Abstract
MicroRNAs (miRNAs), which play a role in tumorigenesis, may also serve as diagnostic or prognostic biomarkers. However, studies on human miRNA profiles in plasma from nasopharyngeal carcinoma (NPC) patients are in their infancy. Here, we used microarrays to perform systematic profiling of human miRNAs in plasma from NPC patients. We subsequently used real-time quantitative polymerase chain reaction (Q-PCR) to validate miRNAs with aberrant expression that could serve as potential biomarkers. By comparing the plasma miRNA profiles of 31 NPC patients and 19 controls, 39 of 887 human miRNAs were found to be aberrantly expressed. Considering the fold change and P value, miR-548q and miR-483-5p were validated in 132 samples from 82 NPC patients and 50 controls. Moreover, high expression of miR-548q and miR-483-5p was further found in 3 NPC cell lines and clinical biopsy tissues from 54 NPC patients and 22 controls. Our results revealed that miR-548q and miR-483-5p are potential biomarkers of NPC. Combining the receiver operating characteristic (ROC) analyses of these 2 miRNAs, an area under the ROC curve (AUC) of 0.737 with 67.1% sensitivity and 68.0% specificity were obtained, showing the preliminary diagnostic value of plasma miRNAs. Moreover, most NPC patients with a poor outcome exhibited high expression (> median) of miR-548q (70.6%) and miR-483-5p (64.7%) in tissue samples, indicating their prognostic value. The high expression levels of miR-548q and miR-483-5p in plasma, cell lines, and clinical tissues of NPC patients indicate that their roles in NPC should be explored in the future.
Keywords: Nasopharyngeal carcinoma (NPC), microRNA profiles, potential biomarkers
Nasopharyngeal carcinoma (NPC) is a highly invasive and metastatic cancer that is widely prevalent in southern China. To date, three major etiologic factors, genetic susceptibility, endemic environmental factors, and Epstein–Barr virus (EBV) infection, are believed to induce NPC[1]. Due to its sensitivity to both chemotherapy and radiotherapy, NPC can be successfully treated if the tumor is locally confined to the nasopharynx at diagnosis. Unfortunately, many NPC patients are usually diagnosed at advanced stages. Partially distant metastases and recurrence after treatment often occur in these patients, leading to poor prognosis[2]. Therefore, it is important to identify novel biomarkers associated with tumor progression[3].
Currently, endoscopic examination of the nasopharynx and histological examination of tissues are usually used to diagnose NPC. However, clinical biopsies of the nasopharynx are highly invasive and inconvenient, and repeated examinations are not clinically practical. Radiologic examinations are useful for determining the extent of the disease. However, these imaging techniques are generally costly and time-consuming. Antibodies against EBV antigens are frequently used for NPC screening because serologic assays are relatively inexpensive and noninvasive. However, the results of these serologic tests alone are insufficient to accurately diagnose NPC[4]. At the same time, differences in clinical outcomes have been reported in patients who are at the same stage and receive similar treatment regimens [5]. These findings suggest that the present staging system is inadequate for prognosis. Driven by this demand, various biomarkers associated with prognosis have been reported. Nevertheless, new diagnostic and prognostic biomarkers are still needed for NPC.
In general, biomarkers that are ideal for clinical application should be easily accessible. Therefore, biomarkers that can be sampled from body fluids such as plasma and serum are particularly desirable[6]. MicroRNAs (miRNAs) are ideal biomarkers because they are commonly present in blood-based samples[7],[8]. Some human tumors have been successfully identified through miRNA expression profiling[9],[10]. miRNAs are non-coding RNA molecules that are approximately 22 nucleotides in length and regulate a variety of cellular processes including cell proliferation, differentiation, and apoptosis. miRNAs play an important role in carcinogenesis through either oncogenic or tumor-suppressive functions[11]. The extensive miRNA profile has become an important tool for developing valuable biomarkers and elucidating their roles in tumorigenesis. In NPC, human miRNA profiles from clinical biopsies have been examined[12]–[15]. Based on tissue miRNA profiling, some aberrant miRNAs were discovered, and their roles in NPC were further investigated[16]–[18]. Although plasma and serum miRNA profiles have been extensively studied in other cancers[19]–[27], few studies have examined human miRNA profiles in plasma from NPC patients.
