Abstract
Background:
Tumor lysis syndrome (TLS) is a life-threatening disorder characterized by hyperuricemia and metabolic derangements. The efficacy of rasburicase, administered daily for 5 days, has been well established. However, the optimal duration of therapy is unknown in adults.
Patients and methods:
We evaluated the efficacy of rasburicase (0.15 mg/kg) administered as single dose followed by as needed dosing (maximum five doses) versus daily dosing for 5 days in adult patients at risk for TLS.
Results:
Eighty of the 82 patients enrolled received rasburicase; 40 high risk [median uric acid (UA) 8.5 mg/dl; range, 1.5–19.7] and 40 potential risk (UA = 5.6 mg/dl; range, 2.4–7.4). Seventy-nine patients (99%) experienced normalization in their UA within 4 h after the first dose; 84% to an undetectable level (<0.7 mg/dl). Thirty-nine of 40 (98%) patients in the daily-dose arm and 34 of 40 (85%) patients in single-dose arm showed sustained UA response. Six high-risk patients within the single-dose arm required second dose for UA >7.5 mg/dl. Rasburicase was well tolerated; one patient with glucose-6-phosphate dehydrogenase deficiency developed methemoglobinemia and hemolysis.
Conclusions:
Rasburicase is highly effective for prevention and management of hyperuricemia in adults at risk for TLS. Single-dose rasburicase was effective in most patients; only a subset of high-risk patients required a second dose.
Keywords: hematologic malignancy, hyperuricemia, rasburicase, tumor lysis syndrome
introduction
Tumor lysis syndrome (TLS) is a potentially life-threatening complication resulting from massive lysis of malignant cells and is most frequently observed in patients with rapidly proliferating, bulky, and chemosensitive malignancies [1–3]. TLS is characterized by rapid release of intracellular metabolites from lysed malignant cells into the blood stream. The resulting metabolic derangements include hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia and can lead to acute renal failure, cardiac arrhythmias, seizures, and death [1–3]. Hyperuricemia, a common manifestation of TLS, results from rapid catabolism of purine-containing nucleic acids from tumor cells and can lead to renal insufficiency when uric acid (UA) precipitates into the renal tubules and distal collecting system [3, 4].
The prevention and management of TLS include aggressive hydration and reduction in the plasma UA levels by drugs that decrease production or increase excretion of UA [3, 5, 6]. Allopurinol decreases the new production of UA by inhibiting enzyme xanthine oxidase and blocking oxidation of xanthine and hypoxanthine into UA [3, 7]. Therefore, following allopurinol administration, there is a time lag for reduction in UA levels. In contrast, rasburicase, a recombinant urate oxidase, rapidly reduces UA by catalyzing the conversion of the existing pool of UA into allantoin which is 5–10 times more soluble in urine than UA [3, 7].
While the efficacy of rasburicase has been well established for pediatric patients [8, 9], it has recently been approved in adult patients at high risk for developing TLS, to be administered at 0.2 mg/kg dose once daily for up to 5 days [10, 11]. Although activity has been seen with lower doses of rasburicase and shorter duration of treatment including a single dose, in smaller studies [12–20], the optimal dosing and duration of rasburicase has not been established for adults. Based on this rationale, we designed a study to evaluate the efficacy of rasburicase administered as a single dose as compared with fixed 5-day dosing in adults with hematologic malignancies at risk for TLS.
patients and methods
patients
Patients with hematologic malignancies were eligible if they had Eastern Cooperative Oncology Group (ECOG) performance status 0–3, life expectancy of >3 months, and were at high risk or potential risk for TLS. High risk was defined as presence of hyperuricemia (plasma UA levels ≥7.5 mg/dl) or a diagnosis of very aggressive lymphoma or leukemia based on the Revised European American Lymphoma classification [3, 10, 21, 22]. Potential risk was defined as aggressive lymphoma or leukemia with lactate dehydrogenase more than or equal to upper limit of normal, stage ≥3 disease or stage 1 or 2 disease with at least one lymph node/tumor >5 cm in size. Patients with history of asthma, severe allergy, known glucose-6-phosphate dehydrogenase (G6PD) deficiency, and use of allopurinol within 72 h were excluded. The study was approved by the Institutional Review Board, and written informed consent was obtained from all patients before study entry.
