Abstract
The ‘‘omics sciences’’ (genomics, transcriptomics, proteomics) are often used to study living organisms as a whole system by evaluating the complex expression patterns of genes, miRNA, proteins, and metabolites. This study aimed, through bioinformatics and systems biology, to decipher the cytokinome profile in the evolution of inflammatory processes leading to cancer. The cytokinome was defined as the totality of cytokines and their interactions in and around biological cells. The system biology approach would provide a better understanding of the complex interaction network of cytokines, especially in cancer patients. Acquired knowledge would enable health providers with tools to evaluate disease onset through progression as well as identifying innovative therapeutic strategies. Understanding the role each cytokine plays in the metabolic network is of great importance. This paper reviews our group’s ‘‘omics’’ work. In particular, it addresses the role cytokines play in liver disease in six different scenarios. The first is the role the cytokines play in chronic inflammatory diseases and cancers. The second is the significance of the cytokinome profile. The third is the role of liver cirrhosis as an inflammatory disease. The fourth is the comparison of cytokine levels evaluated in patients with chronic hepatitis C virus (HCV) or with HCV-related cirrhosis. The fifth is the comparison of cytokine levels evaluated in patients with HCV-related cirrhosis in the presence and absence of type 2 diabetes. And lastly, we present a comparison of cytokine levels evaluated in patients with HCV-related cirrhosis in the presence and absence of hepatocellular carcinoma.
Keywords: Cytokinome, Hepatitis C virus, Cirrhosis, Type 2 diabetes, Hepatocellular carcinoma, Interactome, Systems biology
Core tip: In this review we report the usefulness of cytokinome profiling in improving our understanding of the role cytokines play in the evolution of chronic inflammatory processes leading to cancer. The serum levels of cytokines were evaluated in patients with hepatitis C virus infection-related liver diseases in the presence and absence of either hepatocellular carcinoma or type 2 diabetes. In addition, the complex interaction network, between the significant cytokines and their metabolic pathways, was studied using bioinformatics and systems biology approaches.
INTRODUCTION
Inflammation is a physiological process in response to acute tissue damage resulting from physical injury, ischemic injury, infection, exposure to toxins, chemical irritation, and/or several types of trauma[1,2]. The process is a protective attempt, by the organism, to remove the injurious stimuli as well as to initiate the tissue healing process[3,4].
In certain cases, inflammation becomes chronic as a result of a persistent inflammatory stimulus or a dysregulation in the control mechanisms that normally turn the inflammatory process off[2,5,6]. Many cancers may arise from sites of an infection, chronic irritation, or inflammation. The cancer microenvironment, which is largely orchestrated by inflammatory cells and cytokines, is an indispensable participant in the neoplastic process. Moreover, cancer not only alters the metabolic needs of the tissue, it also fosters DNA and protein damage, proliferation, survival, mutagenesis, migration, and metastasis of malignant cells[7,8]. Therefore, many players, belonging to the different “omics” levels (genomics, transcriptomics, proteomics), play very important roles in the progression of chronic inflammation processes leading to cancer. An interesting example for this phenomenon is chronic hepatitis C virus (HCV) that induces, after some years, the development of liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). Recent studies, on the application of genomics, transcriptomics, and proteomics of HCV infections and HCV-induced liver disease, identified new markers that may be useful in improving the diagnosis and the prognosis of these diseases[9-13].
The major physiological difference between cancer and normal cells is the presence of hypoxia, which induces many cytokines and chemokines[14]. Both cytokines and chemokines play numerous roles in the cell-cell communication at the tissue level, where the outcome is determined by a cytokine concentration milieu and cell type[8]. Current understanding describes that activated immune cells provide both anti- and pro-tumorigenic signals. Hence, these cells provide potential targets for the development of therapeutics based on environmental and/or cellular context. Since the control of cytokine production is highly complex and multifactorial, cytokine effects are mediated through multiple regulatory networks. The intricate complexity of cytokine networks conceals the role a single cytokine may play in the pathogenesis of a disease. It is, therefore, informative to investigate the immunopathogenesis of a disease process by analyzing multiple cytokine panels. This approach could provide a comprehensive understanding of the cytokines’ cellular, humoral, and chemotactic roles at a critical time in various cancers, as well as roles cytokines might play in the treatment course of a correlated infection[15].
