Abstract
AIM: To determine intra-hepatic blood flow and liver stiffness in patients with non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) using contrast-enhanced ultrasound and fibroscan.
METHODS: This prospective study included 15 patients with NAFLD, 17 patients with NASH and 16 healthy controls. In each patient, real-time ultrasound was used to locate the portal vein (PV) and the right liver lobe, and 5 mL of SonoVue® was then injected intravenous in a peripheral vein of the left arm over a 4-s span. Digital recording was performed for 3 min thereafter. The recording was subsequently retrieved to identify an area of interest in the PV area and in the right liver parenchyma (LP) to assess the blood flow by processing the data using dedicated software (Qontrast®, Bracco, Italy). The following parameters were evaluated: percentage of maximal contrast activity (Peak%), time to peak (TTP, s), regional blood volume (RBV, cm3), regional blood flow (RBF, cm3/s) and mean transit time (MTT, s). At 24-48 h post-injection, liver stiffness was evaluated using Fibroscan and measured in kPa. The statistical evaluation was performed using Student’s t test.
RESULTS: In the PV, the Peak%, RBV and RBF were significantly reduced in the NAFLD and NASH patients compared with the controls (Peak%: NAFLD 26.3 ± 6.6, NASH 28.1 ± 7.3 vs controls 55.8 ± 9.9, P < 0.001; RBV: NAFLD 4202.3 ± 3519.7, NASH 3929.8 ± 1941.3 vs controls 7473 ± 3281, P < 0.01; RBF: NAFLD 32.5 ± 10.8, NASH 32.7 ± 12.1 vs controls 73.1 ± 13.9, P < 0.001). The TTP in the PV was longer in both patient groups but reached statistical significance only in the NASH patients compared with the controls (NASH 79.5 ± 37.8 vs controls 43.2 ± 30, P < 0.01). In the LP, the Peak%, RBV and RBF were significantly reduced in the NAFLD and NASH patients compared with the controls (Peak%: NAFLD 43.2 ± 7.3, NASH 41.7 ± 7.7 vs controls 56.6 ± 6.3, P < 0.001; RBV: NAFLD 4851.5 ± 2009, NASH 5069.4 ± 2292.5 vs controls 6922.9 ± 2461.5, P < 0.05; RBF: NAFLD 55.7 ± 10.1, NASH 54.5 ± 12.1 vs controls 75.9 ± 10.5, P < 0.001). The TTP was longer in both patient groups but did not reach statistical significance. The MTT in both the PV and LP in the NAFLD and NASH patients was not different from that in the controls. Liver stiffness was significantly increased relative to the controls only in the NASH patients (NASH: 6.4 ± 2.2 vs controls 4.6 ± 1.5, P < 0.05).
CONCLUSION: Blood flow derangement within the liver present not only in NASH but also in NAFLD suggests that a vascular flow alteration precedes liver fibrosis development.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Contrast-enhanced ultrasound, Fibroscan, Hepatic blood flow, Liver stiffness
Core tip: The use of contrast-enhanced ultrasound (CEUS) assisted by dedicated software (Qontrast®) in combination with Fibroscan examination could provide a non-invasive tool to evaluate the level of fatty-liver disease. In this study, we found that there were reductions in portal and intra-parenchymal blood flow in patients affected by non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH), whereas liver stiffness was increased only in NASH patients. Qontrast®-assisted CEUS could be used to quantify early changes in intra-parenchymal liver flow before the onset of fibrosis.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease worldwide[1,2]. Liver biopsy, which is the gold standard for diagnosing NAFLD is an invasive procedure with potential adverse effects and large inter- and intra-observer variability[3]. NAFLD cannot be diagnosed reliably without clear imaging or biopsy evidence of hepatic steatosis and without excluding excessive alcohol consumption, viral hepatitis and medications. NAFLD is further divided into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). NAFL is simple steatosis with no evidence of hepatocellular injury, whereas NASH is steatosis with inflammation, hepatocellular injury and possible fibrosis. NASH can lead to cirrhosis and hepatocellular carcinoma, whereas NAFLD has a very slow, if any, progression to NASH. NAFL and NASH, therefore, can be considered different steps in the same histological disease spectrum[3,4]. The pathogenesis of NAFLD is not completely known[5,6]. The fat accumulation occurring in NAFLD is key to the onset of vascular impairment[7]. The fat accumulation is responsible for liver structural and functional changes, leading to increased hepatic vascular resistance and finally to portal hypertension.
