Abstract
There is no consensus as to what symptoms or quality-of-life (QOL) domains should be measured as patient-reported outcomes (PROs) in ovarian cancer clinical trials. A panel of experts convened by the National Cancer Institute reviewed studies published between January 2000 and August 2011. The results were included in and combined with an expert consensus-building process to identify the most salient PROs for ovarian cancer clinical trials. We identified a set of PROs specific to ovarian cancer: abdominal pain, bloating, cramping, fear of recurrence/disease progression, indigestion, sexual dysfunction, vomiting, weight gain, and weight loss. Additional PROs identified in parallel with a group charged with identifying the most important PROs across cancer types were anorexia, cognitive problems, constipation, diarrhea, dyspnea, fatigue, nausea, neuropathy, pain, and insomnia. Physical and emotional domains were considered to be the most salient domains of QOL. Findings of the review and consensus process provide good support for use of these ovarian cancer–specific PROs in ovarian cancer clinical trials.
Ovarian cancer is the leading cause of gynecologic cancer deaths in the United States (1). The majority of women are diagnosed with advanced disease. After extensive cytoreductive surgery followed by combination taxane/platinum–based chemotherapy (2,3), most women develop recurrent disease and are managed with palliative chemotherapy. The disease and its treatment give rise to a multitude of symptoms and substantial impairments in domains of quality of life (QOL). Knowledge of these symptoms and impairments should guide aggressive symptom management to improve patients’ overall and domain-specific QOL and monitor patients’ ability to tolerate and continue to receive treatment.
It is generally accepted that the valid assessment of symptoms and QOL impairments requires the use of patient-reported outcomes (PROs) (4,5). In a clinical trial, PROs are a means of evaluating treatment benefit or risk in a way that complements the typical primary outcome of survival (5). Currently, there is no consensus on which specific PROs (ie, symptoms and other QOL domains) should be assessed in cancer clinical trials, including trials in ovarian cancer. In recognition of this, the National Cancer Institute’s Symptom Management and Health-Related Quality of Life Steering Committee initiated independent reviews of the published literature by expert panels and a data-driven consensus-seeking process designed to culminate in recommendations for 1) a core set of symptoms to be assessed routinely in cancer clinical trials that include a PRO and 2) those symptoms and QOL domains to be routinely assessed in clinical trials for three specific cancer sites: ovary, head and neck, and prostate. This brief report summarizes the recommended core set of symptoms and QOL domains for ovarian cancer clinical trials.
The expert panel was comprised of patient representatives and health professionals from gynecologic oncology, medical oncology, nursing, and psychology. In this brief communication, we present methodological detail unique to deriving the core set of symptoms and QOL domains for ovarian cancer clinical trials. Detailed methods reflective of our data-driven, consensus-building process, including the multistakeholder clinical trial planning meeting and efforts before and after, are described in the companion article in this issue of the Journal by Reeve et al. (6).
Electronic database searches of journal articles from January 2000 through August 2011 were conducted. The principal medical subject heading search terms used were “ovarian neoplasms” AND “quality of life” OR “symptoms” OR “clinical trials (phase II–IV). Study abstracts were screened based on two eligibility criteria: 1) publication in a peer-reviewed, English language journal; 2) reporting of results based on the systematic assessment of patient-reported symptoms or QOL in ovarian cancer. The information extracted using a standardized form included study purpose and design, participant demographic and clinical characteristics, and PROs, including the outcome measures used, the prevalence and severity of symptoms assessed, and the QOL domains assessed.
A total of 1528 abstracts were identified, and 23 publications were ultimately included in the final analysis (Figure 1). Fourteen of these studies (7–20) originated in the United States, four studies (21–24) originated in Canada, four studies (25–28) originated in Europe, and one study (29) originated in Hong Kong. The stated purpose of the majority of studies was to evaluate the effect of active treatment on women’s symptom experience and/or QOL. The purpose of approximately one-third of the studies was to evaluate the psychometric properties of new or existing outcome measures of QOL and/or symptom experience in ovarian cancer. The remaining studies were observational in nature and aimed primarily to examine various psychosocial variables, including QOL and symptoms, in ovarian cancer patients or survivors. With respect to study design, the 23 publications included 17 longitudinal studies, five cross-sectional studies (10,11,14–16), and one study that pooled data across multiple time points (25). Among the longitudinal studies, three (12,13,20) were phase II clinical treatment trials, and 10 reported data from phase III trials (8,15–19,21,23,26,27).
Figure 1.
