Abstract
There is growing evidence that obesity is a risk factor of cancer incidence and mortality. Hence, the identification of the mechanistic links between obesity and cancer progression is emerging as a topic of widespread interest. Recently, several groups have addressed the functional roles of leptin, an adipocyte-derived adipokine, for mammary tumor progression. In this issue of Endocrine-Related Cancer, Zheng et al. study the role of leptin on tumor growth in a xenograft model of MMTV-Wnt1-derived cancer cells. They study growth of these cancer cells in the context of obese animals, such as ob/ob mice (lacking leptin) and db/db mice (lacking functional leptin receptors (LEPR)) and find that leptin triggers LEPR-positive cancer stem cell differentiation, thereby promoting tumor cell survival. These findings highlight the therapeutic potential for leptin and leptin signaling in the context of mammary tumor growth.
Introduction
A large number of epidemiological studies on cancer incidence link the propensity to develop certain types of cancers (e.g. colon, thyroid, esophagus, renal, endometrial, and postmenopausal breast cancer) with an individual's excess body fat/obesity (Bianchini et al. 2002, Calle et al. 2003). Adipokines, i.e. adipocyte-derived secretory proteins, represent likely candidates to mediate at least in part the increased cancer risk and enhanced progression associated with obesity. Other contributors to obesity-related cancer progression are the insulin/IGF1 pathways and sex hormones (summarized in Park et al. (2011a)). Among the adipokines, leptin is the most intensively studied factor, both in metabolism in general and in obesity-related cancers due to the fact that leptin levels increase in proportion to fat mass. In this issue of Endocrine-Related Cancer, Zheng et al. (2011) show that tumor cells derived from a widely used mammary tumor model, the MMTV-Wnt1 mouse, grow less effectively in leptin-deficient, obese mice (generally referred to as ‘ob/ob mice’) compared with obese mice with an intact leptin pathway. This suggests that leptin signaling plays an essential role in MMTV-Wnt1 tumor cell growth and survival. They conclude that these leptin effects are mediated through a comprehensive set of responses of leptin receptor (LEPR)-positive tumor cells that include a cancer stem cell (CSC) population defined by characteristic cell surface markers that expresses the LEPR as well (Zheng et al. 2011).
Leptin: just in your head?
Leptin is encoded by the ob gene and is a well-established adipokine influencing appetite control and energy expenditure through its actions on the hypothalamus and other regions in the brain where LEPR are highly expressed (Frederich et al. 1995, Halaas et al. 1995, Vaisse et al. 1996). A single transcript encoded by the db gene produces at least five different variants of the LEPR protein through alternative splicing (Lee et al. 1996). However, only the long form of LEPR-B has a cytoplasmic domain that transduces the leptin-mediated downstream signaling events, such as activation of the PI3K, ERK1/2, and Jak2/Stat3 pathways (Baumann et al. 1996, Morris & Rui 2009). In addition to the neuronal actions, leptin also exerts other physiological responses in peripheral tissues. These include effects on the immune response, angiogenesis, reproduction, and an intracellular cross talk with signaling pathways of growth hormones, such as insulin, and lipid metabolism pathways (Sierra-Honigmann et al. 1998, Margetic et al. 2002, Muoio & Lynis Dohm 2002). Adipose tissue with its well-appreciated endocrine functions takes advantage of leptin as a potent signaling molecule that profoundly impacts multiple peripheral tissues. Tumor tissues have been demonstrated to have cells that are leptin responsive, including tumor cells. A number of reports indicate that LEPR are highly abundant in many tumor tissues compared with benign or normal tissues. Leptin-responsive tumors include mammary carcinoma, pancreatic tumor, and gastrointestinal tumors, such as esophageal, gastric, and colon cancer cells (Ishikawa et al. 2004, Garofalo et al. 2006, Howard et al. 2010).
Leptin and the breast cancer axis
Leptin is a growth factor that plays an important role in development, differentiation, and cell growth under normal physiological conditions. It affects a number of cell types, including neuronal cells, immune cells, pancreatic β-cells, endothelial cells, and adipocytes. Leptin exerts its effects through LEPR-B-mediated downstream pathways, such as PI3K, ERK1/2, and Jak2/Stat3 (Morris & Rui 2009). With respect to breast cancer, leptin is an attractive target due to its involvement in cell proliferation, migration, and invasion, giving rise to more aggressive and metastatically more potent tumor cells (Cirillo et al. 2008). In vitro studies using human breast cancer cell lines indicate that differential leptin responses among various cell lines may primarily depend on receptor levels of LEPR-B among those cell lines studied.