study drug
Rasburicase was provided by Sanofi-aventis, in the vials of 1.5 mg of active substance to be reconstituted in 1 ml of reconstitution solution. The predetermined dose of rasburicase was mixed with 0.9% of sterile sodium chloride to achieve the final volume of 50 ml.
study design
In this randomized, controlled open-label trial, the eligible patients were stratified based on their risk for TLS (high risk or potential risk) and randomized 1 : 1 into arm A or arm B. Rasburicase was administered at a dose of 0.15 mg/kg i.v. over 30 min at least 4 h (no more than 24 h) before initiation of anticancer therapy. Patients on arm A received a single dose on day 1. Any subsequent doses (maximum five doses) were administered only if plasma UA levels were >7.5 mg/dl. Patients on arm B received rasburicase at the same dose daily for 5 days. Patients were monitored for adverse events with frequent clinical assessments. Plasma samples for UA were collected at baseline before rasburicase, 4- and 24-h post-rasburicase, and daily during treatment. The blood samples for UA during the course of treatment were collected in prechilled tubes containing heparin and immediately immersed and transported on ice and analyzed within 4 h of collection. Serum electrolytes, blood urea nitrogen, creatinine, calcium, and phosphorous were monitored daily during this period.
study end points
The primary end point of the study was the plasma UA response rate, defined as normalization of plasma UA levels within 48 h after the start of study drug and maintaining within the normal range after the final drug infusion on day 5. Secondary end points included evaluation of plasma UA exposure as assessed by the area under curve (AUC) of UA over 5-day period, the number of doses required (during days 2–5) to maintain plasma UA within normal range in arm A, renal insufficiency, electrolyte abnormalities, and clinical safety.
statistical analysis
This study was designed to estimate the UA response rate of arm A (investigational arm). The control arm (arm B) was used to avoid selection bias. Therefore, power calculations were based on exact binomial test for the single proportion. Forty assessable patients in the arm A provide 95% power to positively detect 85% UA response rate against an assumed response rate of 99% in arm B, also containing 40 patients, with a one-sided confidence at the 0.05 significance level. Alternatively stated, with 40 patients per arm, the 95% two-sided confidence interval of the difference in response rate between the two treatment arms were predicted to have a width of 0.12, assuming the UA response rate of the control arm being 0.99 and the investigational arm reaching 0.85. The areas under the plasma UA concentration versus time (days 1–5) curves were calculated using trapezoidal rule [23]. Comparisons of total AUC between arm A and arm B were conducted using standard t-test. All statistical analyses were conducted under the intent-to-treat principle using SAS (SAS version 9.1.3; SAS Institute, Cary, NC). All tests were two-sided at a significance level of 0.05.
results
patients
Eighty-two patients were enrolled between February 2008 and February 2010 (Figure 1). Two patients withdrew consent and 80 patients were randomized; 40 to the arm A and 40 to the arm B. The patients in both arms were balanced with regards to gender, age, diagnosis, and their TLS risks (Table 1), with slightly higher proportion of patients with ECOG performance status of ≥2 on arm A. About half the patients in each arm were classified as high risk for TLS, and over one-third of the patients (35%–38%) exhibited hyperuricemia at baseline. Over 90% of patients had diagnosis of ‘aggressive’ or ‘very aggressive’ lymphoma/leukemia and received anthracycline-based chemotherapy.
Figure 1.