To study blood flow in the liver, pulsed continuous Doppler ultrasound (US) is used as the first-line imaging investigation. Doppler US can evaluate the blood flow in large and small vessels but fails to analyze the flow in the capillaries or sinusoids, where the velocity of the red blood cells is too slow to produce a Doppler signal[8]. Hence, changes in the hepatic microcirculation may be assessed using contrast-enhanced ultrasonography (CEUS) that consists of an intravenously administered suspension of gas-filled microbubbles that remain entirely within the intravascular space, thus acting as a blood pool tracer[9,10]. The obtained data can be processed using a post-processing computational tool (Qontrast®, Esaote, Florence, Italy) that includes a suite of software applications for image analysis designed to use alternative representations to extract and present brightness information that is already present in the image.
Liver fibrosis directly affects the mechanical properties of the liver parenchyma, such as stiffness, which indicates tissue resistance to deformation under mechanical stress. A greater stiffness corresponds to a higher tissue resistance to deformation. Liver stiffness can be studied using three physical measurements: two measures based on sonographic techniques, such as Fibroscan[11] and acoustic radiation force impulse[12,13], and one that is MR-based, such as magnetic resonance elastography[14]. Regardless of the specific technique, the measured parameter is correlated with the histological fibrosis stage, and the results can be used to accurately predict moderate to severe fibrosis[10,11,15].
In NASH and NAFLD, it remains unclear whether early changes in intrahepatic blood flow are associated with an early production of fibrous tissue. Therefore, the aim of this study was to evaluate the liver blood flow in the large and small intra-parenchymal vessels and fibrosis using CEUS and Fibroscan in patients with NAFLD and NASH compared with healthy controls.
MATERIALS AND METHODS
Populations
The study population was enrolled from August 2010 to December 2013. All of the participants were Caucasian and underwent physical examinations, laboratory tests for liver function, upper and lower abdominal real-time ultrasonography (RUS) and computed tomography (CT) scan when necessary.
Sixteen healthy controls and 32 patients with US-documented steatosis were recruited (Table 1). Fifteen patients affected by NAFLD as defined according the latest guidelines established by the American Association for the study of liver diseases[3] and 17 patients with NASH defined as having fatty liver on abdominal ultrasound examination and either aspartate aminotransferase or alanine aminotransferase more than 1.5 times the upper normal limit on two occasions during the six months before enrollment were included. Exclusion criteria were laboratory data and image studies as assessed with ultrasound or CT scan when necessary, compatible with hepatitis B and C, autoimmune hepatitis, sclerosing cholangitis, Wilson’s disease, alpha-1 anti-trypsin deficiency, hemochromatosis and hepatic cirrhosis[16]. Additional exclusion criteria were patients with medical histories of malignancy, previous abdominal or thoracic surgery and history of heart and pulmonary disease that may impair the flow of the contrast bubbles to the liver as well as severe concomitant diseases. Finally, patients with pregnancy and breastfeeding as well as pediatric patients were also excluded.
Table 1.