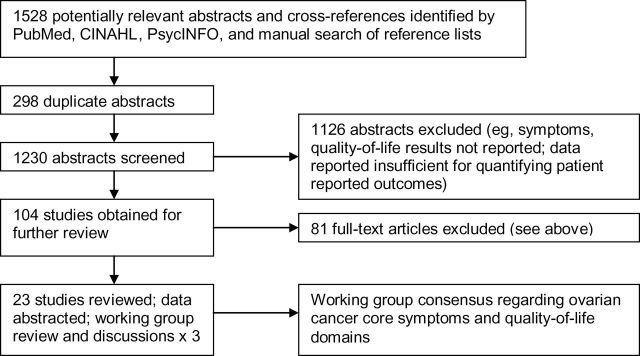
Study identification.
Sample sizes ranged from 17 to 502 patients. Ten studies (8–11,14,15,24,26,27,29) reported the sample’s mean age, with means ranging from 55 to 62 years, whereas eight studies (12,13,20–23,29) reported median age, ranging from 55 to 64 years. Across studies, the majority of patients had stage III or IV disease. Among the eight studies that included stage I disease in addition to stages II, III, and IV (8–10,14,24,25,27,28), all but one (27) were aimed at either evaluating a measure’s psychometric properties or examining select psychosocial variables. All of the studies involved women in active treatment with chemotherapy, either for initial, refractory, or recurrent disease. A minority of these studies involved women undergoing initial treatment for their disease. Fifteen of the 23 studies reported patients’ Eastern Cooperative Oncology Group performance status; the majority included patients with a performance status score between 0 and 2; four studies included a small percentage (between 0.5% and 7%) of patients with performance score 3 (requiring rest in bed or chair for more than half of the waking day) (7,11,21,27).
Across studies, the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system (30) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ) (31) family of outcome measures predominated. The most commonly administered outcome measure from the FACIT measurement system was the multidimensional Functional Assessment of Cancer Therapy (FACT)–Ovarian (FACT-O) (7), which is comprised of the FACT–General (FACT-G) (30) plus a 12-item ovarian cancer–specific scale. With respect to the EORTC-QLQ, six studies (22–24,26,27,29) used the core questionnaire (EORTC-QLQ-C30), a 30-item self-report measure (31) of QOL that includes both one-item and multi-item symptom scales, and three studies (21,25,28) used a version of the provisional ovarian cancer–specific module (EORTC-QLQ-OV28) (25), a subscale specific to ovarian cancer, in addition to the core questionnaire. Additionally, four studies (10,11,14,20) used either a commonly administered symptom checklist such as the Memorial Symptom Assessment Scale (32) or a symptom assessment measure such as the Symptom Representation Questionnaire for Assessing Cancer Symptoms (33).
Eight studies (10,11,14,15,20,22,25,28) supported the identification of particular symptoms based on prevalence and severity (see Supplementary Table 1, available online), including anorexia (appetite loss), dyspnea, fatigue, pain, cognitive problems, and insomnia, which are prevalent in ovarian cancer as well as other cancer types. We also identified abdominal symptoms, including abdominal pain, bloating, nausea, vomiting, constipation, cramping, indigestion, weight gain, and weight loss. This is consistent with the knowledge that abdominal symptoms are frequently associated with the presence of advanced disease (34). Finally, we identified neuropathy, sexual dysfunction, and fear of recurrence/disease progression as PROs that were noteworthy in terms of prevalence and/or severity in ovarian cancer. Whereas more general states such as anxiety or worry are prevalent across cancer types, fear of recurrence or progression in ovarian cancer seems to reflect the high likelihood of disease recurrence. Similarly, sexual problems, although not uncommon in other types of cancer, in ovarian cancer reflect the anatomical and physiological realities of the disease and its treatment. These ovarian cancer–specific PROs and the symptoms common to most cancers also were supported in the literature by several studies (8,11,17–19,22,23,26,27) focused on describing the effects of different treatment protocols on symptom experience and QOL. Still other studies (8,9,12–14,16,22) identified these symptoms as having a meaningful effect on function or QOL.
Eleven studies (8,9,12,13,15,16,18–21,26) identified physical and emotional well-being domains of QOL as especially salient to women with ovarian cancer. Overall QOL was also differentially affected depending on the cancer treatment type (19,23), where worse physical and functional well-being had an adverse effect on overall QOL (9,12,13,16,22).
We used a data-driven, consensus-building process to identify the most important symptoms for women with ovarian cancer. Ten of these—anorexia, constipation, diarrhea, dyspnea, fatigue, nausea, neuropathy, pain, cognitive problems, and insomnia—are included in a core set of 12 symptoms identified in a parallel process by the group charged with identifying the most important symptoms across cancer types. We also identified several ovarian cancer–specific symptoms: abdominal pain, bloating, cramping, fear of recurrence, indigestion, sexual dysfunction, vomiting, weight gain, and weight loss. We also identified physical and emotional domains of QOL that appear to be most commonly affected by treatments for ovarian cancer. The panel recommends that researchers and clinicians consider including these symptoms and QOL domains in future ovarian cancer clinical trials.