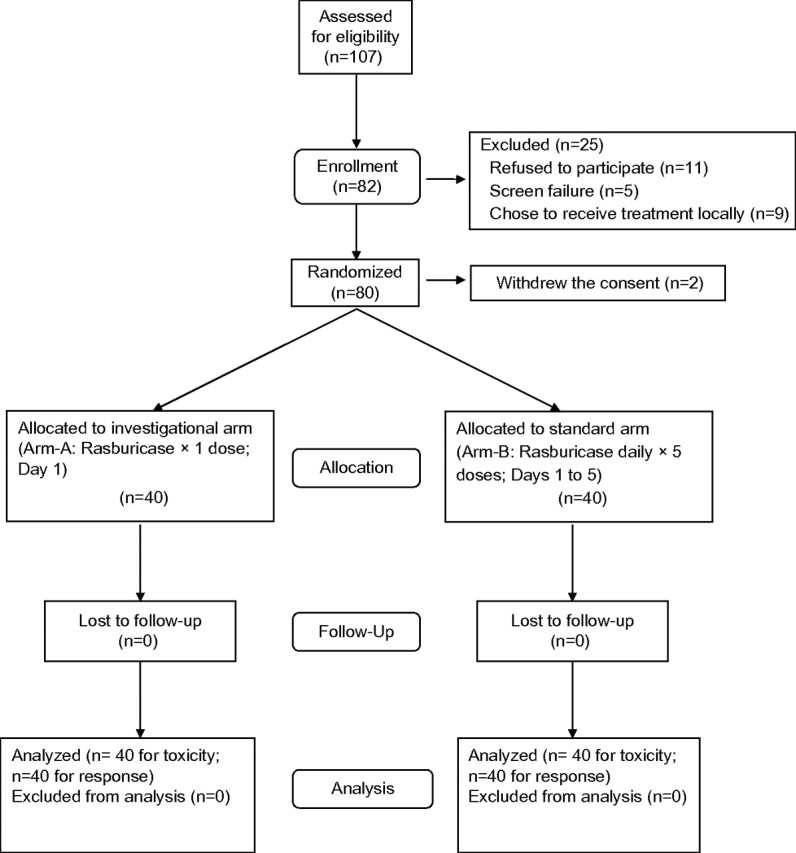
CONSORT diagram. Patient enrollment and randomization.
Table 1.
Patient characteristics
| Arm A (single dosing) | Arm B (daily dosing) | |
| Number of patients | 40 | 40 |
| Male/female (N) | 30/10 | 30/10 |
| Median age (range), years | 62 (27–82) | 58 (27–81) |
| ECOG ≥2 | 16 (40) | 11 (27.5) |
| Diagnosis | ||
| Diffuse large B-cell lymphoma | 27 (67.5) | 23 (57.5) |
| Mantle cell lymphoma | 7 (17.5) | 9 (22.5) |
| Burkitt’s lymphoma | 1 (2.5) | 2 (5) |
| Others | 5 (12.5) | 6 (15) |
| Risk for TLS | ||
| High risk | 21 (51.5) | 19 (47.5) |
| Potential | 19 (47.5) | 21 (51.5) |
| Baseline plasma uric acid | ||
| >7.5 mg/dl | 15 (38) | 14 (35) |
| ≤7.5 mg/dl | 25 (62) | 26 (65) |
| Baseline LDH > 2× ULN | 11 (27.5) | 12 (30) |
| Baseline creatinine elevated (>ULN) | 10 (25) | 6 (15) |
| Spontaneous TLSa | ||
| Laboratory TLS | 5 (12.5) | 1 (2.5) |
| Anthracyclin-based chemotherapy | 37 (92.5) | 35 (87.5) |
Values are given as N (%) unless stated otherwise.
Presence of tumor lysis syndrome before initiating chemotherapy.
ECOG, Eastern Cooperative Oncology Group; TLS, tumor lysis syndrome; LDH, lactate dehydrogenase; ULN, upper limit of normal.
plasma UA response to rasburicase
All 80 patients received at least one dose of rasburicase and were assessable for clinical safety and response to rasburicase. The plasma UA levels rapidly declined within 4 h to undetectable levels in the vast majority (84%) of patients and normalized in all but one patient (n = 79, 99%) within 24 h after the first dose (Figure 2). Plasma UA remained low during the study period in 98% of patients (39 of 40) receiving daily dosing of rasburicase (arm B). In the single-dose arm (arm A), the UA also remained within normal range in 85% (34 of 40) of patients, including all at potential risk and the majority of patients in the high-risk group (Figure 2). The AUC (1–120 h in mg/dl × h) for plasma UA was significantly lower in patients in arm B than in arm A (90.7 ± 5.3 versus 203.4 ± 13.9, P < 0.0001) as shown in Figure 2. Arm B patients experienced a lower exposure of plasma UA as compared with arm A both in the high-risk subgroup (101.7 ± 10.3 versus 242.6 ± 21.3, P < 0.0001) and the potential-risk group (80.8 ± 3.0 versus 157.7 ± 9.3, P < 0.0001).
Figure 2.