CHALLENGE AND SIGNIFICANCE OF THE CYTOKINOME PROFILE
The complexity of the cytokine system in humans can be elucidated using the cytokinome, which evaluates the complex network of interactions used to regulate either cytokine synthesis or their cognate receptors. In addition, the cytokinome provides an insight into both antagonistic and synergistic interactions among different cytokines, which occur in many different and often redundant ways (Figure 1)[16]. An intriguing problem related to the cytokine family and their receptors is the existence of pleiotropy in the cytokine system. Pleiotropy enables one cytokine to activate various receptors and many different cytokines to activate the same receptor. Elucidation of these complex cytokine interactions results in the production of more efficient and selective targeted therapeutics against cancer via direct interaction with the receptors instead of cytokines.
Figure 1.
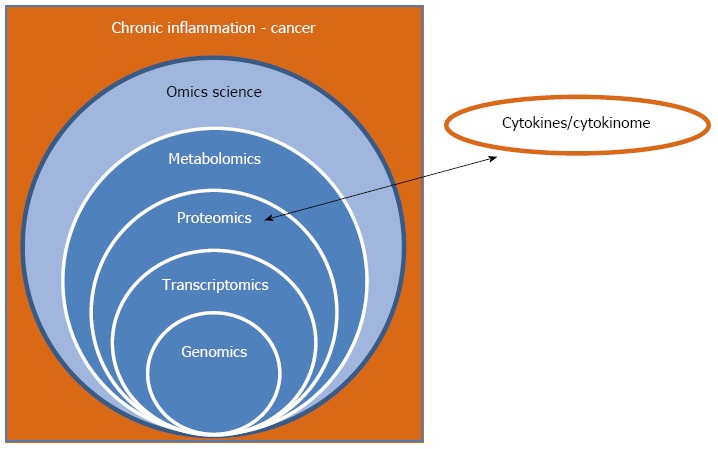
Relationship of the “omics sciences”, chronic inflammation, and cancer. The scheme shows (1) the necessity to integrate data derived from all the “omics sciences” for obtaining a complete picture of the system; and (2) the importance of including the cytokinome profile, at the proteomics level, due to the crucial role of cytokines in the chronic inflammation process leading to cancer.
An accurate and comprehensive understanding of cytokine functions can be obtained from simultaneous and coherent measurements of cytokine concentrations in serum[17,18]. However, this approach is limited by the inherently difficult simultaneous measurement of the various cytokine concentrations, in the same biological fluid, due to large differences in concentrations spanning several magnitude orders. At present, a broad-spectrum bead-based multiplex immunoassay is a viable approach to effectively characterize cytokine levels in serum[19-22].
Systems biology approaches are powerful tools in analyzing the system as a whole. The enormous amount of data derived by “omics” disciplines, computational methods, and algorithms, resulted in a comprehensive and integrated vision of the biological phenomenon under investigation. In fact, until the last century, the common approach in the biological sciences was to break down the object of study down to its elementary parts, and study all the singular units, in order to explain the life processes. These analytical and reductionist approaches have provided significant functional understanding specific molecular mechanisms and properties of living organisms, such as genes, proteins, and metabolites. However, this single component focused approach was not able to predict the behavior of the system as a whole. Novel methodologies study the living organism as a whole, even if the laws regarding the organizational forces of these systems are not yet well understood. The essence is to understand the collective phenomena in the framework of the functionality of the whole system[23].
The definition and evaluation of the human cytokinome is an important tool in analyzing the cytokine interaction network of healthy subjects as well as cancer patients. Computational models can provide a broader understanding of the effects exerted by drugs that act on cytokine populations and/or their receptors in the regression of chronic inflammation or cancer progression. In addition, knowledge of cytokine levels may be used with diagnosticorprognostic tools by the physicians.