Study populations
| Population characteristics | Controls | NAFLD | NASH |
| Number | 16 | 15 | 17 |
| Male/female | 8/8 | 12/3 | 16/1 |
| Mean age (range) | 37 yr (26-69 yr) | 48 yr (26-75 yr) | 45 yr (20-74 yr) |
| AST (mean ± SD) | 20.6 ± 4.5 | 19.3 ± 5b | 45.2 ± 22.1d |
| ALT (mean ± SD) | 24.4 ± 7.0 | 27.4 ± 8.1b | 86.4 ± 55.7d |
| GGT (mean ± SD) | 18.3 ± 10.1 | 25.6 ± 20b | 73.1 ± 43d |
| ALP (mean ± SD) | 144.4 ± 45.4 | 154.1 ± 38.3 | 176.5 ± 57.4 |
P < 0.001, NAFLD vs NASH;
P < 0.001, NASH vs controls. AST: Aspartate aminotransferase: reference range 0-37 (IU/L); ALT: Alanine aminotransferase: reference range 0-40 (IU/L); GGT: Gamma-glutamyl transferase: reference range 7-50 (IU/L); ALP: Alkaline phosphatase: reference range 98-279 (IU/L); NASH: Non-alcoholic steatohepatitis; NAFLD: Non-alcoholic fatty liver disease.
Contrast-enhanced ultrasound and dedicated software
The patients were examined after fasting for 8 h and after having obtained written informed consent. The CEUS examination was always performed by the same expert operator using RUS with a 3.5-MHz convex probe (MyLab70 XVision, Esaote, Ansaldo, Italy) through a longitudinal intercostal scan, in which the portal vein (PV) and right liver parenchyma (LP) could be easily identified while keeping the patient or subject in the supine position. The US contrast medium (SonoVue®, Bracco Spa, Milan, Italy) consisted of 2.5 μm sulfur hexafluoride-filled microbubbles (hence, they are smaller than red blood cells, which have a diameter of 7 μm) stabilized by a lipid monolayer membrane[9]. The microbubbles can generate a nonlinear harmonic response to a low mechanical index (MI), thus permitting continuous real-time imaging. In our study, we used a signal-processing algorithm installed on the ultrasonographic machine (Contrast-Tuned Imaging™, CnTI™, Esaote, Genoa, Italy) that automatically sets a low MI of 0.06 and holds this value constant during the entire CEUS procedure. These features allow the contrast medium microbubbles to travel through the smallest blood vessels without bursting. SonoVue, a blood-pool contrast agent, has no cellular uptake; thus, it enhances only the US image generated by the blood vessels[9]. In CEUS studies, there are 3 overlapping vascular phases: the arterial phase, which starts within 20 s after the injection and lasts 30-45 s; the portal venous phase, which usually lasts until 2 min after the injection; and the late phase, which corresponds to the clearance of the US contrast agent from the circulation. The CEUS screen, because of the low gain, shows signals only from intensely reflective structures, which limits the ability to identify the proper scan area. To overcome this problem, a split-screen display was used on the ultrasound machine to show the conventional B-mode image beside the CEUS image (Figure 1). Using contrast-processed data, the blood flow through the small capillaries of the liver interstitial tissue could be measured in terms of the volume and flow.
Figure 1.
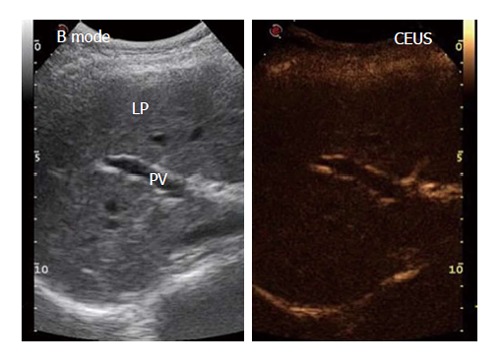
Example in a healthy control of the split-screen display during the contrast-enhanced ultrasound procedure upon the injection of SonoVue® using a low mechanical index. Left: B-mode frame; Right: Contrast-enhanced ultrasound frame. PV: Portal vein; LP: Liver parenchyma. The B-mode frame shows more detail because of the higher gain.