Herein, we aimed to examine the miRNA expression profiles in plasma samples from NPC patients to reveal potential biomarkers and further explore the malignancy of NPC cell lines and clinical tissues.
Materials and Methods
Study populations
We collected plasma and tissue samples from participants who underwent nasopharyngeal biopsy at the Sun Yat-sen University Cancer Center (SYSUCC). The plasma samples were collected from April 2010 to August 2010, and the tissue samples were collected from April 2002 to August 2010. Most of the participants had biopsy-proven NPC, whereas some had chronic nasopharyngitis and could therefore serve as clinical controls. The patients were newly diagnosed with NPC and had no history of cancer or cancer treatment. Finally, plasma samples from 82 NPC patients and 50 controls were collected. According to their chronologic order, they were divided into a microarray cohort (n = 50) and a validation cohort (n = 82). Moreover, biopsy tissues were collected from 54 NPC patients and 22 controls. This study was approved by the Human Ethics Committee of SYSUCC, and all participants provided informed consent.
Cell lines
Three NPC cell lines (CNE1, CNE2, and HNE1) and one immortalized nasopharyngeal epithelial cell line (NP69) were provided by professor Mu-Seng Zeng, Department of Experimental Research, SYSUCC. NP69 was cultured in defined, serum-free keratinocyte medium (Invitrogen, Carlsbad, CA, USA) as described previously[28], and the other three cell lines were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum. The cells were maintained in a humidified incubator at 37°C with 5% CO2.
Plasma preparation
For the plasma preparation, peripheral blood (3 mL) was drawn into EDTA tubes. Within 30 min, the tubes were centrifuged at 820 ×g for 10 min. Then, 1-mL aliquots of the plasma were transferred to 1.5-mL tubes and centrifuged at 16,000 ×g for an additional 10 min to remove any remaining cellular debris. Subsequently, the supernatants were transferred to fresh tubes and immediately stored at –80°C.
RNA extraction
RNA was isolated from 400 µL of plasma using the mirVana PARIS miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer's instructions. Total RNA from cells and clinical tissues was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The RNA concentration was quantified using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Waltham, MA, USA).
MicroRNA profiling using microarrays
Microarrays from Agilent Technologies (Santa Clara, CA, USA) were used to profile human miRNAs and identify differentially expressed miRNAs using plasma samples from 31 NPC patients and 19 controls (microarray cohort). The details of the microarray hybridizations were described previously[23]. The raw miRNA data are available in Gene Expression Omnibus (GEO) as GSE43329. The raw signals obtained for single-color CY3 hybridization were normalized to a stable endogenous control, miR-454, which was chosen due to its low coefficient of variance (CV) and positive signal in the microarray. Then, log10 transformation was performed before data analysis.
Reverse transcription
For all samples, total RNA (40 ng) was used for reverse transcription with a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. Reverse transcription reactions were conducted with custom stem-loop primers (Applied Biosystems) that were specific to the corresponding mature sequence obtained from miRBase (www.miRBase.org).
Quantitative polymerase chain reaction (Q-PCR)
To test the candidate miRNAs discovered using the microarrays, Q-PCR was performed using TaqMan microRNA assays (Applied Biosystems). miR-454, which was the control used in the microarray, was selected as the endogenous control in the plasma, whereas RNU6B was used as the endogenous control in the NPC cell lines and tissues. All cycle threshold (Ct) values were determined in real time with the ABI 7900HT system and analyzed with SDS Relative Quantification Software 2.2.3 (Applied Biosystems). All reactions were performed in triplicate.
Statistical analyses
SPSS 16.0 software was used to perform the statistical analyses. All P values were two-sided, and values less than 0.05 were considered significant. The relative expression of the miRNAs as determined using Q-PCR was analyzed using the 2−ΔΔCt method. The area under the receiver operating characteristic (ROC) curve (AUC) was used as an accuracy index for evaluating the diagnostic performance of the selected miRNA combinations.
Results
Characteristics of the study population
A total of 132 plasma samples and 76 biopsy tissues were collected in this study. The characteristics of the study population are shown in Table 1. The cancer stage is usually evaluated by clinical doctors based on the comprehensive results of magnetic resonance imaging (MRI), histopathologic type, and clinical symptoms. Although some patients at our cancer center had biopsy-proven NPC, they were moved to other hospitals for further diagnosis and treatment; thus, examinations such as MRI for these patients were not conducted at our cancer center. Therefore, in our study, the cancer stage information associated with 32 plasma samples and 13 clinical tissue samples was lost, and only histopathologic information was obtained.