EXAMPLE OF AN INFLAMMATORY DISEASE: LIVER CIRRHOSIS
Liver cirrhosis (LC) represents the fifth leading cause of death in the Western world. The condition arises from complications associated with various liver diseases and is characterized by an abnormal liver structure and function. A majority of liver diseases injure and kill liver cells, while inflammation and cellular repair processes generate scar tissue[24]. The morphology of LC is determined by the amount of time that has elapsed from the onset of parenchymal extinction. If active injury continues, new lesions of extinction will coexist with old lesions. In the case of an inactive and remote primary disease, the liver contains only lesions in the late stages of repair. Once LC has developed, severity may either progress or regress towards normal. Progression depends on the activity of the primary disease and on the secondary obstructive events in portal and hepatic veins. Hence, the LC is permanent and irreversible liver damage, which can go from minimal signs to the advanced symptoms as liver function decreases[25]. The LC is characterized by protein blood flow disturbances due to progressive disruption of normal vascular anatomy and physiology[26]. Cirrhotic patients are recognized as being at risk of developing HCC at a rate of 1% to 4% per year after cirrhosis has been established.
The production of cytokines function as immune response key players in cell proliferation and apoptosis suppression, increases the risk of cancer[3]. The evaluation of a large panel of cytokines in cirrhotic patients may provide physicians with information necessary for better prognosis. In addition, this information could potentially elucidate the correlation between LC and its involvement in the development of liver cancer in presence and absence of other complications like HCV or type 2 diabetes.
CYTOKINE EVALUATION IN CHRONIC HCV AND LC PATIENTS
The serum levels of 32 cytokines, chemokines and growth factors [β-nerve growth factor (NGF), chemokine (C-C motif) ligand (CCL) 3, CCL27, CXCL [chemokine (C-X-C motif) ligand] 1, CXCL9, CXCL10, CXCL12, interferon (IFN)-2, hepatocyte growth factor (HGF), interleukin (IL)-1α, IL-1β, IL-1ra, IL-2R, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-12p40, IL-16, IL-17, IL-18, leukemia inhibitory factor (LIF), monocyte chemoattractant protein (MCP)-3, macrophage colony-stimulating factor (M-CSF), macrophage migration inhibitory factor (MIF), stem cell factor (SCF), stem cell growth factor (SCGF)-β, tumor necrosis factor (TNF)-α, TNF-related apoptosis inducing ligand (TRAIL)] were evaluated in 30 patients with chronic HCV, in 30 patients with HCV-related LC, and in 20 healthy donors by multiplex biometric ELISA-based assays[27]. General patient demographics are as follows: 15 women and 15 men had HCV; 16 women and 14 men had HCV-related LC; and 11 women and 9 men were used as healthy controls. Differences in the cytokine serum levels between diseased patients and healthy controls are reported in Table 1.
Table 1.
Differences in the serum levels of pro-inflammatory molecules between patients with either hepatitis C virus orliver cirrhosis and the healthy controls
| Controls vs HCV | Controls vs LC | |
| IL-1α | a↑ | b↑ |
| IL-1β | b↑ | b↑ |
| IL-2R | a↑ | b↑ |
| IL-6 | b↑ | b↑ |
| IL-8 | b↑ | b↑ |
| CXCL1 | b↑ | b↑ |
| CXCL9 | b↑ | b↑ |
| CXCL10 | b↑ | b↑ |
| CXCL12 | a↑ | a↑ |
| MIF | a↑ | b↑ |
| β-NGF | b↑ | b↑ |
| HGF | b↑ |
P values obtained for all significant molecules in chronic hepatitis C virus (HCV) and HCV-related cirrhosis (LC) patients with respect to controls using the nonparametric Mann-Whitney U test. aP value < 0.05, bP < 0.01, up arrows indicate an increase in patients’ cytokine serum level vs healthy controls. HCV: Hepatitis C virus; LC: Liver cirrhosis; IL: Interleukin; NGF: Nerve growth factor; HGF: Hepatocyte growth factor; MIF: Migration inhibitory factor.