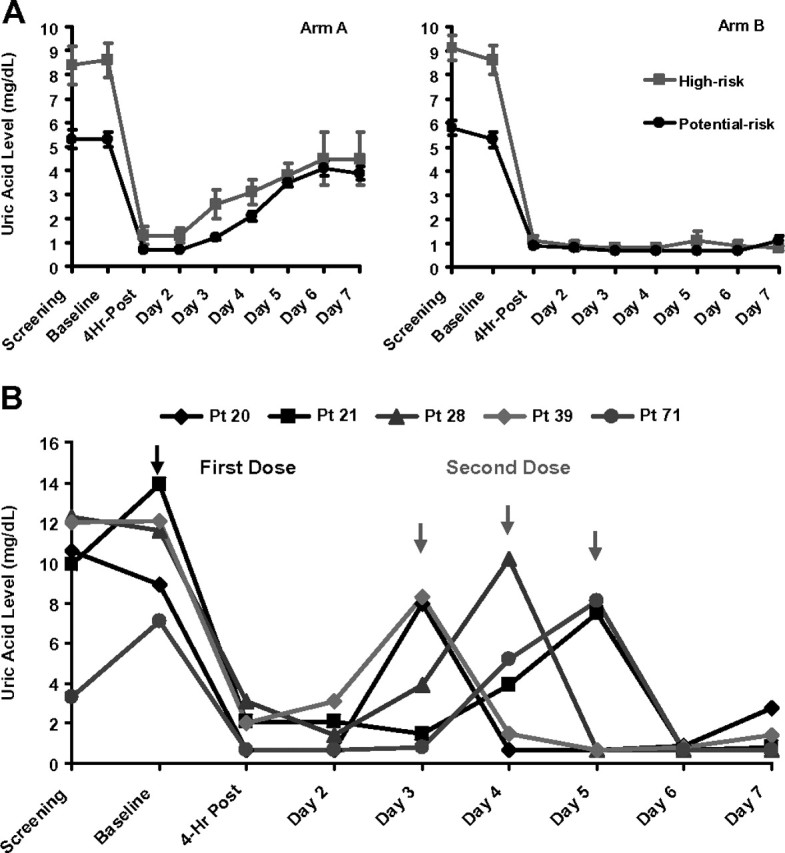
(A) Plasma uric acid (UA) levels (mean ± SEM) during the study period. Left panel shows plasma UA profile over the study period in arm A stratified by the risk group. Right panel shows plasma UA profile over the study period in arm B stratified by the risk group. (B) Timing of a second dose of rasburicase in a subset of high-risk patients within arm A requiring additional therapy. Five patients needed a second dose of rasburicase during the study period (days 2–5). Two patients on day 3, one patient on day 4, and two patients on day 5.
The UA response (i.e. normalization and maintenance within the normal range during study) was seen in 39 of 40 patients (98%) in arm B. The single patient who was classified as ‘nonresponder’ was removed from the study after first dose due to development of a serious adverse event. Within arm B, UA response rate was 100% (21 of 21 patients) in the potential-risk group and 95% (18 of 19 patients) in the high-risk group.
The UA response in the single-dose arm (arm A) was observed in 85% of patients (34 of 40 patients). The response rate was 100% (19 of 19 patients) in the potential-risk group and no patient required a second dose. In the high-risk group, 15 of 21 patients (71.4%) experienced a sustained UA response to a single dose; only 5 patients in this subgroup required a second dose for UA >7.5 mg/dl (Figure 2) during days 2–5 (2 patients on day 3, 1 patient on day 4, and 2 patients on day 5). All five patients had very aggressive lymphoma and/or bulky disease and none of these patients required more than two doses (Table 2). Furthermore, one patient with baseline evidence of TLS, in whom chemotherapy was delayed by 4 days, required two doses upon chemotherapy initiation.
Table 3.