The procedure started with a 5-mL contrast medium injection (always performed by the same expert nurse) using a 20-gauge (G) needle cannula over a 4-s span into the antecubital vein of the left arm with the patient in the supine position. The line was then flushed with a 5-mL bolus of saline solution, also injected over a 4-s span. During the contrast medium injection, digital recording was started and performed for 3 min; during this operation, the patients were asked to breathe slowly to minimize respiration-related movements. The video recordings were then analyzed by the same trained operator using Qontrast® software (Esaote, Florence, Italy), which performs a parametric analysis of perfusion within a selected set of higher signal intensity frames in the region of interest (ROI). In each patient, we evaluated two ROIs: one in the PV and one in the right LP. To correct for translational movements in the ROI, a Gamma variate (bolus)-corrected parametric curve model was selected. The Qontrast® software was then allowed to process the perfusion in each of the previously determined ROIs, calculate the parameters automatically and plot the measured and calculated curves. The following parameters were generated (Figure 2): Peak%, the maximum signal intensity (SI) reached during SonoVue® bolus transit at time T, where T was the time to peak (TTP, s), the time to reach the maximum SI; regional blood volume (RBV, cm3), the blood volume in the ROI, proportional to the area under the time intensity curve; mean transit time (MTT, s), the contrast medium mean transit time in the ROI; and regional blood flow (RBF, cm3/s), the RBV to MTT ratio. The reproducibility of the data obtained by Qontrast® analysis of CEUS was tested according to the method of Ridolfi et al[17].
Figure 2.

Example of region of interest selection in the portal vein and Qontrast®-assisted contrast-enhanced ultrasound analysis of portal vein parameters in healthy control. A: Region of interest (ROI) drawn in a region of the portal vein (PV) in a selected set of higher signal intensity frames 1 min and 10 s after SonoVue® injection; B: A gamma variate (bolus)-corrected parametric curve for the translational movement caused by breathing activity. SI (%): Signal intensity; PEAK (%): Maximum signal intensity reached during SonoVue® bolus injection; TTP (s): Time to peak; RBV (cm3): Regional blood volume; RBF (cm3/s): Regional blood flow; MTT (s): Mean transit time.
Transient elastography
Transient elastography was performed 24-48 h after CEUS using a Fibroscan device (Echosens, Paris, France). Fibroscan consists of a 5-MHz US transducer probe installed on the axis of a vibrator that generates a 50-Hz vibration (completely painless to the patient) that causes an elastic shear wave to propagate through the skin and subcutaneous tissue and finally to the LP, the stiffness of which is directly related to the velocity of the wave. Fibroscan measures the stiffness of a cylindrical volume 1 cm in diameter, 4 cm in length and 25 to 45 cm from the skin. Acquisition was performed by the same expert operator through an intercostal scan, in which the probe was placed perpendicular to an area free of large vascular structures. During acquisition, the patient lay in the supine position with the right arm in abduction. Liver stiffness was determined by computing the median value of 10 successful acquisitions in kPa.
Statistical analysis
Continuous variables (laboratory values, SonoVue® data processed by Qontrast® software and elastosonography data) are expressed as group means ± SDs. Age was analyzed as a mean. Comparisons of all gathered data among the groups were tested by a Welch-corrected unpaired t test. P values were two-tailed, and all P values less than 0.05 were considered statistically significant. All statistical relationships were assessed using correlation analysis. The statistical analyses were performed using the GraphPad Prism software, version 3.00 (GraphPad Software, San Diego, California, Unites States).
RESULTS
Qontrast
We could not analyze two PV ROIs in NAFLD patients and three in NASH patients because of poor video recording due to liver steatosis that interfered with the returning echoes to the US probe.
The PV analysis showed a significantly shorter Peak% (Figure 3A) and decreased RBV and RBF (Figure 3C and D) in both the NASH and NAFLD patients compared with the controls. The TTP in the PV was longer in both patient groups but reached significance only in the NASH patients (Figure 3B).
Figure 3.