Table 1. Characteristics of the study population.
| Variable | Plasma sample group |
Tissue sample group |
||||
| Microarray cohort |
Validation cohort |
|||||
| NPC (n = 31) | Controls (n = 19) | NPC (n = 51) | Controls (n = 31) | NPC (n = 54) | Controls (n = 22) | |
| Age (years) | ||||||
| Mean | 46.23 | 43.26 | 46.72 | 42.19 | 44.5 | 40.4 |
| Median | 45 | 41 | 47 | 40 | 45 | 40 |
| Maximum | 62 | 59 | 73 | 73 | 63 | 64 |
| Minimum | 27 | 28 | 14 | 14 | 18 | 18 |
| Gender (cases) | ||||||
| Male | 21 | 12 | 46 | 19 | 39 | 9 |
| Female | 10 | 7 | 5 | 12 | 15 | 13 |
| Cancer stage (cases) | ||||||
| Stage I | 0 | N/A | 0 | N/A | 1 | N/A |
| Stage II | 2 | N/A | 8 | N/A | 8 | N/A |
| Stage III | 10 | N/A | 16 | N/A | 25 | N/A |
| Stage IV | 7 | N/A | 7 | N/A | 7 | N/A |
| Unknown | 12 | N/A | 20 | N/A | 13 | N/A |
| VCA-IgA titer (cases) | ||||||
| Negative | 0 | 15 | 3 | 14 | 0 | 16 |
| 1:10 - 1:20 | 0 | 4 | 3 | 17 | 2 | 6 |
| 1:40 - 1:80 | 3 | 0 | 12 | 0 | 12 | 0 |
| 1:160 | 28 | 0 | 33 | 0 | 40 | 0 |
| EA-IgA titer (cases) | ||||||
| Negative | 2 | 19 | 19 | 31 | 10 | 22 |
| 1:10 - 1:20 | 11 | 0 | 22 | 0 | 30 | 0 |
| 1:40 - 1:80 | 18 | 0 | 10 | 0 | 14 | 0 |
| 1:160 | 0 | 0 | 0 | 0 | 0 | 0 |
NPC, nasopharyngeal carcinoma; VCA, Epstein-Barr virus (EBV) viral capsid antigen; EA, EBV early antigen; N/A, not applicable.
Candidate biomarkers discovered using 50 microarrays
By comparing miRNA expression profiles using samples from the microarray cohort, 39 miRNAs with significantly different expression levels were identified. A total of 37 miRNAs were up-regulated in the NPC patients compared with the controls, whereas 2 miRNAs were down-regulated. There were 14 miRNAs with a fold change > 2 and 11 miRNAs with P < 0.005. Overall, 9 miRNAs (miR-548q, miR-1290, miR-630, miR-765, miR-135a*, miR-940, miR-483-5p, miR-29c, and miR-1915) with both a fold change > 2 and P < 0.005 had great potential for use in NPC diagnosis (Table 2).
Table 2. A total of 39 altered microRNAs were found using 50 microarrays.