Incidence of tumor lysis syndrome and renal events
| Arm A (single dosing), N (%) | Arm B (daily dosing), N (%) | |
| Number of patients | 40 (50) | 40 (50) |
| Clinical tumor lysis syndrome [7] | 0 (0) | 3 (7.5) |
| Laboratory tumor lysis syndrome [7] | 14 (35) | 11 (27.5) |
| Hyperuricemia | 13 (32.5) | 11 (27.5) |
| Hyperphosphatemia | 16 (40) | 12 (30) |
| Hypocalcemia | 28 (70) | 20 (50) |
| Hyperkalemia | 1 (2.5) | 1 (2.5) |
| Renal events | ||
| Increased serum creatinine | 0 (0) | 4 (10) |
| Renal events requiring dialysisa | 1 (2.5) | 1 (2.5) |
Both patients had preexisting chronic renal insufficiency.
incidence of emergent TLS, renal insufficiency, and clinical safety parameters
A small proportion of patients had evidence of spontaneous laboratory TLS (12.5% on arm A and 2.5% on arm B) before initiation of chemotherapy (Table 1). During the study period, several patients developed evidence of clinical TLS (none on arm A and 7.5% on arm B) and/or laboratory TLS (35% on arm A and 27.5% on arm B) (Table 3). All patients with elevated baseline serum creatinine levels (25% on arm A and 15% on arm B) experienced decreases of serum creatinine levels toward normal during rasburicase treatment; 75% (12 of 16 patients) achieved serum creatinine within a normal range. Notably, only two patients (one on each arm) required dialysis on the study.
Table 2.
Clinical features of five high-risk patients requiring two doses of rasburicase (arm A)
| Patient Acc No. | Sex | Age (years) | Diagnosis | Ki-67% positive Cells* | C-MYC** | Baseline uric acid (mg/dl) | Baseline creatinine (mg/dl) | Baseline LDH (IU/l) |
| 20 | F | 71 | DLBCL (high grade) | >95% | + | 8.9 | 1.1 | 4867 |
| 21 | M | 61 | DLBCL (transformed) | 80%–90% | + | 13.9 | 1.4 | 1994 |
| 28 | M | 27 | Burkitt-like lymphoma | 100% | + | 11.6 | 1.1 | 2931 |
| 39 | M | 58 | DLBCL (high grade) | 95% | + | 12.1 | 0.9 | 1356 |
| 71 | M | 51 | Burkitt lymphoma | >95% | + | 7.1 | 0.9 | 687 |
LDH, lactate dehydrogenase; DLBCL, diffuse large B-cell lymphoma; F, female; M, male.
Proliferative index by immunohistochemical stain for Ki-67.
C-MYC gene rearrangement positive by fluorescence in situ hybridization studies.
One patient (arm A) with spontaneous TLS and baseline plasma UA of 18.4 mg/dl responded to rasburicase with normalization of UA within 1 day; however, his chemotherapy was delayed by 4 days after rasburicase. This patient developed hyperkalemia (serum K, 7.1 meq/l) on day 2 of chemotherapy and hyperuricemia (UA = 22.1 ng/ml) and required slow low-efficiency dialysis (SLED) alternating with hemodialysis. His UA levels became undetectable after two doses of rasburicase and his electrolytes normalized.
Another patient (arm B), who presented with history of hypertension and chronic renal insufficiency, high-tumor burden, spontaneous TLS with hyperuricemia (UA = 16.1 ng/ml), and elevated serum creatinine (2.3 mg/dl) at initial assessment. This patient was placed on continuous SLED in anticipation of worsening renal function. This patient’s UA normalized (UA = 3.9 ng/ml) within 4 h of first dose of rasburicase. However, he subsequently developed methemoglobinemia (methemoglobin, 7.6%) and hemolytic anemia (hemoglobin, 9.2 g/dl) characterized by hypoxia with O2 saturation 80% that did not improve with 100% non-rebreathing O2 mask. In this patient, rasburicase was discontinued and methylene blue was administered, which resulted in a decrease in the methemoglobin levels to 3.8%. However, the treatment was associated with hemolysis as evidenced by a decline in hemoglobin from 9.2 to 6.5 g/dl, increases in free hemoglobin and urine hemosiderin, decrease in serum haptoglobin, and peripheral smear showing precipitated hemoglobin in >80% of erythrocytes. The patient was subsequently found to have previously unrecognized G6PD deficiency and was managed with ascorbic acid and red cell transfusions.
