Abstract
Background
Retrospective studies have demonstrated high response rates among patients with advanced pancreatic neuroendocrine tumors (PNETs) treated with capecitabine and temozolamide (CapTem), while responses are infrequently seen among non-PNETs. The objective of the study was to describe progression free survival (PFS) among neuroendocrine tumor (NET) patients treated with CapTem, and to identify factors associated with better activity.
Methods
Patients who were referred to one of five provincial cancer treatment centers between 2009 and 2013 for advanced NETs and initiated CapTem were included. Patients received Cap 1,500 mg/m2 on days 1-14 and TMZ 200 mg/m2 on days 10-14 every 28 days. Their characteristics and outcomes were retrospectively analyzed.
Results
In our cohort, 29 patients (16 males) with a median age of 59 (range 26-76) received palliative CapTem, 15 of them as first-line chemotherapy. Primary tumors included pancreas (48.3%), small bowel (20.7%), lung (10.3%), unknown (10.3%), rectum (6.9%) and appendix (3.4%). Median number of cycles was three. Fifteen patients (51.7%) received CapTem as first-line chemotherapy and 14 (48.3%) as subsequent lines. Median PFS for the entire cohort was 4.7 months. PNETs had a median PFS of 4.9 months compared to 2.8 months for non-PNETs (P=0.178). Patients with PNETs who received CapTem in the first-line setting had a median PFS of 15.9 months as compared to only 3.1 months for the remainder [P=0.047, hazard ratios (HR) 0.342]. Patients with Ki67 above 5% and ≤5% had median PFS of 4.0 and 4.7 months, respectively (P=0.260).
Conclusions
CapTem showed good activity among PNETs, but its broader role in the treatment of carcinoid tumors remains unclear.
Keywords: Carcinoid tumors, pancreatic neuroendocrine tumors (PNETs), pancreatic endocrine tumors, carcinomas, chemotherapy
Introduction
Neuroendocrine tumors (NETs), defined as epithelial neoplasms with predominant neuroendocrine differentiation, are rare cancers which can arise in multiple organs. These tumors have unique clinical and biological behavior according to the site of origin, hormone production and histological differentiation (1). Recent evidence supports the notion that pancreatic NETs (PNETs) are more sensitive to cytotoxic chemotherapy than other types of neuroendocrine malignancies, including carcinoid tumors of the small intestine (2).
Streptozocin-based combination therapy was once considered the standard treatment for patients with advanced PNETs, however, the toxicity profile associated with streptozocin has limited its incorporation into clinical practice (3,4). Temozolomide is a less toxic orally active alkylating agent, which has also showed activity among PNETs (4-7). One of those trials combined temozolamide with thalidomide and demonstrated an objective response rate (RR) of 45% in PNETs versus only 7% in metastatic carcinoid tumors (6). More recently, a retrospective study involving 30 patients with advanced PNETs revealed an encouraging RR of 70% when temozolamide was combined with capecitabine (8). Unfortunately little is known regarding the efficacy of this latter regimen among other NETs.
The benefit of chemotherapy for non-PNETs remains debatable, particularly for gastrointestinal primaries. Streptozocin, 5-fluorouracil (5-FU), and doxorubicin have already been tested as monotherapy in metastatic carcinoid tumors, but demonstrated only modest activity (9). Although single agent capecitabine did not lead to an objective response in a small phase II conducted among 19 patients with carcinoid tumors (12 from small bowel, one from ovary, and six unknown primary), 68% achieved stable disease radiographically, including 4 with long-lasting disease stability for over 12 months (10). Temozolamide was also studied in a retrospective series that included 44 carcinoid tumor patients treated with temozolomide-based regimens. Disappointingly, only one (2%) had an objective tumor response (11). While the majority of these patients had primary gastrointestinal carcinoids, the only patient with objective response had a bronchial primary.