| MicroRNA | Mean signal intensity |
Fold change | P | |
| NPC (n = 31) | Controls (n = 19) | |||
| hsa-miR-548q | 496.31 | 158.39 | 3.13 | < 0.001 |
| hsa-miR-483-5p | 615.53 | 238.09 | 2.59 | 0.002 |
| hsa-miR-122 | 817.00 | 330.46 | 2.47 | 0.012 |
| hsa-miR-630 | 292.53 | 122.13 | 2.40 | 0.001 |
| hsa-miR-135a* | 252.26 | 106.13 | 2.38 | 0.001 |
| hsa-miR-572 | 692.77 | 297.65 | 2.33 | 0.006 |
| hsa-miR-940 | 586.09 | 256.28 | 2.29 | 0.001 |
| hsa-miR-29c | 365.32 | 164.43 | 2.22 | 0.005 |
| hsa-miR-22 | 2,963.42 | 1,430.17 | 2.07 | 0.035 |
| hsa-miR-1915 | 1,671.75 | 806.96 | 2.07 | 0.005 |
| hsa-miR-762 | 2,212.08 | 1,089.96 | 2.03 | 0.048 |
| hsa-miR-1290 | 253.28 | 124.91 | 2.03 | 0.001 |
| hsa-miR-765 | 261.43 | 129.60 | 2.02 | 0.001 |
| hsa-miR-92a | 2,196.96 | 1,097.13 | 2.00 | 0.050 |
| hsa-miR-486-5p | 4,726.64 | 2,438.34 | 1.94 | 0.040 |
| hsa-miR-638 | 11,792.79 | 6,090.35 | 1.94 | 0.010 |
| hsa-miR-140-3p | 276.09 | 143.45 | 1.92 | 0.009 |
| hsa-miR-1246 | 1,151.10 | 606.00 | 1.90 | 0.020 |
| hsa-miR-15b | 284.72 | 153.94 | 1.85 | 0.013 |
| hsa-miR-30d | 211.89 | 115.65 | 1.93 | 0.018 |
| hsa-miR-19a | 264.51 | 149.60 | 1.77 | 0.035 |
| hsa-miR-126 | 242.84 | 141.28 | 1.72 | 0.022 |
| hsa-miR-20a | 301.29 | 175.73 | 1.71 | 0.042 |
| hsa-miR-185 | 175.68 | 105.20 | 1.67 | 0.004 |
| hsa-miR-93 | 160.50 | 99.09 | 1.62 | 0.002 |
| hsa-miR-101 | 161.92 | 104.49 | 1.55 | 0.006 |
| hsa-miR-187* | 118.51 | 78.63 | 1.51 | 0.041 |
| hsa-miR-498 | 158.63 | 109.54 | 1.45 | 0.007 |
| hsa-miR-1234 | 181.41 | 125.49 | 1.45 | 0.042 |
| hsa-miR-1975 | 137.34 | 98.82 | 1.39 | 0.012 |
| hsa-miR-575 | 136.09 | 98.68 | 1.38 | 0.020 |
| hsa-miR-17 | 123.00 | 89.62 | 1.37 | 0.022 |
| hsa-miR-130a | 147.93 | 108.78 | 1.36 | 0.038 |
| hsa-miR-363 | 137.47 | 104.69 | 1.31 | 0.048 |
| hsa-miR-301a | 127.85 | 99.89 | 1.28 | 0.009 |
| hsa-miR-192 | 120.74 | 98.97 | 1.22 | 0.018 |
| hsa-miR-338-3p | 115.82 | 99.60 | 1.16 | 0.021 |
| hsa-miR-1277 | 214.86 | 309.95 | 0.69 | 0.039 |
| hsa-miR-584 | 115.88 | 199.49 | 0.58 | 0.040 |
miR-548q and miR-483-5p were validated in 132 samples using Q-PCR
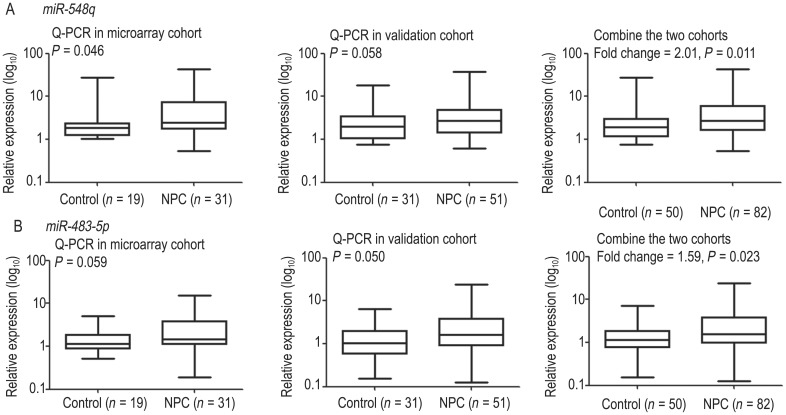
miR-548q and miR-483-5p were the top two miRNAs with a fold change >2 and P value < 0.005. Therefore, they were selected first for validation using Q-PCR. The validations were conducted in the microarray cohort and the validation cohort. Increased expression of miR-548q and miR-483-5p was observed in the microarray cohort (P = 0.046 for miR-548q; P = 0.059 for miR-483-5p) and the validation cohort (P = 0.058 for miR-548q; P = 0.050 for miR-483-5p). The differences in miR-548q in the validation cohort and miR-483-5p in the microarray cohort were not quite significant, most likely due to the sample size. Finally, the increased expression levels of miR-548q (fold change = 2.01, P = 0.011) and miR-483-5p (fold change = 1.59, P = 0.023) were validated by combining the two cohorts (Figure 1).