In general, treatment with rasburicase was well tolerated. The most frequent (experienced by >10% of patients) adverse events reported were gastrointestinal in origin, in these patients that were also receiving chemotherapy. These side-effects were mild to moderate in severity and lower in frequency in the single-dose arm as compared with the daily-dosing arm, with nausea (12.5% versus 32.5%), constipation (15% versus 32.5%), diarrhea (5% versus 12.5%), and vomiting (0% versus 15%). The incidence of hypersensitivity reactions was low; two patients had skin rash/pruritus, three patients experienced shortness of breath during infusion, nonetheless, no patient developed anaphylactic reactions. In addition, one patient with no prior history of gout experienced an acute episode of gouty arthritis of right elbow 10 days after a dose of rasburicase; this was during rising UA level after UA had been undetectable for 5 days. The diagnosis of gout was confirmed by the presence of urate crystals in the fluid aspirated from elbow joint.
discussion
TLS and hyperuricemia are serious complications with significant morbidity and potential mortality in patients with hematologic malignancies undergoing anticancer therapy [1–4]. Allopurinol has been used for many years in the prevention and management of TLS-related hyperuricemia. However, allopurinol should be administered for ≥3 days for the achievement of significant reduction in UA levels. Rasburicase offers potential advantage over allopurinol by its rapid onset of action, reducing preexisting pool of UA within few hours [3].
Rasburicase was initially approved by the US Food and Drug Administration (FDA) for the management of TLS in the pediatric setting, based on a randomized controlled phase III clinical trial whereby rasburicase (0.15 or 0.2 mg/kg) was administered for five consecutive days [8, 9]. This drug was also used in adult patients in an ‘off label’ manner for several years, based on unmet medical need mainly for the management of adults with TLS. More recently, rasburicase at a dose of 0.2 mg/kg for up to 5 days was FDA approved in adult patients based on phase III study involving multiple-day dosing [10, 11]. However, several smaller noncontrolled studies involving retrospective case series have suggested shorter duration of treatment to minimize the cost [12–20]. Our study was focused on patients who have intermediate risk or high risk for TLS in which debate persists on the usage of single versus multiple doses of rasburicase. The results of our randomized study showed that single dose of rasburicase was effective in maintaining the UA levels within the normal range in the vast majority of patients and only small subset with aggressive disease and high-risk features required a second dose.
In this study, all patients except for one normalized their UA within 4 h, including high-risk patients and those with laboratory evidence for TLS. In fact, UA declined to undetectable levels in 84% following a single dose and remained undetectable in the daily-dosing arm. Although persistently high-UA levels are associated with renal insufficiency [4, 24, 25], the potential advantage of undetectable UA levels, if any, are unknown at present. In this study, serum creatinine normalized in 12 of 16 patients who presented with elevated levels. Despite evidence for laboratory TLS in about one-third, only two patients required dialysis, one due to history of chronic renal insufficiency and multiple comorbidities and the other due to the emergence of clinical TLS with his chemotherapy being delayed by 4 days post-rasburicase. Thus, rasburicase appeared to be effective in preventing renal complications by controlling UA; this is consistent with previous observations of the suggested ability of rasburicase to prevent or reverse acute kidney injury in pediatric patients at risk or with TLS [8, 26].
The treatment with rasburicase was generally well tolerated with minimal gastrointestinal toxicity, observed in daily dosing slightly more than in the single-dose arm. The serious adverse events were equal in both arms and were mostly related to the treatment of the underlying disease. A similar pattern of adverse events were observed in the prior studies [8–10]. One patient with previously unknown glucose-6-phosphate deficiency, in the daily-dosing arm, developed methemoglobinemia and hemolysis that was exacerbated with methylene blue. The use of rasburicase is contraindicated in G6PD-deficient patients since their erythrocytes cannot generate nicotinamide adenine dinucleotide phosphate efficiently [11]. Although it is recommended to screen patients at higher risk for G6PD deficiency (e.g. patients with African or Mediterranean ancestry) before rasburicase, it is not practical to wait for the test results and delay therapy in high-risk patients. In clinical studies, the incidence of methemoglobinemia and/or hemolysis is very low (<1%) [27–30]. However, the treatment with methylene blue in G6PD-deficient patients is contraindicated as it may precipitate hemolysis due to increased oxidative stress, as was seen in our patient. In addition, we observed acute episode of gouty arthritis in a patient with no prior history of gout, during rising UA level. It is well known that sudden changes in UA levels in patients with gouty diathesis can precipitate an acute attack of gout [31, 32]. To our knowledge, this is the first report describing this phenomenon in patients receiving rasburicase, suggesting the need for careful monitoring of UA over time.