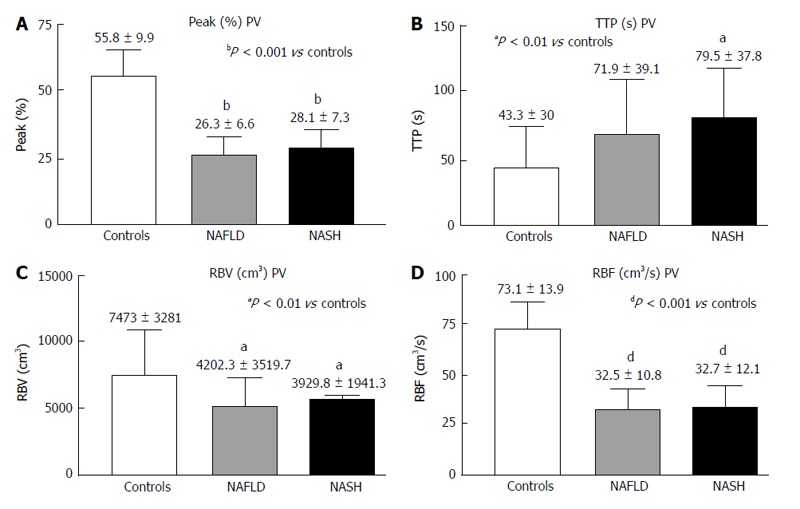
Contrast-enhanced ultrasound of the portal vein in controls, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. A: Peak (%); B: TTP (s); C: RBV (cm3); D: RBF (cm3/s); Peak (%): Maximum signal intensity (SI) reached during SonoVue® bolus injection; TTP (s): Time to peak; RBV (cm3): Regional blood volume; RBF (cm3/s): Regional blood flow; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
The LP analysis yielded similar results, with Peak% (Figure 4A), RBV and RBF (Figure 4C and D) significantly reduced in both the NASH and NAFLD groups compared with the normal controls. The TTP was longer in both NASH and NAFLD patients compared with the controls but did not reach significance (Figure 4B). The MTT in both the PV and LP in the NAFLD and NASH patients was similar to that in the controls (Figure 5).
Figure 4.
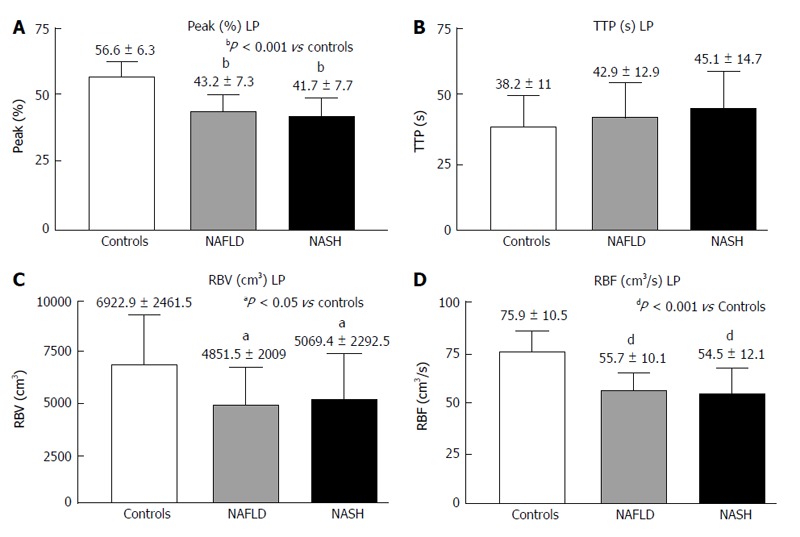
Contrast-enhanced ultrasound of liver parenchyma in controls, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. A: Peak (%); B: TTP (s); C: RBV (cm3); D: RBF (cm3/s); Peak (%): Maximum signal intensity (SI) reached during SonoVue® bolus injection; TTP (s): Time to peak; RBV (cm3): Regional blood volume; RBF (cm3/s): Regional blood flow; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
Figure 5.