Significant up-regulation of IL-1α, IL-1β, IL-2R, IL-6, IL-8, CXCL1, CXCL9, CXCL10, CXCL12, MIF, and β-NGF was seen in HCV and LC patients compared to healthy controls. The only exception was hepatocyte growth factor (HGF), which was up-regulated in LC but not in HCV patients. HGF is a multifunctional growth factor that regulates growth and cell motility, exerts mitogenic effects on hepatocytes and epithelial cells and plays diverse roles in organ development, tissue regeneration, and cancer progression[28]. Moreover, it has been implicated, along with IL-6, IL-8 and IL-1, in the hepatic stellate cell activation pathway. However, numerous reports have examined the relationship between HGF and the occurrence of HCC facilitation/suppression, and have suggested that this growth factor could be used as an index of cellular growth and HCC development in LC patients[29]. In fact, HGF levels were significantly different in LC patients in respect to both healthy controls and chronic inflammation patients, and its concentration in HCC patients was higher than in LC patients. This strongly suggests the importance of HGF role in the progression of chronic inflammation leading to LC and cancer as well as being used as a specific marker for predicting the occurrence of HCC in chronic HCV-related liver diseases[27].
Comparison between chronic HCV and LC patients
Since IL-1α, IL-1β, IL-2R, IL-6, IL-8, CXCL1, CXCL9, CXCL10, CXCL12, MIF, and β-NGF were increased in both HCV and LC patients, their mean concentrations were compared by t test. Figure 2 shows that the concentrations of all the proteins and, in particular, IL-8, CXCL9 and β-NGF were higher (with P < 0.05) in patients with LC than in those with HCV. Subsequent comparison of serum levels for all pro-inflammatory molecules, in HCV and LC patients compared to HCC patients, showed the mean concentrations of all molecules was higher in HCC patients than in LC patients[30]. This indicates that the expression of pro-inflammatory molecules tends to increase in chronic inflammation progression leading to LC and HCC, making these molecules potential markers for prognostic studies. The serum levels of statistically significant cytokines, chemokines, and growth factors in HCV and LC patients were correlated with clinical data using the Pearson correlation coefficient. Patients with HCV had higher levels of IL-1β, IL-2R, MIF, and β-NGF than controls, resulting in a significant positive correlation with the transaminase values. Therefore, these proteins can be considered indices of immune activation. These results are in agreement with literature data on the participation of IL-1 and IL-2R in the progression of liver injury to fibrosis[31] as well as β-NGF involvement in liver cancer growth and metastasis. All three molecules could potentially be used as an index of chronic infection leading to LC and HCC[32]. An increase in MIF serum levels has previously been reported in hepatitis B patients[33]. Our work suggests MIF may also play a role in HCV-related chronic inflammation patients. CXCL1, CXCL9, CXCL10, and HFG, in LC patients, showed a significant negative correlation with albumin values. Since HGF was the only molecule that was statistically different between HC and LC patients, these data suggested that the four proteins could be useful for diagnostic/prognostic purposes.
Figure 2.