In summary, the results of our randomized study demonstrate that rasburicase is a highly effective uricosuric agent for the prevention and management of hyperuricemia associated with TLS. All patients at potential risk and majority of high-risk patients responded to a single dose, indicating that in appropriately monitored patients single dose followed by dosing as needed can be cost saving. In patients with high-risk features and/or the presence of TLS, several doses may be necessary as per the clinical judgment, based on the biological abnormalities. Methemoglobinemia and hemolysis are rare, yet important toxic effects that need prompt diagnosis and management.
funding
This work was supported in part by Sanofi-Aventis (study number 2006-0918 registered at clinicaltrials.gov, NCT00628628); SV-R, MD received funding from Sanofi-Aventis to support this clinical study.
disclosure
SV-R, MD and JEC, MD received funding from Sanofi Aventis to support clinical research. NS, MD was an employee of the Sanofi-Aventis. All the other authors declare no conflict of interest.
Acknowledgments
Presented in part at the 51st Annual Meeting of the American Society of Hematology, December 5–8, 2009, and International MASCC Symposium, June 24–26, 2010.
References
- 1.Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. Am J Med. 1993;94:133–139. doi: 10.1016/0002-9343(93)90174-n. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg J, Cairo MS. Tumor lysis syndrome: current perspective. Haematologica. 2008;93:9–13. doi: 10.3324/haematol.12327. [DOI] [PubMed] [Google Scholar]
- 3.Mughal TI, Ejaz AA, Foringer JR, Coifffier B. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36:164–176. doi: 10.1016/j.ctrv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Shimada M, Johnson RJ, May WS, Jr, et al. A novel role for uric acid in acute kidney injury associated with tumor lysis syndrome. Nephrol Dial Transplant. 2009;24:2960–2964. doi: 10.1093/ndt/gfp330. [DOI] [PubMed] [Google Scholar]
- 5.Coiffer B, Altman A, Pui C-H, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 6.Tosi P, Barosi G, Lazzaro C, et al. Consensus conference on the management of tumor lysis syndrome. Haematologica. 2008;93:1877–1885. doi: 10.3324/haematol.13290. [DOI] [PubMed] [Google Scholar]
- 7.Cairo MS, Bishop M. Tumor lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia and high-risk for tumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]
- 9.Smalley RV, Guaspari A, Haase-Statz, et al. Allopurirnol: intravenous use for prevention and treatment of hyperuricemia. J Clin Oncol. 2000;18:1758–1763. doi: 10.1200/JCO.2000.18.8.1758. [DOI] [PubMed] [Google Scholar]
- 10.Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter Phase III Study. J Clin Oncol. 2010;28:4207–4213. doi: 10.1200/JCO.2009.26.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elitek® (rasburicase) [Package Insert] Bridgewater, NJ: Sanofi-Aventis; 2008. [Google Scholar]
- 12.Liu CY, Sims-McCallum RP, Schaffer CA. A single dose of rasburicase is sufficient for the treatment of hyperuricemia in patients receiving chemotherapy. Leuk Res. 2005;29:463–465. doi: 10.1016/j.leukres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell AM, Lenz KL, Frei-Lahr DA, et al. Single-dose rasburicase 6 mg in the management of tumor lysis syndrome in adults. Pharmacotherapy. 2006;26:806–812. doi: 10.1592/phco.26.6.806. [DOI] [PubMed] [Google Scholar]
- 14.Reeves DJ, Bestul DJ. Evaluation of a single fixed dose of rasburicase 7.5 mg for the treatment of hyperuricemia in adults with cancer. Pharmacotherapy. 2008;28:685–690. doi: 10.1592/phco.28.6.685. [DOI] [PubMed] [Google Scholar]
- 15.Campara M, Shord SS, Haaf CM. Single-dose rasburicase for tumor lysis syndrome in adults: weight-based approach. J Clin Pharm Ther. 2009;34:207–213. doi: 10.1111/j.1365-2710.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 16.Trifillo S, Gordon L, Singhal S, et al. Reduced-dose rasburicase (recombinant xanthine oxidase) in adult cancer patients with hyperuricemia. Bone Marrow Transplant. 2006;37:997–1001. doi: 10.1038/sj.bmt.1705379. [DOI] [PubMed] [Google Scholar]