Figure 1. Plasma miR-548q and miR-483-5p expression levels.
Increased expression levels of miR-548q (A) and miR-483-5p (B) were validated in the microarray cohort (left), the validation cohort (middle), and the combined cohort (right).
High expression of miR-548q and miR-483-5p was further validated in NPC cell lines and biopsy tissues
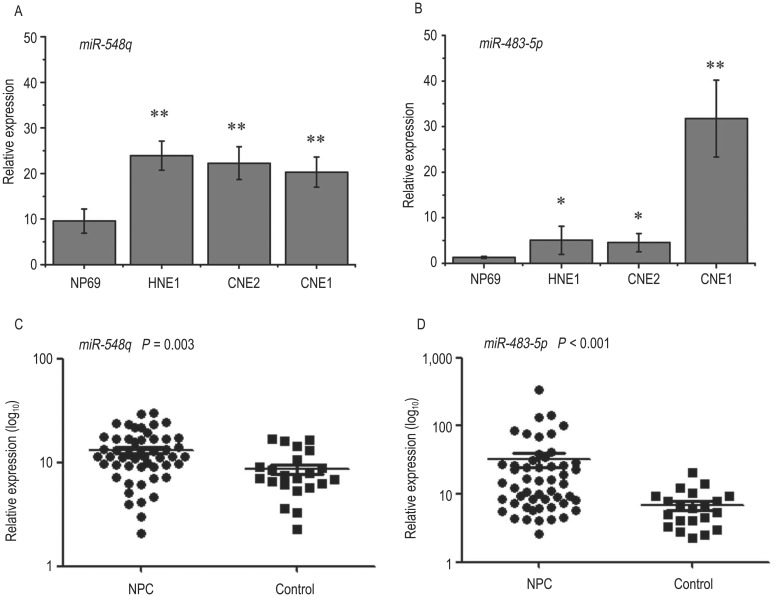
Compared with its expression level in NP69 cells, the expression level of miR-548q was significantly increased in the NPC cell lines, including HNE1 (fold change = 2.51, P < 0.001), CNE2 (fold change = 2.34, P < 0.001), and CNE1 (fold change = 2.13, P < 0.001) (Figure 2A). Similarly, increased expression of miR-483-5p was also observed in HNE1 (fold change = 3.94, P = 0.03), CNE2 (fold change = 3.55, P = 0.01), and CNE1 cells (fold change = 24.87, P < 0.001) (Figure 2B).
Figure 2. The expression levels of miR-548q and miR-483-5p in cell lines and biopsy tissues.
Increased expression levels of miR-548q and miR-483-5p were validated in cell lines (A, B) and biopsy tissues (C, D) from nasopharyngeal carcinoma (NPC) patients. The P values were calculated using an independent two-tailed t-test (*P < 0.05, **P < 0.005; n = 6 per group).
Consistent with the trend observed in plasma and cell lines, increased expression of miR-548q (fold change = 3.56, P = 0.003) (Figure 2C) and miR-483-5p (fold change = 11.18, P < 0.001) (Figure 2D) was also found in NPC biopsy tissues compared to controls.