Our review focused primarily on symptoms and other QOL domains among women with advanced disease, and chemotherapeutic trials for advanced ovarian cancer predominated. However, treatment continues to evolve, and we should anticipate that the nature of symptoms and QOL domains affected will evolve as well. Novel agents, beyond conventional cytotoxic chemotherapy, are increasingly being incorporated into the treatment paradigm (35), and exposure to biologic agents, for example, may result in a distinctly different profile of side effects than those typically seen with ovarian cancer treatment. For example, bevacizumab, an agent targeted at vascular endothelial growth factor, is associated with hypertension, proteinuria, and intestinal perforation. These adverse effects, uncommon among more conventional agents, may have substantial acute and chronic sequelae and a relatively unique impact on QOL. Other antiangiogenesis agents may be associated with other adverse effects, including peripheral edema and encephalopathy, effects not commonly associated with ovarian cancer treatment. Other targeted therapies, including poly ADP-ribose polymerase inhibitors, multikinase inhibitors, insulin-like growth factor inhibitors, and monoclonal antibodies, are currently under investigation. As experience with these agents grows, so, too, will our knowledge of the prevalence and severity of symptoms and QOL impairments associated with these therapies.
The lack of data associated with biologic and other evolving therapies and the predominance in the literature of studies involving advanced disease are but two limitations inherent in this work. Further, we recognize that any final selection of PROs for a trial will be driven by study-specific hypotheses, concerns related to patient burden, and available clinical trial resources [for a complete discussion of limitations and implementation considerations, see the companion article by Reeve et al. (6)]. Nevertheless, the identification of a core set of symptoms and QOL domains to be assessed in ovarian cancer clinical trials represents an important step forward in the science of PRO measurement by promoting the potential for increased consistency across trials.
Funding
This work was supported by the National Cancer Institute.
The authors have no conflicts of interest to report.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29 [DOI] [PubMed] [Google Scholar]
- 2. Bookman MA. Standard treatment in advanced ovarian cancer in 2005: the state of the art. Int J Gynecol Cancer. 2005;15(Suppl 3):212–220 [DOI] [PubMed] [Google Scholar]
- 3. Ozols RF. Maintenance therapy in advanced ovarian cancer: progression-free survival and clinical benefit. J Clin Oncol. 2003;21(13):2451–2453 [DOI] [PubMed] [Google Scholar]
- 4. Cleeland CS, Sloan JA, Group AO. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manage. 2010; 39 (6):1077–1085 [DOI] [PubMed] [Google Scholar]
- 5. US Department of Health & Human Services Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf
- 6. Reeve BB, Mitchell SA, Dueck AC, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst. 2014;XX(XX):XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19(6):1809–1817 [DOI] [PubMed] [Google Scholar]
- 8. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13(6):741–748 [DOI] [PubMed] [Google Scholar]
- 9. de Moor JS, de Moor CA, Basen-Engquist K, Kudelka A, Bevers MW, Cohen L. Optimism, distress, health-related quality of life, and change in cancer antigen 125 among patients with ovarian cancer undergoing chemotherapy. Psychosom Med. 2006;68(4):555–562 [DOI] [PubMed] [Google Scholar]
- 10. Donovan HS, Hartenbach EM, Method MW. Patient-provider communication and perceived control for women experiencing multiple symptoms associated with ovarian cancer. Gynecol Oncol. 2005;99(2):404–411 [DOI] [PubMed] [Google Scholar]
- 11. Jensen SE, Rosenbloom SK, Beaumont JL, et al. A new index of priority symptoms in advanced ovarian cancer. Gynecol Oncol. 2011;120(2):214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kushner DM, Connor JP, Sanchez F, et al. Weekly docetaxel and carboplatin for recurrent ovarian and peritoneal cancer: a phase II trial. Gynecol Oncol. 2007;105(2):358–364 [DOI] [PubMed] [Google Scholar]
- 13. Schmeler KM, Vadhan-Raj S, Ramirez PT, et al. A phase II study of GM-CSF and rIFN-gamma1b plus carboplatin for the treatment of recurrent, platinum-sensitive ovarian, fallopian tube and primary peritoneal cancer. Gynecol Oncol. 2009;113(2):210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Supp Care Cancer. 2005;13(4):219–227 [DOI] [PubMed] [Google Scholar]