- 17.Hummel M, Buchheidt D, Reiter S, et al. Recurrent chemotherapy-induced tumor lysis syndrome (TLS) with renal failure in a patient with chronic lymphocytic leukemia—successful treatment and prevention of TLS with low-dose rasburicase. Eur J Haematol. 2005;75:518–521. doi: 10.1111/j.1600-0609.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee AC, Li CH, So KT, Chan R. Treatment of impending lumor lysis with single-dose rasburicase. Ann Pharmacother. 2003;37:1614–1617. doi: 10.1345/aph.1D111. [DOI] [PubMed] [Google Scholar]
- 19.Hummel M, Reiter S, Adam K, et al. Effective treatment and prophylaxis of hyperuricemia and impaired renal function in tumor lysis syndrome with low doses of rasburicase. Eur J Haematol. 2008;80:331–336. doi: 10.1111/j.1600-0609.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- 20.Hutcherson DA, Gammon DC, Bhatt MS, Faneuf M. Reduced-dose rasburicase in the treatment of adults with hyperuricemia associated with malignancy. Pharmacotherapy. 2006;26:242–247. doi: 10.1592/phco.26.2.242. [DOI] [PubMed] [Google Scholar]
- 21.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 22.Harris NL, Jaffe ES, Diebold J, et al. Lymphoma classification—from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11(Suppl 1):3–10. [PubMed] [Google Scholar]
- 23.He J. SAS Programming to Calculate AUC in Pharmacokinetic Studies—Comparison of Four Methods in Concentration Data. SAS Institute 2008. http://www.lexjansen.com/pharmasug/2008/sp/sp06.pdf (10 November 2011, date last accessed) [Google Scholar]
- 24.Ejaz AA, Beaver TM, Shimada M, et al. Uric Acid: a novel risk factor for acute kidney injury in high-risk cardiac surgery patients? Am J Nephrol. 2009;30:425–429. doi: 10.1159/000238824. [DOI] [PubMed] [Google Scholar]
- 25.Cairo MS, Casciano R, Morris E, et al. Uric acid (UA) level is a significant prognostic factor in the development of tumor lysis syndrome (TLS) and renal events (RE) in non-Hodgkin's lymphoma (NHL) in patients admitted for inpatient chemotherapy (IC) Ann Oncol. 2002;13:174. (Abstr 624) [Google Scholar]
- 26.Goldman S, Lynch J, Harrison L, et al. Preliminary results of the addition of rasburicase to the reduction cycle and rituximab to the induction and consolidation cycles of FAB Group C chemotherapy in children and adolescents with advanced stage (bone Marrow ±CNS) mature B-Cell non-Hodgkin lymphoma (B-NHL): a Children’s Oncology Group report. Blood. 2009;114:22. (Abstr 104) [Google Scholar]
- 27.Kizer N, Martinez E, Powell M. Report of two cases of rasburicase-induced methemoglobinemia. Leuk Lymphoma. 2006;47:2648–2650. doi: 10.1080/10428190600967204. [DOI] [PubMed] [Google Scholar]
- 28.Jeha S, Kantarjian H, Irwin D, et al. Efficacy and safety of rasburicase, a recombinant urate oxidase (Elitek), in the management of malignancy-associated hyperuricemia in pediatric and adult patients: final results of a multicenter compassionate use trial. Leukemia. 2005;19:34–38. doi: 10.1038/sj.leu.2403566. [DOI] [PubMed] [Google Scholar]
- 29.Coiffier B, Mounier N, Bologna S, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin's lymphoma: results of the GRAAL1 (Groupe d'Etude des Lymphomes de l'Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol. 2003;21:4402–4406. doi: 10.1200/JCO.2003.04.115. [DOI] [PubMed] [Google Scholar]
- 30.Browning LA, Kruse J. Hemolysis and methemoglobinemia secondary to rasburicase administration. Ann Pharmacother. 2005;39:1932–1935. doi: 10.1345/aph.1G272. [DOI] [PubMed] [Google Scholar]
- 31.Landis RC, Haskard DO. Pathogenesis of crystal-induced inflammation. Curr Rheumatol Rep. 2001;3:36–41. doi: 10.1007/s11926-001-0049-7. [DOI] [PubMed] [Google Scholar]
- 32.Neogi T. Gout. N Engl J Med. 2011;364:443–452. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]