miR-548q and miR-483-5p contributed to diagnosis and prognosis
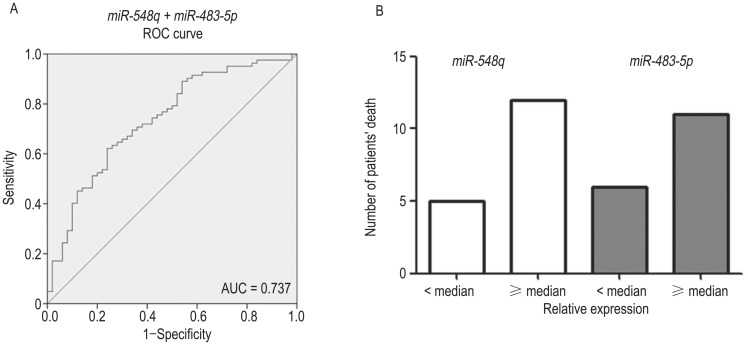
In plasma samples, we determined a sensitivity of 62.2% and a specificity of 60.0% using miR-548q (AUC, 0.631) in ROC analysis. Using miR-483-5p (AUC, 0.600), we observed a sensitivity of 62.2% and a specificity of 58.0% (data not shown). The combination of miR-548q and miR-483-5p produced an increased AUC of 0.737, with 67.1% sensitivity and 68.0% specificity, indicating an additive effect in the diagnostic value of these 2 miRNAs (Figure 3A). In biopsy tissue samples from NPC patients, the expression levels of miR-548q and miR-483-5p were classified into low and high groups according to their medians. A total of 17 NPC patients died; of these 17 patients, 12 (70.6%) had an miR-548q expression level higher than its median and 11 (64.7%) had an miR-483-5p expression level higher than its median. These results indicate that high expression of miR-548q and miR-483-5p might contribute to poor prognosis (Figure 3B).
Figure 3. miR-548q and miR-483-5p contributed to diagnosis and prognosis.
A, the combination of miR-548q and miR-483-5p produced an area under the receiver operating characteristic (ROC) curve (AUC) of 0.737, with 67.1% sensitivity and 68.0% specificity. B, based on the analysis of the tissue samples from NPC patients, most of the patients who died exhibited high expression (≥ median) of miR-548q and miR-483-5p.
Discussion
In this study, a total of 39 altered miRNAs were found using 50 microarrays containing 887 miRNAs. The increased expression levels of miR-548q and miR-483-5p were further validated by Q-PCR in plasma, cell lines, and biopsy tissues from NPC patients. These consistent results pave the way for further exploration of the roles of these miRNAs in tumorigenesis.
Recently, efforts have been made to examine human miRNA profiles in the serum of NPC patients. In 2012, miRNA profiling of serum samples pooled from 20 NPC patients or 20 controls was conducted to explore the diagnostic value of serum miRNAs using a TaqMan Low-Density Array, and miR-17, miR-20a, miR-29c, and miR-223 were found to be differently expressed[29]. In 2013, miRNA profiling of serum samples from 8 NPC patients with a long survival and 8 patients with a shorter survival was conducted to explore the prognostic value of serum miRNAs, and miR-22, miR-572, miR-638, and miR-1234 were found to be altered between these two groups[30]. In our study, 50 microarrays of plasma samples from 31 NPC patients and 19 controls were analyzed, and a comprehensive miRNA profile was obtained. A large sample size may aid in discovering more potential biomarkers. In fact, all the miRNAs reported in serum except miR-223 were discovered in our microarray (Table 2).
miR-548q was initially identified in epithelial ovarian cancer samples through next-generation sequencing[31]. To date, no reports have described this miRNA in other tumors. miR-483-5p was initially identified as a predictor of poor prognosis in adrenocortical cancer in 2009[32]. Later, miR-483-5p was shown to serve as a biomarker of malignancy that could be used to accurately categorize adrenocortical tumors as benign or malignant [33]. In the current study, the expression levels of miR-548q and miR-483-5p were validated to be higher in plasma from NPC patients than in plasma from controls (Figure 1).
Interestingly, higher expression levels of miR-548q and miR-483-5p were found in NPC cell lines (CNE1, CNE2, and HNE1) compared to an immortalized nasopharyngeal epithelial cell line (NP69) and clinical biopsy tissues (Figure 2), suggesting that these miRNAs might be involved in NPC development. Because the numbers of males and females in the NPC patient and control groups were not well balanced (Table 1), the difference between males and females in each group was compared using plasma samples and biopsy tissue samples. No significant differences were observed between genders; however, nearly significant and significant differences were observed between controls and NPC patients of the same gender. These results further demonstrated that the differences observed were due to tumorigenesis.
