigned to the high-food treatment and the other eight to low-food. Only six fish per treatment per block were used in behaviour trials; the other two were reserves in case of any deaths during the treatment period. A reserve was used on only two occasions. Feeding treatments were thus applied to 160 females, 120 of which were used in behaviour trials.
Fish were fed 1 ml of the appropriate food solution on six mornings per week for 4 weeks. The "high food" treatment fish each received the equivalent of 40 μl wet volume of brine shrimp (Artemia spp.) nauplius larvae per ml solution, and "low food" females received 6 μl of shrimp per ml.
Measuring female mate choice behaviour
We measured mate choice by observing male courtship and female response in an open aquarium [31,35]. This is a standard method used to study mate choice in guppies, and is the most biologically relevant method available because it allows the full range of male courtship and female response behaviours, and is a reliable indicator of male mating success [36,37]. One limitation of this method is that one cannot distinguish individual females, and so only a single true replicate measure of female responsiveness (mean) or preference (common slope) can be obtained in a given trial. The results that we present are, however, strongly consistent with results from trials where individual females were observed interacting with males enclosed behind glass partitions (Syriatowicz, unpublished data).
The floor of the test aquarium (30 cm × 75 cm × 36 cm high) was covered by a layer of gravel, and the back and sides were covered in brown paper. During behaviour trials, light came from two reading lamps (Crompton Craft 60 W daylight incandescent bulbs) placed above and behind the observer. Light intensity at the water surface ranged from 1.0 to 1.9 μmol.sec-1.m-2. The day before and each day during behaviour trials, we fed females from both treatments on "low-food" rations to distinguish hunger from dietary condition as a reason for any differences in mate choice behaviour.
The six females from a given treatment and block were placed in an aquarium with six randomly-drawn stock males on the night before observations began. Males are distinguished by their unique colour patterns. Each replicate involved 70 minutes of observation per day on two consecutive days. Observations began between 07h00 and 09h00 and involved watching each male for five minutes in random order, and then again in a different random order, followed by a ten minute scanning period in which the observer shifted attention regularly from one focal male to another. We scored the female responses to each male sigmoid display after the methods of Houde [35,38]. The proportion of all male displays that elicit at least a "glide" response is a convenient and reliable predictor of male mating success [36,37]. We also noted the number of male displays that ended in mating (genital contact) in order to compare female willingness to mate in the two treatments. The same six males were then used with the six females from the second treatment on the next two days. We alternated treatment order between blocks. A new set of six males was used for each block of females, such that sixty males were used in the entire experiment.
Measuring male ornamentation
After his last behaviour trial had ended, we anaesthetised each male by dipping him in an ice slurry for a few seconds, and then laying him on white waterproof paper on his right side. We then photographed his left side using a Nikon Coolpix 950 digital camera, including a ruler in the photograph for calibration. We used Measure Master 3.44 (Leading Edge Pty Ltd Adelaide, Australia) to measure male body and tail area and the area of each colour spot from the photograph. We calculated the proportion of the body area covered by black, fuzzy black, orange and iridescent spots, and transformed these proportions to angles for analysis.
Measuring female choosiness and preference functions
Responsiveness of the females in a behaviour trial was defined as the mean response to the displays of all the males in the trial. We used paired-sample t-tests to assess whether there were differences between good and poor condition females in responsiveness and in the rate at which males display to females. In these analyses and those described in the next paragraph, there is one measure per treatment per block, and the good and poor condition measures in a block are paired.
Female preference functions for male traits were estimated as the linear regression slope coefficients of female response on the male trait being evaluated (after Brooks & Endler [3]). We estimated the female preference function for each male trait within each replicate tank separately. We then compared mean female preference slopes in the two treatments using paired-sample t-tests, and we assessed the significance of overall preferences of females in both treatments using a one-sample t- test to compare the observed slopes with a mean of zero.
To test whether females from the two treatments prefer the same males, we estimated the Pearson's product-moment correlation between the attractiveness of individual males to high and low food treatment females. We standardised male attractiveness (z-transformation) within trials to prevent differences in mean female responsiveness among trials from generating spurious correlations. We used a Chi-square test of whether the number of observed matings was independent of treatment.