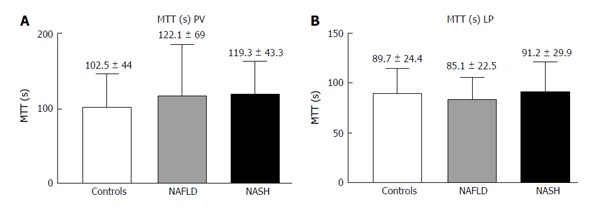
Contrast-enhanced ultrasound of the portal vein (A) and liver parenchyma (B) in controls, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Mean transit time (s). NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
Fibroscan
The values of liver stiffness measured in kPa were found to be significantly greater in the NASH patients compared with the control group (Figure 6).
Figure 6.
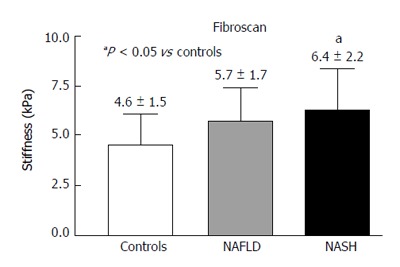
Fibroscan in controls, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
Adverse effects
CEUS studies were performed successfully in all of the patients and were well tolerated, with no side or adverse effects reported.
DISCUSSION
Our study showed that blood flow, as assessed by Qontrast®-assisted CEUS analysis of the PV and LP, was decreased in patients affected by NAFLD and NASH. We also found that liver stiffness, as assessed by Fibroscan, was increased only in NASH patients.
Based on the data obtained by the Qontrast® analysis of ROIs in the PV and LP, significant reductions in the Peak%, RBV and RBF were found in both groups of patients, whereas a delayed TTP was found only in the PV of the NASH group. Our results suggest the hypothesis that in patients with NAFLD, there is a reduced vascular compliance in the liver due to augmented hepatic vascular resistance to portal blood flow and an increased hepatic vascular tone that starts before the onset of fibrosis. This change was previously demonstrated by Francque et al[18] in an experimental animal model; in their study, Wistar rats fed with a methionine- and choline-deficient diet for four weeks developed severe steatosis associated with a significant increase in intrahepatic resistance before the onset of fibrosis and inflammation. These changes involved functional (liver endothelial dysfunction and vasoconstrictor overproduction) and structural (sinusoidal altered microvascular architecture) factors. Another study by Pasarín et al[19], performed on rats fed with a cafeteria diet for one month, showed that the impaired response to endothelial-dependent vasodilation caused endothelial dysfunction, leading to augmented intrahepatic resistance and reduced portal flow. Even in this study, the functional features of intrahepatic vascular changes preceded the onset of fibrosis and inflammation[19].
Another interesting observation is that our data obtained by Qontrast®-assisted CEUS were similar to those from other studies that analyzed liver blood flow with CEUS using SonoVue® as the contrast medium in cirrhotic patients. Lin et al[20] studied the flow in the right PV by means of color Doppler and CEUS, and they found that the arrival time of SonoVue® in the right PV was prolonged, whereas the velocity and flow volume were decreased. Similar results were found in another study by Ridolfi et al[17], who evaluated liver blood flow in the PV and in the parenchyma by means of CEUS and subsequent analysis by Qontrast® in cirrhotic patients and healthy subjects. They found a reduced Peak% and prolonged TTP and MTT in cirrhotic patients compared with controls. These data suggest that NASH and, more interestingly, NAFLD might be considered precursors of liver cirrhosis due to the presence of similar hemodynamic changes in liver blood flow.