So far, the combination of capecitabine and temozolamide (CapTem) has not been studied among non-PNETs in randomized trials. The aims of the present study were to investigate the activity of CapTem among all NETs and to explore whether primary tumor location (PNETs versus non-PNETs) matters for progression free survival (PFS) among our population of patients.
Patients and methods
The British Columbia Cancer Agency is a multi-centre institution treating the majority of oncology patients for the province of British Columbia, Canada. It consists of five major cancer centers and their satellite clinics. All patients with metastatic NETs who received at least one cycle of CapTem from July 2009 to January 2013 in our institution were identified using the pharmacy database. Patients were eligible if they had biopsy proven metastatic NET with measurable disease based on either computed tomography (CT) or magnetic resonance imaging (MRI), had radiological evidence of disease progression prior to starting CapTem, and had initiated at least one cycle of CapTem. In our institution, CapTem is only approved for patients with adequate bone marrow reserve (neutrophils greater than or equal to 1.5×109/L, platelets greater than 100×109/L), renal function (creatinine less than or equal to 1.5× upper limit normal), and liver function (bilirubin less than or equal to 35 micromol/L; aspartate aminotransferase (AST)/alkaline phosphatase less than or equal to 5× upper limit normal). Moreover, the baseline Eastern Cooperative Oncology Group (ECOG) performance status should not be equal or greater than 2.
Treatment consisted of capecitabine 1,500 mg/m2 on days 1-14 and temozolamide 200 mg/m2 on days 10-14 every 28 days until disease progression, unacceptable toxicities or patient preference. Complete blood counts, creatinine and liver function tests were obtained before each chemotherapy cycle in all patients. For patients on warfarin, weekly international normalized ratio (INR) was also obtained until a stable warfarin dose was established. Baseline demographics, tumor characteristics and treatment details were abstracted to an anonymous database and analyzed. Toxicity information was collected retrospectively from the medical records. This study was approved by the local Institutional Review Board.
Statistical analysis
Statistical analysis was performed using SPSS version 14.0 for Windows® (SPSS, Chicago, IL, USA). Overall survival (OS) was calculated in months from the time of initiation of CapTem to date of death or last follow-up. PFS was calculated from the time of initiation of CapTem to date of disease progression based on local radiologist assessment, death or last follow-up. Kaplan-Meier (KM) curves for PFS and OS were generated. The log-rank test was used to assess statistical differences among variables and P values <0.05 were considered statistically significant. Multivariable survival analyses were performed using Cox-proportional hazards models in order to explore the effect of variables on PFS. Hazard ratios (HR) and 95% confidence intervals were calculated to estimate risk of progression.
Results
In our cohort, 29 patients (16 males and 13 females) with a median age of 59 (range, 26-76) began palliative CapTem. Their baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics.
| Number | % | |
|---|---|---|
| Age (median) | 59 | |
| Gender | ||
| Male | 16 | 55.2 |
| Female | 13 | 44.8 |
| Ethnicity | ||
| Caucasian | 28 | 96.6 |
| African-American | 1 | 3.4 |
| Primary | ||
| Pancreas | 14 | 48.3 |
| Small bowel | 6 | 20.7 |
| Unknown | 3 | 10.3 |
| Lung | 3 | 10.3 |
| Rectum | 2 | 6.9 |
| Appendix | 1 | 3.4 |
| Metastatic sites | ||
| Liver | 27 | 93.1 |
| Lymph nodes | 17 | 58.6 |
| Peritoneum | 6 | 20.7 |
| Bones | 6 | 20.7 |
| Lungs | 5 | 17.2 |
| Orbit | 1 | 3.4 |
| Heart | 1 | 3.4 |
| Chromogranin A (median) | 98 | |
| Ki67 (median) | 5 |
Primary tumor location included pancreas (48.3%), small bowel (20.7%), lung (10.3%), unknown (10.3%), rectum (6.9%) and appendix (3.4%). Median chromogranin A was 98 (range, 9-6,000) while median ki67 was 5% (range, 1-40). Ki67 was missing in 4 patients. Among the 25 patients with available ki67, 16 (64%) had ki67 ≤5% while 9 (36%) had ki67 above 5%. Only 3 patients had ki67 above 20%. The most common metastatic sites were liver (93.1%), lymph nodes (58.6%), peritoneum (20.7%), bones (20.7%) and lungs (10.3%).