Measuring female condition
After behaviour trials, we measured several aspects of female somatic condition: growth (length, weight), reproductive status (number of ova), fat reserves (lipid analysis) and physiological performance (critical swimming speed). We anaesthetised each fish by immersing her in iced water and then laying her on a ruler to measure total and standard length (tip of snout to the end of the caudal lobe and caudal peduncle respectively). We then weighed her to the nearest 0.1 mg before reviving her in vigorously aerated room-temperature water. All fish made a rapid and complete recovery.
We measured critical swimming speed, Ucrit, of females from five of the ten treatment blocks in a flow chamber designed after Nicoletto [39]. It comprised a horizontal clear PVC pipe 1.2 m long and 2.5 cm in diameter (see Figure 1). This chamber had water pumped through it by a submersible pump at a velocity controlled using a tap and measured using a flowmeter (2–10 l/min). A block of 6 cm long straws at the entrance of the chamber was inserted to produce a straightened, laminar flow.
Figure 1.
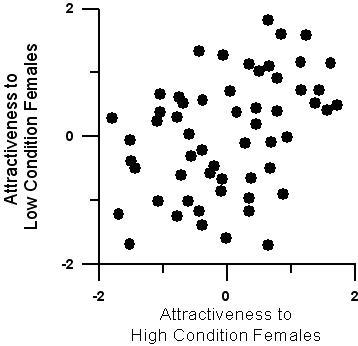
Attractiveness of males (standardized within trials) to females from the high and low-condition treatment.
Guppies did not at first swim against the current in such an apparatus, but this was quickly remedied by placing each fish in a tub of water that had a small inverted bucket in the centre, and a small submersible pump that generated a gentle flow, for two hours immediately before the trial. Critical swimming speed measures commenced with the animal being placed in the flow chamber and water velocity beginning at 7.5 cm.s-1. All fish were able to swim at this speed for three minutes. We then progressively increased the flow by 3.75 cm.s-1 every three minutes until the fish touched a mesh screen at the chamber's outflow end. At this point the trial was immediately ended and the flow turned down. The time each fish spent at the highest velocity was recorded and critical swimming speed was calculated after Brett [40] as:
![]()
Where Ui is the highest velocity maintained for a full three minutes, Uii is the velocity increment, Ti is the time elapsed at fatigue velocity and Tii is the time interval between velocity increments. Each fish's Ucrit was then corrected for body size by dividing it by standard length [39].
After critical swimming speed testing (in the first five blocks) or after behaviour (blocks 6–10), we killed each female by immersion in an ice slurry, dissected her and counted her mature ova. We then estimated her fat reserves using a method adapted from [41]. The fins and contents of the abdominal cavity were removed, and the fish dried overnight in an oven at 60°C and then the carcass was weighed to the nearest 0.1 mg. It was then immersed in a bath of diethyl ether (which dissolves triglycerides) for 24 hours and then weighed. This step is repeated until the fish reaches a constant mass (maximum three extractions). The carcass was then re-dried overnight and re-weighed. The percentage mass loss between the first dry weight and the dry weight after fat extraction is a measure of stored body fat [41].
Two-way ANOVA was used to test for a significant effect of treatment (fixed factor) and block (random factor) on condition measures. All Two-way ANOVAs used in this study were performed using a Type III model in SPSS v10.0 and manually recalculating the appropriate F-ratios for block effect as block MS / treatment * block MS (according to the equations provided in Zar [42], Table 12.3, p. 243).
Thirty eight reserve females remained after two were used to replace females that died during the four week experimental feeding period. We paired these with males and waited for them to give birth in order to test whether females from both treatments were capable of producing offspring, which they all did.
Author's contributions
This study was jointly conceived and designed. AS did all of the laboratory work, the preliminary analysis and wrote an honours thesis on this experiment. RB contributed to the final analysis and the final manuscript was written jointly.
Acknowledgments
Acknowledgements
The protocols described here, including the feeding treatments complied with University and Australian animal ethics regulations (UNSW ACEC# 01/018). Many thanks to Mark Blows, John Endler, Megan Head, John Hunt, Anna Lindholm, Lisa Miller and anonymous referees for assistance and for helpful comments on earlier drafts of the manuscript. This research was supported by the Australian Research Council.