Numerous attempts have been made to evaluate leptin effects on breast cancer progression in vivo. Genetic loss-of-function mutants for leptin or the LEPR (i.e. ob/ob or db/db mice) develop systemic metabolic abnormalities that include obesity, diabetes, infertility, and immune defects (Friedman 2009). Manipulation of leptin levels with these genetic mouse models in MMTV–transforming growth factor α (TGFα) mice failed to develop mammary tumors because these mice completely lack a ductal mammary epithelium. As most tumors arise from the ductal epithelium, it is not possible to use these mice for the study of mammary tumor development (Cleary et al. 2003, 2004). Recently, more compelling in vivo evidence was provided with a hypothalamic LEPR-B reconstitution in db/db mice (db/dbNse+/+), which restores metabolic abnormalities in db/db mice, such as obesity, diabetes, and infertility. These mice also develop a normal mammary epithelium (Chua et al. 2004). Therefore, they can be crossed with a mammary tumor model, such as the MMTV-PyMT mouse. Results from these crosses suggest that an LEPR-B-mediated signal promotes tumor growth and metastasis. Cancer cell metabolism is affected in these mice by orchestrating downstream pathways, such as PI3K, ERK1/2, and Jak2/STAT3 (Park et al. 2011b). In addition, diet-induced obese mouse models have been used to modulate leptin levels in vivo. From these diet studies, it is apparent that mammary tumors grow faster under high-fat diet conditions. Consistent with a possible involvement of leptin, obese MMTV–TGFα mice do indeed display elevated circulating leptin levels (Dogan et al. 2007). However, for obvious reasons, it is challenging to discern distinct leptin effects from other metabolic changes associated with obesity-induced metabolic dysregulation.
Xenografts of MMTV-Wnt1 breast cancer cells transplanted into diet-induced obese mice (which maintain high levels of circulating leptin over prolonged periods of time) grow faster, in further support of a tight association between obesity and mammary tumor growth (Nunez et al. 2008). To assess whether this obesity-associated increase is solely due to leptin or a combined effect of other metabolic parameters that change under these conditions, Zheng et al. (2011) in this issue of Endocrine-Related Cancer demonstrate that xenografts of MMTV-Wnt1 cancer cells transplanted into leptin-deficient obese mice (ob/ob) displayed a stunted tumor growth. In contrast, transplants into obese db/db mice (lacking the LEPR and as a result displaying high leptin levels) augmented tumor growth. This is a clear indication that tumor cell behavior heavily relies on leptin signaling, even if cancer cells are exposed to other mitogenic signals and excessive nutrients, such as hyperinsulinemic, hyper-glycemic, and hyperlipidemic conditions prevailing in obesity (Zheng et al. 2011).
LEBR-B+ cell populations in mammary tumor tissues
Cancer progression is a multistep process that involves tumor initiation, primary tumor growth, invasion, and metastasis, with minimally the latter three relying on interactions with stromal tumor components that include endothelial cells, immune cells, fibroblasts, and adipocytes (Hanahan & Weinberg 2011). The mechanistic details underlying the association between obesity and cancer as they relate to leptin are still elusive despite the vast literature on the topic. A key question remains as to whether leptin contributes to cancer initiation or whether its role is restricted to promoting the growth of existing tumor cells? Numerous in vitro studies with breast cancer cell lines indicate that leptin directly contributes to LEPR-B-positive cancer cell proliferation, migration, and invasion. Furthermore, leptin has been known to regulate the immune response and angiogenesis through targeting immune cells and endothelial cells respectively (Sierra-Honigmann et al. 1998, La Cava & Matarese 2004), an effect that clearly affects cancer cell growth as well, albeit only indirectly. Despite these potent leptin-induced cellular changes on mammary tumor progression at various stages, the specific leptin-responsive cell populations in tumor tissues have not yet been adequately defined.
As xenografts of MMTV-Wnt1 cell into ob/ob mice failed to thrive, Zheng et al. (2011) analyzed these regressed tumor tissues and compared them to tumor cells isolated from wild-type mice to identify a leptin-responsive cell population missing in the ob/ob population but present in the wild-type isolates (Zheng et al. 2011). Xenograft tumor tissues from transplants into ob/ob mice were analyzed by fluorescent-activated cell sorting for markers characteristic of CSC-rich populations, such as CD29 (integrin β1), CD49f (integrin α6), and CD24 (heat stable antigen) on depletion of CD45- and Ter119-positive cells (Mani et al. 2008, Charafe-Jauffret et al. 2009). Interestingly, they found that the survival of CD29+CD24− CSC population is efficiently increased in response to leptin. They measured this by using a ‘tumor sphere formation’ assay. A leptin-responsive CD29+CD24− CSC population expresses high levels of LEPR-B. These findings are highly provocative but will require a more in-depth evaluation of this CSC population to strengthen the hypothesis that leptin is a mammary tumor-initiating factor on the basis of its ability to stimulate CSC survival.