Thus far, some miRNAs have been found to be altered in cells or tissues after conducting tissue miRNA microarray experiments[12]–[15]. Further, the roles of some miRNAs in tumorigenesis have been explored[16]–[18],[34]–[40]. However, the examination of miRNA expression profiles in tissue samples is inconvenient, and these profiles cannot be used to screen high-risk populations. By contrast, plasma microRNA expression profiles provide valuable biomarkers for the diagnosis of NPC. Currently, few miRNAs are known to be altered in both plasma and clinical tissues. With the development of personalized therapy, the combination of diagnostic application and functional regulation may be focused on one biomarker. Given the overexpression of miR-548q and miR-483-5p in both plasma and tissue, we also examined the potential target pathways and typical genes using bioinformatic tools. For example, genes involved in ubiquitin-mediated proteolysis (VHL, UBE3A, and UBE3B) were targeted by miR-483-5p, and genes involved in apoptosis (BCL2L2, BCL2L11) were targeted by miR-548q. Interestingly, genes involved in the cell cycle (CDKN2A and CDKN2B by miR-548q; CDKN1A by miR-483-5p) and p53 signaling (HIPK2 and TP53INP2 by miR-548q; HIPK2 by miR-483-5p) were targeted by both miR-548q and miR-483-5p (data not shown). Despite the limitations of this study, our results provide preliminary insight into the mechanisms underlying miRNA-related tumorigenesis. Based on the positive results of this preliminary study, further functional investigation of miR-548q and miR-483-5p should be conducted in the future.
Based on the results of our 50 microarrays, we found that 39 miRNAs exhibited significant expression differences (Table 2). In the first phase of the study, only two potential biomarkers (miR-548q and miR-483-5p) were selected for validation based on their fold change and P value (Figure 1). Our data suggested that either biomarker alone or in combination may not provide sufficient predictive accuracy (Figure 3A). However, a miRNA panel has been reported to greatly improve the performance of a single biomarker. For example, a panel of 7 miRNAs was successfully constructed to diagnose hepatitis B virus-related hepatocellular carcinoma[23]. In addition, a combination of 10 miRNAs was used as a novel biomarker for non-small cell lung cancer diagnosis[24]. Therefore, other miRNAs with significant differences should be validated, and a better predictive panel may be constructed in the future. Due to the loss of patient information and limitation of sample size, the prognostic value of miR-548q and miR-483-5p could not be accurately evaluated in this study. Only 17 patients with NPC died, and most of the patients who died had high expression levels of miR-548q and miR-483-5p (Figure 3B). Our preliminary results indicated that miR-548q and miR-483-5p might play an important role in tumorigenesis and have an effect on prognosis. However, further study is needed to fully understand their contribution to prognosis.
In conclusion, in this study, we found that miR-548q and miR-483-5p are potential biomarkers of NPC. Specific miRNAs are consistently found in cells, clinical tissues, and the blood circulation; thus, their role in tumorigenesis can be easily explored. The plasma miRNA expression profiles were important resources for revealing biomarkers of malignancy.
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB504303), the Ministry of Science and Technology of China (2011ZX11307), and the Natural Science Foundation of Guangdong Province (9151008004000002).
References
- 1.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Razak AR, Siu LL, Liu FF, et al. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010;46:1967–1978. doi: 10.1016/j.ejca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.He ML, Luo MX, Lin MC, et al. MicroRNAs: potential diagnostic markers and therapeutic targets for EBV-associated naso-pharyngeal carcinoma. Biochim Biophys Acta. 2012;1825:1–10. doi: 10.1016/j.bbcan.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Chan KCA, Lo YMD. Circulating EBV DNA as a tumor marker for nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:489–496. doi: 10.1016/s1044579x02000913. [DOI] [PubMed] [Google Scholar]
- 5.Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance image. Int J Radiat Oncol Biol Phys. 2009;73:1326–1334. doi: 10.1016/j.ijrobp.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, den Boon JA, Chen IH, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci U S A. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Chen JX, Fu XP, et al. MicroRNA expression profiling of nasopharyngeal carcinoma. Oncol Rep. 2011;25:1353–1363. doi: 10.3892/or.2011.1204. [DOI] [PubMed] [Google Scholar]