Contributor Information
Alexandra Syriatowicz, Email: allie@squiz.net.
Robert Brooks, Email: rob.brooks@unsw.edu.au.
References
- Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev. 1997;72:283–327. doi: 10.1017/S0006323196005014. [DOI] [PubMed] [Google Scholar]
- Widemo F, Sæther SA. Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends in Ecology and Evolution. 1999;14:26–31. doi: 10.1016/S0169-5347(98)01531-6. [DOI] [PubMed] [Google Scholar]
- Brooks R, Endler JA. Female guppies agree to differ: phenotypic and genetic variation in mate-choice behaviour and the consequences for sexual selection. Evolution. 2001;55:1644–1655. doi: 10.1111/j.0014-3820.2001.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Wagner WEJ. Measuring female mating preferences. Anim Behav. 1998;55:1029–1042. doi: 10.1006/anbe.1997.0635. [DOI] [PubMed] [Google Scholar]
- Reinhold K, Reinhold K, Jacoby KJ. Dissecting the repeatability of female mate choice in the grasshopper Chorthippus biguttulus. Anim Behav. 2002;64:245–250. doi: 10.1006/anbe.2002.3061. [DOI] [Google Scholar]
- Holland B, Rice WR. Chase-away selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Endler JA. Predation, light intensity and courtship behaviour in Poecilia reticulata (Pisces: Poeciliidae) Anim Behav. 1987;35:1376–1385. [Google Scholar]
- Magurran AE. Sexual conflict and evolution in Trinidadian guppies. Genetica. 2001;112–113:463–464. doi: 10.1023/A:1013339822246. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, Gross MR, Coombs MJ. Environmental conditions and male morphology detemine alternative mating behaviour in Trinidadian guppies. Anim Behav. 1993;45:145–152. doi: 10.1006/anbe.1993.1013. [DOI] [Google Scholar]
- Gamble S, Lindholm AK, Endler JA, Brooks R. Environmental variation and the maintenance of polymorphism: The effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecology Letters. 2003;6:463–472. [Google Scholar]
- Lesna I, Sabelis MW. Diet-dependent female choice for males with 'good genes' in a soil predatory mite. Nature. 1999;401:581–584. doi: 10.1038/44125. [DOI] [Google Scholar]
- Partridge L. The rare-male effect; what is its evolutionary significance? Philosophical Transactions of the Royal Society of London Series B. 1988;319:525–539. doi: 10.1098/rstb.1988.0063. [DOI] [PubMed] [Google Scholar]
- Bakker TCM, Milinski Sequential female choice and the previous male effect in sticklebacks. Behav Ecol Sociobiol. 1991;29:205–210. [Google Scholar]
- Milinski M, Bakker TCM. Costs influence sequential mate choice in sticklebacks Gasterosteus aculeatus. Proc R Soc Lond B. 1992;250:229–233. [Google Scholar]
- Luttbeg B, Towner MC, Wandesforde-Smith A, Mangel M, Foster SA. State-dependent mate-assessment and mate-selection behaviour in female threespine sticklebacks (Gasterosteus aculeatus, Gasterosteiformes: Gasterosteidae) Ethology. 2001;107:545–558. doi: 10.1046/j.1439-0310.2001.00694.x. [DOI] [Google Scholar]
- Backwell PRY, Passmore NI. Time constraints and multiple choice criteria in the sampling behaviour and mate choice of the fiddler crab, Uca anulipes. Behav Ecol Sociobiol. 1996;38:407–416. doi: 10.1007/s002650050258. [DOI] [Google Scholar]
- Hedrick AV, Dill LM. Mate choice by female crickets is influenced by predation risk. Anim Behav. 1993;46:193–196. doi: 10.1006/anbe.1993.1176. [DOI] [Google Scholar]
- Poulin R. Mate choice decisions by parasitized female upland bullies, Gobiomorphus breviceps. Proc R Soc Lond B. 1994;256:183–187. [Google Scholar]
- López S. Parasitized female guppies do not prefer showy males. Anim Behav. 1999;57:1129–1134. doi: 10.1006/anbe.1998.1064. [DOI] [PubMed] [Google Scholar]
- Bakker TCM, Künzler R, Mazzi D. Condition-related mate choice in sticklebacks. Nature. 1999;401:234. doi: 10.1038/45727. [DOI] [Google Scholar]