In our patients affected by NASH, the delay in reaching the maximum signal intensity (TTP), only present in the PV, together with the reduction in blood flow in either the PV or LP, could be the consequence of not only intra-parenchymal microcirculation variations but also increased liver stiffness. Liver stiffness data in our patients with NAFLD and NASH could be included among those with no fibrosis (NAFLD) or mild fibrosis (NASH) as classified by Wong et al[21]. These authors studied a large cohort of patients with hepatic steatosis using Fibroscan and liver histology. They found that patients with no fibrosis or mild fibrosis showed liver stiffness values (kPa) that were consistent with those of our patients with NAFLD (no fibrosis) and NASH (mild fibrosis), respectively. They also identified some patients with steatosis with liver stiffness values that were much higher than those found in this present study. These patients were classified at histology as having a fibrosis pattern compatible with early cirrhosis, which was an exclusion criterion in our study.
The major limitation of this present study was the small number of patients that were examined; further studies in a much larger population are required to draw definitive conclusions regarding the value of the digital data generated by Qontrast®. However, the differences between the control, NAFLD and NASH groups for the main measure of the analysis (Peak% in the PV and LP) were so large that the statistical power of the study could be considered satisfactory. The absence of liver biopsy data in our study was another limitation, although other authors have found a significant correlation between US and histopathologic data in the evaluation of steatosis[22-24]. The CEUS procedure may be incorrectly applied when the US machine does not meet the criteria of good sensitivity, good tissue suppression and good temporal and spatial resolution as reported in the Guidelines of European Federation of Societies for Ultrasound in Medicine and Biology[25]. In our study, we obtained a good tissue suppression by means of CnTI™, which maintains a low MI throughout the study and avoids microbubble bursting as well as bioeffects in the target organs.
In conclusion, CEUS evaluated by Qontrast® might be able to quantify functional vascular liver changes not otherwise detectable with any other non-invasive procedure and before the development of fibrosis. The combined use of Fibroscan and Qontrast®-assisted CEUS could be helpful in assessing the level of disease and could be potentially useful for monitoring the effects of therapeutic interventions.
COMMENTS
Background
Non-alcoholic fatty liver disease (NAFLD) is among the most common cause of chronic liver disease worldwide. NAFLD is further subdivided into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). Whereas NASH is represented by steatosis with inflammation, hepatocellular injury and possible fibrosis, NAFL is a simple steatosis with no evidence of hepatocellular injury. Fat accumulation within the hepatocytes leads to narrowing and distortion of the sinusoidal lumen, leading to increased hepatic vascular resistance and finally to portal hypertension and fibrosis that are among the stigmata of hepatic cirrhosis. Whether these vascular hemodynamic changes are present in NAFLD and NASH remains unclear. Pulsed continuous Doppler ultrasound (US) is the first-line imaging tool for studying blood flow in the liver and allows for the evaluation of flow in the great hepatic vessels but fails to analyze the flow in the capillaries or sinusoids, where the velocity of the red blood cells is too slow to produce a Doppler signal. Hence, to assess changes in hepatic microcirculation, the authors used US to analyze a Doppler signal generated by an intravenously administered suspension of gas-filled microbubbles (each bubble is one-third the diameter of a red blood cell) stabilized by a lipid monolayer membrane; these features allow these bubbles to remain entirely within the intravascular space, thus acting as a blood pool tracer. The obtained data can be processed with a post-processing computational tool (Qontrast®, Esaote, Firenze, Italy), which allowed them to extrapolate objective and quantitative parameters of microvascular damage in the liver. Liver fibrosis directly affects the mechanical properties of the liver parenchyma and may also contribute to portal hypertension. Liver stiffness can be studied with Fibroscan that consists of measuring the resistance of the liver tissue to the propagation of a US beam within the tissue.
Research frontiers
Considering the increasing prevalence of NAFLD with potentially severe outcomes and the limitations of the actual gold standard (liver biopsy) as a diagnostic procedure, the development of a non-invasive technique that allows for an early assessment of liver damage in terms of the derangement of intrahepatic microcirculation and the development of fibrosis appears to be a stimulating research field. This approach also has therapeutic implications in terms of the development of new drugs and monitoring of their therapeutic effects.