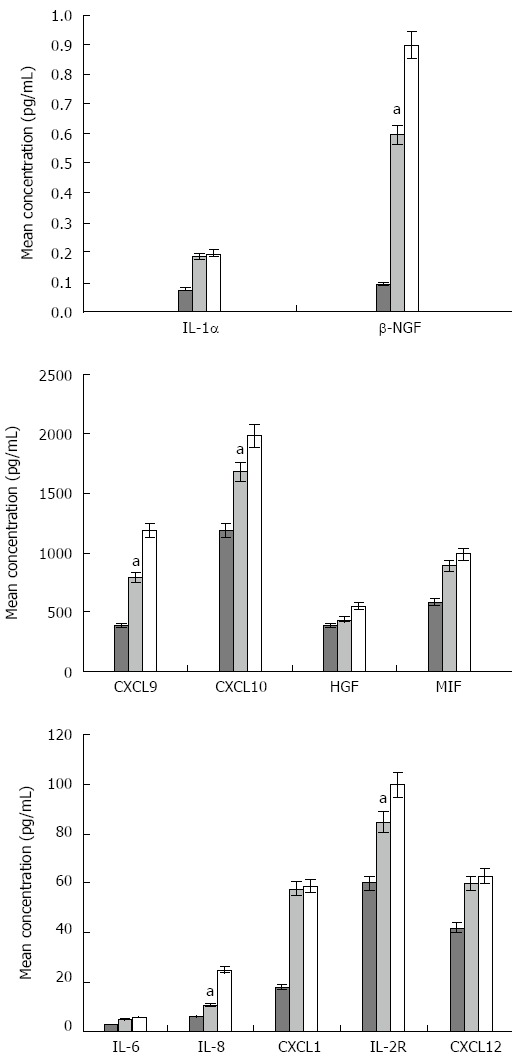
Differences in the concentration (pg/mL) of significant cytokines, chemokines, and growth factors among hepatitis C virus, hepatitis C virus-related liver cirrhosis patients, and healthy controls. The mean concentrations, for each significant molecule, in healthy control subjects, HCV, and HCV-related LC patients were calculated using a t test. Healthy control - light grey; HCV patients - cyan, HCV-related LC patients - green bars. The cytokine expression was statistically different between HCV and HCV-related LC patients (aP < 0.05). HCV: Hepatitis C virus; LC: Liver cirrhosis; IL: Interleukin; NGF: Nerve growth factor; HGF: Hepatocyte growth factor; MIF: Migration inhibitory factor.
CYTOKINE EVALUATION IN LC PATIENTS IN THE PRESENCE OR ABSENCE OF TYPE 2 DIABETES
Type 2 diabetes has recently been recognized as a cofactor that may modify the course of HCV infection, as well as functioning as an independent predictor of HCC. Recent studies have shown that type 2 diabetes is associated with hepatocarcinogenesis in patients with HCV infection without LC[34]. Hence, the search for specific immunological markers, which follow the progression of HCV to LC and HCC in association with type 2 diabetes, has become a topic of great scientific interest. The serum levels of 33 cytokines, chemokines, adipokines, and growth factors [adiponectin, adipsin, β-NGF, CCL27, C-peptide, CXCL1, CXCL9, CXCL12, ghrelin, gastric inhibitory polypeptide (GIP), glucagon- like peptide [glp]-1, glucagon, HGF, IFN-α2, IL-1α, IL-2R, IL-3, IL-12p40, IL-16, IL-18, insulin, leptin, LIF, MCP-3, M-CSF, MIF, plasminogen activator inhibitor (PAI)-1, resistin, SCF, SCGF-β, TNF-α, TRAIL, visfatin] were evaluated at the same time by a BioPlex panel. The serum levels of (1) patients with LC, HCV-related cirrhosis and type 2 diabetes (LCD);(2) patients with HCV and type 2 diabetes (CHD); and (3) healthy controls were used to identify specific molecules at the various stages of disease allowing for differentiation amongst them[35]. Twenty patients (12 women, 8 men) with LC, 10 patients (4 women, 6 men) with CHD, 10 patients (4 women, 6 men) with LCD, and 20 healthy control subjects (11 women, 9 men) were used for this study.
Higher levels of IL-1α, IL-2R, IL-12, IL-18, CXCL1, CXCL9, MIF, β-NGF, HGF, C-peptide, GIP, insulin, PAI-1, adiponectin, leptin, resistin, and adipsin were found in patients with LCD and LC compared to healthy controls (Table 2). Ghrelin concentration decreased in both LCD and LC patients compared to healthy controls (Table 2). Hence, the same molecules seem to be significant for both LC and LCD patients except for glucagon levels, which were increased in LCD patients but not LC (P < 0.05). Significant molecules were similar between LCD and LC patients, with the exception of IL-2R, IL-18, and glucagon, which were higher in LCD patients. This indicates that the lower levels of these three molecules in LC patients are most likely due to the presence of type 2 diabetes. Moreover, in LC patients, glucagon and HGF showed a significant positive correlation with glycemia and body mass index values and a negative correlation with albumin values, which are lower in these patients compared to controls. Since type 2 diabetes can be associated with HCC in HCV patients without LC[36], the levels of the significant molecules in CHD and LC patients were compared using a Student’s t test. The comparison allowed for separation of cytokines in two groups (similar or different) so molecules could be used as specific markers of the two different processes. In particular, β-NGF was higher in LC patients, whereas glucagon and IL-18 were higher in CHD patients (with P < 0.05). β-NGF is a pro-inflammatory protein as well as an index of inflammatory process, related to chronic infection, causing the progression to LC and cancer. Glucagon and IL-18 are mainly associated with diabetes, occurring as part of the metabolic syndrome, with an increased risk of HCC.