- Reynolds JD, Gross MR. Costs and benefits of female mate choice: is there a lek paradox? Am Nat. 1990;136:230–243. doi: 10.1086/285093. [DOI] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B. 1996;263:1415–1421. [Google Scholar]
- Hingle A, Fowler K, Pomiankowski A. The effect of transient food stress on female mate preference in the stalk-eyed fly Cyrtodiopsis dalmanii. Proc R Soc Lond B. 2001;268:1239–1244. doi: 10.1098/rspb.2001.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cratsley CK, Lewis SM. Female preference for male courtship flashes in Photinus ignitus fireflies. Behav Ecol. 2003;14:135–140. doi: 10.1093/beheco/14.1.135. [DOI] [Google Scholar]
- Clark DC, deBano S, Moore AJ. The influence of environmental quality on sexual selection in Nauphoeta cinerea. Behav Ecol. 1997;8:46–53. [Google Scholar]
- Godin J-GJ, Dugatkin LA. Variability and repeatability of female mating preference in the guppy. Anim Behav. 1995;49:1427–1433. doi: 10.1016/0003-3472(95)90063-2. [DOI] [Google Scholar]
- Brooks R. Copying and the repeatability of mate choice. Behav Ecol Sociobiol. 1996;39:323–329. doi: 10.1007/s002650050296. [DOI] [Google Scholar]
- Kodric-Brown A, Nicoletto PF. Repeatability of female choice in the guppy: response to live and videotaped males. Anim Behav. 1997;54:369–376. doi: 10.1006/anbe.1996.0420. [DOI] [PubMed] [Google Scholar]
- Kotiaho JS. Testing the assumptions of conditional handicap theory: costs and condition dependence of a sexually selected trait. Behav Ecol Sociobiol. 2000;48:188–194. doi: 10.1007/s002650000221. [DOI] [Google Scholar]
- Wilkinson GS, Taper M. Evolution of genetic variation for condition-dependent traits in stalk-eyed flies. Proc R Soc Lond B. 1999;266:1685–1690. doi: 10.1098/rspb.1999.0832. [DOI] [Google Scholar]
- Houde AE. Sex, Color and Mate Choice in Guppies. Princeton, N.J.: Princeton University Press; 1997. [Google Scholar]
- Kelly CD, Godin J-GJ, Wright JM. Geographical variation in multiple paternity within natural populations of the guppy (Poecilia reticulata) Proc R Soc Lond B. 1999;266:2403–2408. doi: 10.1098/rspb.1999.0938. [DOI] [Google Scholar]
- Ortigosa A, Rowe L. The effect of hunger on mating behaviour and sexual selection for male body size in Gerris buenoi. Anim Behav. 2002;64:369–375. doi: 10.1006/anbe.2002.3065. [DOI] [Google Scholar]
- Kokko H, Brooks R, Jennions MD, Morley J. The evolution of mate choice and mating biases. Proc R Soc Lond B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde AE. Mate choice based upon naturally occurring colour-pattern variation in a guppy population. Evolution. 1987;41:1–10. doi: 10.1111/j.1558-5646.1987.tb05766.x. [DOI] [PubMed] [Google Scholar]
- Houde AE. The effects of female choice and male-male competition on the mating success of male guppies. Anim Behav. 1988;36:888–896. [Google Scholar]
- Brooks R, Caithness N. Intersexual and intrasexual selection, sneak copulation and male ornamentation in guppies (Poecilia reticulata) South African Journal of Zoology. 1999;34:48–52. [Google Scholar]
- Endler JA, Houde AE. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49:456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Nicoletto PF. The relationship between male ornamentation and swimming performance in the guppy, Poecilia reticulata. Behav Ecol Sociobiol. 1991;28:365–370. [Google Scholar]
- Brett JR. The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can. 1964;21:1183–1226. [Google Scholar]
- Reznick DN, Braun B. Fat cycling in the mosquitofish (Gambusia affinis: fat storage as a reproductive adaptation. Oecologia. 1987;73:401–413. doi: 10.1007/BF00385257. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 3. Upper Saddle River, N.J.: Prentice Hall; 1996. [Google Scholar]