Six patients (20.7%) had received previous liver-limited therapies: three patients were treated with chemoembolization (10.3%), two underwent liver surgery as well as radiofrequency ablations (6.9%), and one received radioembolization with Yttrium-90 (3.4%) (Table 2).
Table 2. Treatment characteristics.
| Number | % | |
|---|---|---|
| CapTem as first-line therapy | ||
| Yes | 15 | 51.7 |
| No | 14 | 48.3 |
| Prior systemic therapies | ||
| Cisplatin + etoposide | 6 | 20.7 |
| Streptozocin + doxorubicin | 2 | 6.9 |
| Everolimus | 2 | 6.9 |
| I-131 mIBG | 2 | 6.9 |
| Streptozocin + 5-FU | 1 | 3.4 |
| Sunitinib | 1 | 3.4 |
| Carboplatin + paclitaxel | 1 | 3.4 |
| Carmustine + 5-FU | 1 | 3.4 |
| Prior octreotide-Lar | ||
| Yes | 16 | 55.2 |
| 5-FU, 5-fluorouracil. |
In terms of prior systemic therapies, 6 patients (20.7%) had already received cisplatin and etoposide, 2 (6.9%) had streptozocin and doxorubicin, 2 (6.9%) had everolimus, 2 (6.9%) had I-131-meta-iodobenzylguanidine sulfate (I-131 mIBG), 1 (3.4%) had carboplatin and paclitaxel, 1 (3.4%) had carmustine and 5-FU and 1 (3.4%) had sunitinib. Moreover, 16 patients (55.2%) were previously treated with somatostatin analogs. Fifteen patients (51.7%) received CapTem as first-line chemotherapy and 14 (48.3%) as subsequent lines (Table 2). The median number of cycles was 3 (range, 1-31).
For the entire cohort, median PFS and OS were 4.7 (95% CI, 3.11-6.28) and 20.2 months (95% CI, 9.02-33.37), respectively (Figures 1,2). PNETs had median OS of 18.8 months (versus not reached for non-PNETs, P=0.37), while their PFS was 4.9 months (versus 2.8 months for non-PNETs, P=0.178, HR 0.25) (Figure 3). Six patients (20.7%) were progression-free at 12 months (4 pancreatic, 1 rectal and 1 unknown) while 3 of them remained progression-free at 24 months. Patients with ki67 above 5% had a median PFS of 4.0 versus 4.7 months when compared to the group with lower ki67 (P=0.260, HR 1.683) (Figure 4). Even after excluding patients with ki-67 above 20 or unknown, median PFS remained 4.7 months (95% CI, 1.59-7.80).
Figure 1.
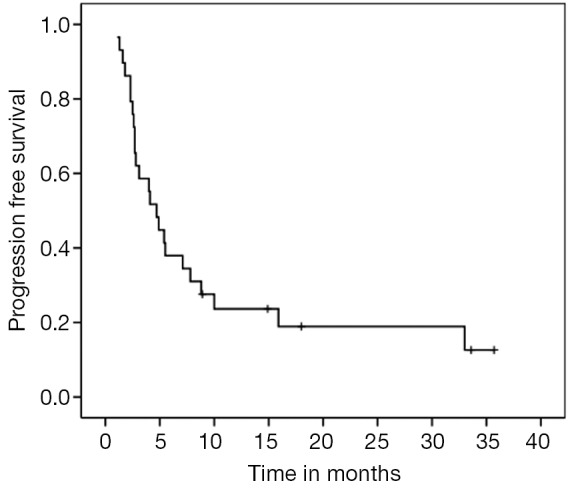
PFS for the entire cohort (median 4.7 months, 95% CI, 3.11-6.28). PFS, progression free survival.