- 14.Chen HC, Chen GH, Chen YH, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100:1002–1011. doi: 10.1038/sj.bjc.6604948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Z, Zhang L, Li Z, et al. An in silico analysis of dynamic changes in microRNA expression profiles in stepwise development of nasopharyngeal carcinoma. BMC Med Genomics. 2012;5:3. doi: 10.1186/1755-8794-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng M, Tang H, Zhou Y, et al. miR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J Cell Sci. 2011;124:2997–3005. doi: 10.1242/jcs.085050. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, He ML, Wang L, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 18.Alajez NM, Lenarduzzi M, Ito E, et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011;71:2381–2391. doi: 10.1158/0008-5472.CAN-10-2754. [DOI] [PubMed] [Google Scholar]
- 19.Wulfken LM, Moritz R, Ohlmann C, et al. MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PLoS One. 2011;6:e25787. doi: 10.1371/journal.pone.0025787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrauder MG, Strick R, Schulz-Wendtland R, et al. Circulating micro-RNAs as potential blood-based markers for early stage breast cancer detection. PLoS One. 2012;7:e29770. doi: 10.1371/journal.pone.0029770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi P, Cheng SQ, Wang H, et al. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. doi: 10.1371/journal.pone.0028486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Todd NW, Zhang H, et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab Invest. 2011;91:579–587. doi: 10.1038/labinvest.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, Huang D, Ni S, et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Hu Z, Wang W, et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for non-small cell lung cancer diagnosis. Int J Cancer. 2012;130:1620–1628. doi: 10.1002/ijc.26177. [DOI] [PubMed] [Google Scholar]
- 26.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 27.Li LM, Hu ZB, Zhou ZX, et al. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- 28.Tsao SW, Wang X, Liu Y, et al. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV 16E6/E7 viral oncogenes. Biochim Biophys Acta. 2002;1590:150–158. doi: 10.1016/s0167-4889(02)00208-2. [DOI] [PubMed] [Google Scholar]
- 29.Zeng X, Xiang JJ, Wu MH, et al. Circulating miR-17, miR-20a, miR-29c, and miR-223 combined as non-invasive biomarkers in nasopharyngeal carcinoma. Plos One. 2012;7:e46367. doi: 10.1371/journal.pone.0046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu N, Cui RX, Sun Y, et al. A four-miRNA signature identified from genome-wide serum miRNA profiling predicts survival in patients with nasopharyngeal carcinoma. Int J Cancer. 2014;134:1359–1368. doi: 10.1002/ijc.28468. [DOI] [PubMed] [Google Scholar]
- 31.Wyman SK, Parkin RK, Mitchell PS, et al. Repertoire of microRNAs in epithelial ovarian cancer as determined by next generation sequencing of small RNA cDNA libraries. PLoS One. 2009;4:e5311. doi: 10.1371/journal.pone.0005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soon PS, Tacon LJ, Gill AJ, et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res. 2009;15:7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 33.Patterson EE, Holloway AK, Weng J, et al. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia H, Ng SS, Jiang S, et al. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem Biophys Res Commun. 2010;391:535–41. doi: 10.1016/j.bbrc.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Deng T, Li X, et al. microRNA-141 is involved in a nasopharyngeal carcinoma-related genes network. Carcino-genesis. 2010;31:559–66. doi: 10.1093/carcin/bgp335. [DOI] [PubMed] [Google Scholar]
- 36.Wong TS, Man OY, Tsang CM, et al. MicroRNA let-7 suppresses nasopharyngeal carcinoma cells proliferation through down-regulating c-Myc expression. J Cancer Res Clin Oncol. 2011;137:415–422. doi: 10.1007/s00432-010-0898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CD, Kuo YS, Wu HC, et al. MicroRNA-1 induces apoptosis by targeting prothymosin alpha in nasopharyngeal carcinoma cells. J Biomed Sci. 2011;18:80. doi: 10.1186/1423-0127-18-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Lv XB, Wang XP, et al. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle. 2012;11:2495–506. doi: 10.4161/cc.20898. [DOI] [PubMed] [Google Scholar]
- 39.Yi C, Wang Q, Wang L, et al. MiR-663, a microRNA targeting p21(WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma. Oncogene. 2012;31:4421–33. doi: 10.1038/onc.2011.629. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LY, Ho-Fun Lee V, Wong AM, et al. MicroRNA-144 promotes cell proliferation, migration and invasion in nasopharyngeal carcinoma through repression of PTEN. Carcinogenesis. 2013;34:454–463. doi: 10.1093/carcin/bgs346. [DOI] [PubMed] [Google Scholar]