Concluding remarks
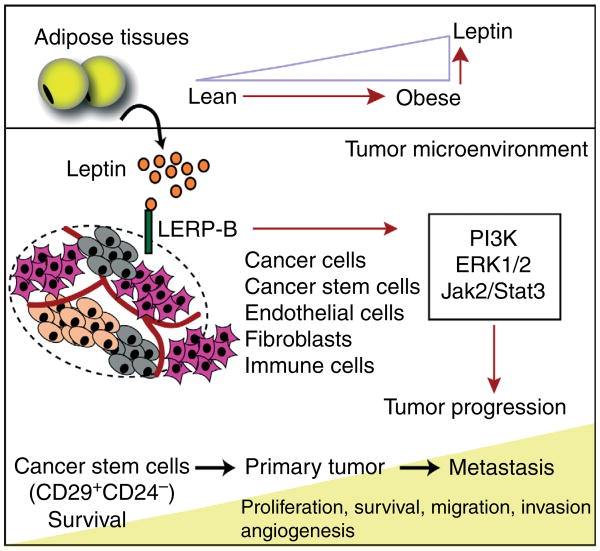
These results shed new light on the role of leptin and its receptor in mammary tumors (and potentially other LEPR-B+ tumor types). These observations touch on the important question whether obesity can be ‘tumor initiating’ and highlights that leptin may be an important contributing factor. Based on these results, an emerging model for the role of leptin on tumor progression is raised (Fig. 1). Obesity via LEPR-B-mediated signaling pathways promotes mammary tumor growth at various stages, affecting different tumor cell types that include a spectrum of cells from early CSCs through metastatic tumor cells. In this model, leptin is involved at early stages in CSC survival. Once the primary tumor is established, leptin triggers cancer cell proliferation, migration, and invasion. Furthermore, it exerts effects on tumor-associated stromal cells, such as endothelial cells, immune cells, and fibroblasts, to enhance angiogenesis and inflammatory processes that support tumor growth. Considering all of these potential roles for leptin on cancer progression, the leptin signaling pathway emerges as an attractive therapeutic target for the obese cancer patients. Can the peripheral leptin actions effectively be targeted without deteriorating the prevailing central leptin resistance under those conditions? In light of the life-threatening circumstances in the context of rapid growth of a tumor mass, a transient deterioration of central leptin action may be a price well worth paying.
Figure 1.
The potential role of leptin in mammary tumor progression. Leptin levels are increased in proportion to adipose tissue mass over the course of obesity. Leptin produced by adipose tissues binds to LEPR-B expressing cells within the tumor microenvironment, which include epithelial cancer cells, cancer stem cells, immune cells, endothelial cells, and potentially fibroblasts. LEPR-B-mediated pathways include activation of downstream kinases, such as PI3K, ERK1/2, and Jak2/Stat3. These pathways contribute to various steps of tumor progression, from cancer stem cell survival and proliferation to metastatic tumor growth.
Acknowledgments
Funding: The authors are supported by the NIH grants R01-DK55758, R01-CA112023, P01DK088761 (P E S), and DK081182 (Jay Horton). J P is supported by a fellowship from the Department of Defense (USAMRMC BC085909).
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. PNAS. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncology. 2002;3:565–574. doi: 10.1016/S1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Research. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SC, Jr, Liu SM, Li Q, Sun A, DeNino WF, Heymsfield SB, Guo XE. Transgenic complementation of leptin receptor deficiency. II. Increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. American Journal of Physiology Endocrinology and Metabolism. 2004;286:E384–E392. doi: 10.1152/ajpendo.00349.2003. [DOI] [PubMed] [Google Scholar]
- Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. Journal of Cellular Biochemistry. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV–TGF-alpha/Lep(ob) Lep(ob) female mice do not develop mammary tumors. Breast Cancer Research and Treatment. 2003;77:205–215. doi: 10.1023/A:1021891825399. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV–TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Experimental Biology and Medicine. 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV–TGF-alpha mice. Breast Cancer Research. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. Journal of Clinical Investigation. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. Leptin at 14 y of age: an ongoing story. American Journal of Clinical Nutrition. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clinical Cancer Research. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Howard JM, Pidgeon GP, Reynolds JV. Leptin and gastro-intestinal malignancies. Obesity Reviews. 2010;11:863–874. doi: 10.1111/j.1467-789x.2010.00718.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clinical Cancer Research. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nature Reviews Immunology. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. International Journal of Obesity and Related Metabolic Disorders. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. American Journal of Physiology Endocrinology and Metabolism. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Practice & Research Clinical Endocrinology & Metabolism. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutrition and Cancer. 2008;60:534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocrine Reviews. 2011a;32 doi: 10.1210/er.2010-0030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. American Journal of Pathology. 2011b;177:3133–3144. doi: 10.2353/ajpath.2010.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nature Genetics. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Smith S, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocrine-Related Cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]