Innovations and breakthroughs
The US contrast medium (SonoVue®, Bracco Spa, Milan, Italy) consisted of 2.5 μm sulfur hexafluoride-filled microbubbles, which are smaller than red blood cells. The microbubbles have no cellular uptake, unlike the contrast media used for computed tomography scan or magnetic resonance, and can travel through the smallest liver blood vessels without bursting. A Doppler signal not otherwise detectable with the standard US machine is therefore generated, and the flow in microvessels can be measured. In addition, the use of a computer program that analyzes the signal intensity within the US image allows for the standardization of data in term of the blood flow and volume. By means of contrast-enhanced ultrasound (CEUS) and computer-assisted determination of flow and volume, it has been possible for the first time to detect a derangement in the microcirculation within the liver parenchyma not only in NASH but also in NAFLD. Fibrosis otherwise appears to be limited only to NASH.
Applications
To non-invasively monitor the development of liver disease and to study the effect of drugs on hepatic micro-circulation and fibrosis.
Terminology
CEUS: contrast-enhanced ultrasound, SonoVue®: microbubbles of 2.5 μm in diameter filled with sulfur hexafluoride that are stabilized by a lipid monolayer membrane. QONTRAST® is a suite of software applications for image analysis designed to extract and present, in alternative representation, brightness information that is already contained within the images. FibroScan® is a sonography-based non-invasive and rapid bedside method for the diagnosis and quantification of hepatic fibrosis (by measuring liver stiffness).
Peer review
This is a clinical study which evaluated the findings of contrast-enhanced US and Fibroscan in patients with NAFLD and control. The authors found some differences of the hepatic hemodynamics and liver stiffness among control NAFL and NASH.
Footnotes
P- Reviewer: Maruyama H, Nagarajan P, Videla LA S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 2.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815–823. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 7.Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291:684–692. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 8.Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383–387. doi: 10.1097/00042737-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Greis C. Technology overview: SonoVue (Bracco, Milan) Eur Radiol. 2004;14 Suppl 8:P11–P15. [PubMed] [Google Scholar]
- 10.Quaia E. Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol. 2011;21:604–615. doi: 10.1007/s00330-010-1965-6. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371–378. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213.e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori M, Fujii H, Ogawa T, Kobayashi S, Iwai S, Morikawa H, Enomoto M, Tamori A, Sawada A, Takeda S, et al. Close correlation of liver stiffness with collagen deposition and presence of myofibroblasts in non-alcoholic fatty liver disease. Hepatol Res. 2011;41:897–903. doi: 10.1111/j.1872-034X.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 16.Barzin G, Merat S, Nokhbeh-Zaeem H, Saniee P, Pedramnia S, Mostashfi Habibabadi A, Nasseri-Moghaddam S. Oral Nitrate Reductase Activity Is Not Associated with Development of Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Pilot Study. Middle East J Dig Dis. 2014;6:23–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Ridolfi F, Abbattista T, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound evaluation of hepatic microvascular changes in liver diseases. World J Gastroenterol. 2012;18:5225–5230. doi: 10.3748/wjg.v18.i37.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francque S, Laleman W, Verbeke L, Van Steenkiste C, Casteleyn C, Kwanten W, Van Dyck C, D’Hondt M, Ramon A, Vermeulen W, et al. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab Invest. 2012;92:1428–1439. doi: 10.1038/labinvest.2012.103. [DOI] [PubMed] [Google Scholar]
- 19.Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7:e32785. doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin LW, Duan XJ, Wang XY, Xue ES, He YM, Gao SD, Yu LY. Color Doppler velocity profile and contrast-enhanced ultrasonography in assessment of liver cirrhosis. Hepatobiliary Pancreat Dis Int. 2008;7:34–39. [PubMed] [Google Scholar]
- 21.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 22.Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- 23.Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, Stevens WR. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619–625. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]