Figure 2.
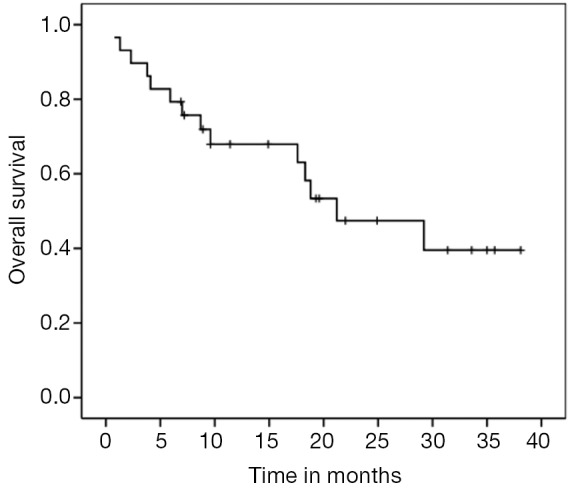
OS for the entire cohort (median 20.2 months, 95% CI, 9.02-33.37). OS, overall survival.
Figure 3.
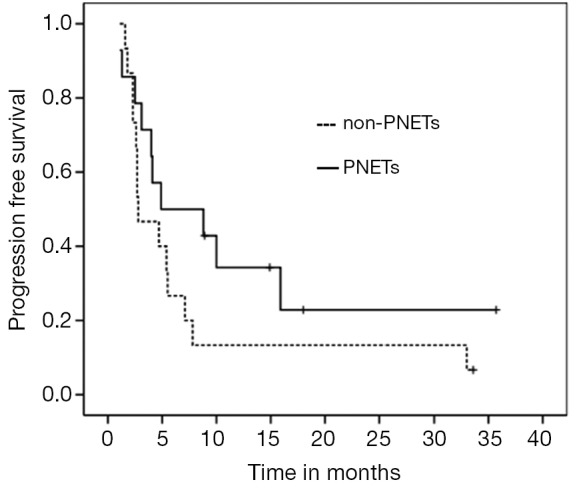
PFS according to tumor location-PNETs vs. non-PNETs (P=0.178, HR 0.25). PFS, progression free survival; PNETs, pancreatic neuroendocrine tumors; HR, hazard ratios.
Figure 4.
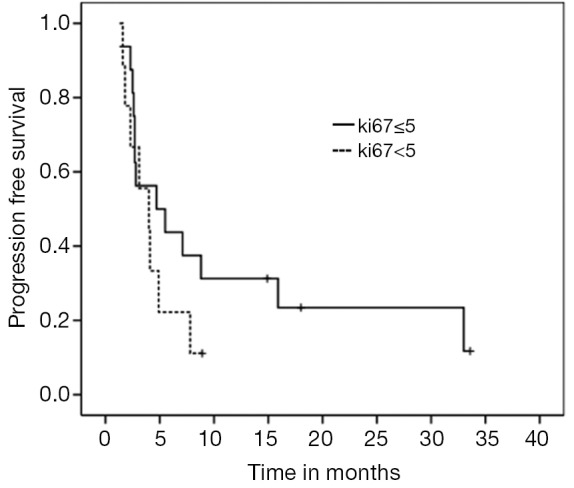
PFS according to ki67 ≤5 versus >5 (P=0.26, HR 0.417). PFS, progression free survival; HR, hazard ratios.