- 15. von Gruenigen VE, Huang HQ, Gil KM, et al. Assessment of factors that contribute to decreased quality of life in gynecologic oncology group ovarian cancer trials. Cancer. 2009;115(20):4857–4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Gruenigen VE, Huang HQ, Gil KM, et al. A comparison of quality-of-life domains and clinical factors in ovarian cancer patients: a Gynecologic Oncology Group study. J Pain Symptom Manage. 2010;39(5):839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wenzel L, Huang HQ, Cella D, Walker JL, Armstrong DK. Validation of FACT/GOG-AD subscale for ovarian cancer-related abdominal discomfort: a Gynecologic Oncology Group study. Gynecologic Oncology. 2008;110(1):60–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wenzel L, Huang HQ, Monk BJ, Rose PG, Cella D. Quality-of-life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23(24):5605–5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(4):437–443 [DOI] [PubMed] [Google Scholar]
- 20. Wolf JK, Bodurka DC, Verschraegen C, et al. A phase II trial of oral capecitabine in patients with platinum—and taxane—refractory ovarian, fallopian tube, or peritoneal cancer. Gynecol Oncol. 2006;102(3):468–474 [DOI] [PubMed] [Google Scholar]
- 21. Bezjak A, Tu D, Bacon M, et al. Quality of life in ovarian cancer patients: comparison of paclitaxel plus cisplatin, with cyclophosphamide plus cisplatin in a randomized study. J Clin Oncol. 2004;22(22):4595–4603 [DOI] [PubMed] [Google Scholar]
- 22. Doyle C, Crump M, Pintilie M, Oza AM. Does palliative chemotherapy palliate? Evaluation of expectations, outcomes, and costs in women receiving chemotherapy for advanced ovarian cancer. J Clin Oncol. 2001;19(5):1266–1274 [DOI] [PubMed] [Google Scholar]
- 23. Hirte H, Vergote IB, Jeffrey JR, et al. A phase III randomized trial of BAY 12–9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy: a National Cancer Institute of Canada Clinical Trials Group Study. Gynecol Oncol. 2006;102(2):300–308 [DOI] [PubMed] [Google Scholar]
- 24. Lakusta CM, Atkinson MJ, Robinson JW, Nation J, Taenzer PA, Campo MG. Quality of life in ovarian cancer patients receiving chemotherapy. Gynecol Oncol. 2001;81(3):490–495 [DOI] [PubMed] [Google Scholar]
- 25. Greimel E, Bottomley A, Cull A, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39(10):1402–1408 [DOI] [PubMed] [Google Scholar]
- 26. Greimel ER, Bjelic-Radisic V, Pfisterer J, Hilpert F, Daghofer F, du Bois A. Randomized study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group comparing quality of life in patients with ovarian cancer treated with cisplatin/paclitaxel versus carboplatin/paclitaxel. J Clin Oncol. 2006;24(4):579–586 [DOI] [PubMed] [Google Scholar]
- 27. Sehouli J, Stengel D, Oskay-Oezcelik G, et al. Nonplatinum topotecan combinations versus topotecan alone for recurrent ovarian cancer: results of a phase III study of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2008;26(19):3176–3182 [DOI] [PubMed] [Google Scholar]
- 28. Cull A, Howat S, Greimel E, et al. Development of a European Organization for Research and Treatment of Cancer questionnaire module to assess the quality of life of ovarian cancer patients in clinical trials: a progress report. Eur J Cancer. 2001;37(1):47–53 [DOI] [PubMed] [Google Scholar]
- 29. Chan YM, Ng TY, Ngan HY, Wong LC. Quality of life in women treated with neoadjuvant chemotherapy for advanced ovarian cancer: a prospective longitudinal study. Gynecol Oncol. 2003;88(1):9–16 [DOI] [PubMed] [Google Scholar]
- 30. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579 [DOI] [PubMed] [Google Scholar]
- 31. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376 [DOI] [PubMed] [Google Scholar]
- 32. Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326–1336 [DOI] [PubMed] [Google Scholar]
- 33. Donovan HS, Ward S, Sherwood P, Serlin RC. Evaluation of the Symptom Representation Questionnaire (SRQ) for assessing cancer- related symptoms. J Pain Symptom Manage.2008;35(3):242–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goff BA, Mandel LS, Melancon CH, Muntz HG. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291(22):2705–2712 [DOI] [PubMed] [Google Scholar]
- 35. Han ES, Lin P, Wakabayashi M. Current status on biologic therapies in the treatment of epithelial ovarian cancer. Curr Treat Options Oncol. 2009;10(1–2):54–66 [DOI] [PubMed] [Google Scholar]