Those with chromogranin A above 100 ng/mL before starting CapTem had a median PFS of 4.1 versus 4.7 months when compared to patients with lower values (P=0.636, HR 1.215). Women had a median PFS of 4.9 months (versus 4.0 months for men, P=0.521, HR 0.767). In addition, patients who received CapTem as part of their initial systemic therapy had a median PFS of 4.7 months while the group who received it in subsequent lines achieved 4.1 months (P=0.333, HR 0.669). When we analyzed the subset of patients with PNETs who received CapTem as first-line chemotherapy, their median PFS was significantly longer when compared to the remainders (15.9 versus 3.1 months, P=0.047, HR 0.342) (Figure 5).
Figure 5.
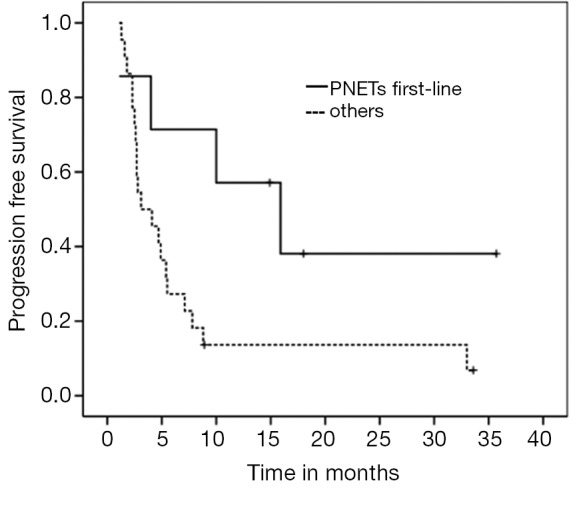
PFS for PNETs in 1st-line versus others (P=0.047, HR 0.342). PFS, progression free survival; PNETs, pancreatic neuroendocrine tumors; HR, hazard ratios.
CapTem was generally well-tolerated. Only three patients had to discontinue treatment due to poor tolerance (two due to intractable nausea and one due to myocardial infarction). There were no treatment-related deaths. Rates of dose reduction and treatment delay were not captured during this retrospective analysis.
Discussion
The results of our study support the prior retrospective study by confirming activity of CapTem among PNETS. In the previous study an objective radiographic response rate of 70% among patients with metastatic PNETs treated with front-line CapTem was observed while their median PFS was 18 months (8). Although we have not assessed tumor response, a similar PFS for patients with PNETs treated first-line CapTem was obtained (15.9 months). However, much less activity was observed among PNETs in subsequent lines of therapy or non-PNETs with a median PFS of only 3.1 months. When PNETs were compared to non-PNETs regardless of lines of therapy, there was a numerical trend towards longer PFS, but it did not reach statistical significance due to the small number of patients (4.9 versus 2.8, P=0.178).
Investigators from an ongoing phase II study have recently reported their interim analysis results in which 28 patients with metastatic well-moderately differentiated NET (ki-67 ≤20%), including those with octreotide scan positive who had shown progression on 60 mg octreotide LAR, were treated with CapTem (12). Of note, temozolamide was given twice a day on days 10 to 14. They reported an overall RR of 43% with a surprising RR of 41% among carcinoid tumors. Median PFS for their entire cohort was above 20 months while patients with carcinoid tumors had median PFS above 22 months (12). This differs significantly from our findings, as the non-PNETs PFS of 2.8 months achieved by our cohort is much shorter than that reported in the above trial. We should note that even when excluding the three patients with ki67 above 20% as well as the four patients with unknown ki67, the PFS remained low at 4.7 months. It is unclear why such a disparity exists, but it certainly warrants further investigation.
Apoptotic synergism for the combination of CapTem in NET cell lines has been demonstrated in vitro, although the precise mechanism remains unknown (12). One potential hypothesis is that the DNA damage induced by capecitabine given in the first place, by incorporation of 5-FdUTP into DNA and reducing thymidine pools by inhibition of thymidylate synthase via 5-FdUMP, synergistically enhances the effect of subsequent temozolomide on O6-alkylguanyl alkyl-transferase (O6-AGAT) (13). Capecitabine may deplete the DNA repair enzyme O6 methylguanine DNA methyltransferase (MGMT) making it more sensitive to the antiproliferative effects of temozolamide (14).
MGMT deficiency, measured by immunohistochemistry, was more common in pancreatic NETs (51%) than in carcinoid tumors (0%) in a retrospective analysis of 97 archival specimens and this may justify why treatment response to CapTem was more prominent in PNETs as compared to non-PNETs in our study (15). However, the reason why well-differentiated non-PNETs also derive less benefit from other systemic chemotherapy when compared to PNETs remains uncertain. Despite the common belief that chemotherapy works better among less well-differentiated tumors (16), our analysis showed no difference in PFS according to Ki67 levels.
Recently inhibitors of signaling pathways targeting vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin (mTOR) have demonstrated considerable activity in two phase III trials of advanced PNETs. Median PFS was 11 months with everolimus as compared to only 4.6 months with placebo (HR 0.35, P<0.001) in the RADIANT-3 trial (17). Sunitinib showed similar activity with a PFS of 11.4 months versus 5.5 months with placebo (HR 0.42, P<0.001) (18). Both drugs are currently being employed as first-line options in advanced PNETs worldwide. Although comparison between studies are not prudent, CapTem seems to offer a longer PFS with better response rate and should therefore be tested against either sunitinib or everolimus in the first-line setting.
Our study has some limitations inherent to all retrospective analyses, especially the potential for selection bias associated with information from chart reviews. Moreover, due to the rarity of metastatic NETs, our sample size of patients receiving CapTem was small and statistical significance could not be obtained for the majority of our analyses. Adverse events related to CapTem could not be extensively reviewed, which would otherwise increase the strength of the present study. Nonetheless, our results are comparable with the published retrospective study of CapTem for PNETs (8), reassuring its activity among those patients. Our data also casts into doubt the activity of CapTem among non-PNETs.
Conclusions
In summary, the combination of CapTem showed promising activity in metastatic PNETs. Randomized clinical trials comparing CapTem versus single-agent everolimus or sunitinib are necessary to establish a standard of care for this rare malignancy. The role of CapTem in patients with progressive non-PNET metastatic carcinoid remains unclear and those patients should be considered for enrollment in trials testing different systemic therapy strategies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Hauser H, Wolf G, Uranüs S, et al. Neuroendocrine tumours in various organ systems in a ten-year period. Eur J Surg Oncol 1995;21:297-300 [DOI] [PubMed] [Google Scholar]
- 2.Kvols LK, Buck M. Chemotherapy of endocrine malignancies: a review. Semin Oncol 1987;14:343-53 [PubMed] [Google Scholar]
- 3.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519-23 [DOI] [PubMed] [Google Scholar]
- 4.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71 [DOI] [PubMed] [Google Scholar]
- 5.Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 1987;47:5846-52 [PubMed] [Google Scholar]
- 6.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol 2006;24:401-6 [DOI] [PubMed] [Google Scholar]
- 7.Chan JA, Stuart K, Earle CC, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol 2012;30:2963-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999;340:858-68 [DOI] [PubMed] [Google Scholar]
- 10.Medley L, Morel AN, Farrugia D, et al. Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocrine tumours. Br J Cancer 2011;104:1067-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 2009;15:338-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine RL, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol 2014;32:abstr 179.
- 13.Fine RL, Fogelman DR, Schreibman MS. Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. J Clin Oncol 2005;23:abstr 4216.
- 14.Murakami J, Lee YJ, Kokeguchi S, et al. Depletion of O6-methylguanine-DNA methyltransferase by O6-benzylguanine enhances 5-FU cytotoxicity in colon and oral cancer cell lines. Oncol Rep 2007;17:1461-7 [PubMed] [Google Scholar]
- 15.Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 2009;15:338-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moertel CG, Kvols LK, O’Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227-32 [DOI] [PubMed] [Google Scholar]
- 17.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13 [DOI] [PubMed] [Google Scholar]