Table 2.
Comparison of cytokine serum levels in all patients and healthy controls
| Cytokines | Controls vs CHD | Controls vs LC | Controls vs LCD |
| Adiponectin | a↑ | a↑ | a↑ |
| Adipsin | a↑ | a↑ | a↑ |
| C-peptide | b↑ | b↑ | b↑ |
| CXCL1 | b↑ | b↑ | b↑ |
| CXCL9 | b↑ | b↑ | b↑ |
| Ghrelin | a↓ | a↓ | a↓ |
| GIP | a↑ | a↑ | a↑ |
| Glucagon | a↑ | b↑ | |
| HGF | b↑ | b↑ | b↑ |
| IL-12 | b↑ | b↑ | b↑ |
| IL-18 | b↑ | b↑ | b↑ |
| IL-1α | b↑ | b↑ | b↑ |
| IL-2R | b↑ | b↑ | b↑ |
| Insulin | b↑ | b↑ | b↑ |
| Leptin | b↑ | b↑ | b↑ |
| MIF | b↑ | b↑ | b↑ |
| PAI-1 | b↑ | b↑ | b↑ |
| Resistin | a↑ | a↑ | a↑ |
| β-NGF | b↑ | b↑ | b↑ |
P values obtained for all significant molecules in patients with (1) HCV hepatitis and type 2 diabetes (CHD); (2) with HCV-related LC, and (3) with HCV-related cirrhosis and type 2 diabetes (LCD), with respect to healthy control subjects, were calculated using the nonparametric Mann-Whitney U test. aP value < 0.05, bP < 0.01; up- and down-arrows indicate if the pro-inflammatory molecule serum concentrations in patient groups increased/decreased vs healthy controls. HCV: Hepatitis C virus; LC: Liver cirrhosis; IL: Interleukin; NGF: Nerve growth factor; HGF: Hepatocyte growth factor; MIF: Migration inhibitory factor; PAI: Plasminogen activator inhibitor.
CYTOKINE EVALUATION IN LC PATIENTS IN THE PRESENCE AND ABSENCE OF HCC
In another study, 30 HCV-related LC patients (16 females and 14 males), 34 HCC patients with HCV-related LC (11 females and 23 males) and 20 healthy control subjects (11 females and 9 males) were enrolled with the aim to study the progression from cirrhosis to cancer (paper submitted). A panel of 46 cytokines, chemokines, growth factors, and cancer biomarkers {adiponectin, adipsin β-NGF, CCL27, C-peptide, CXCL1, CXCL9, CXCL12, fibroblast growth factor (FGF)-basic,follistatin, ghrelin, granulocyte colony-stimulating factor (G-CSF), GIP, glp-1, glucagon, HGF, IFN-α2, IL-1α, IL-2R, IL-3, IL-12p40, IL-16, IL-18, insulin, leptin, LIF, MCP-3, M-CSF, MIF, osteopontin, PAI-1, platelet-derived growth factor-AB/BB,platelet-endothelial cell adhesion molecule (PECAM)-1, prolactin (PRL), resistin, epidermal growth factor receptor, V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 [sHER-2/neu (ErbB2)], SCF, SCGF-β, sIL-6Ra, tyrosine kinase with immunoglobulin and EGF homology domains-2, vascular endothelial growth factor (sVEGFR)-1, sVEGFR-2, TNF-α, TRAIL, visfatin} was evaluated. The data suggest that the expression of sHER-2/neu, sIL-6Ra, PECAM-1, PRL, IL-2R, IL-16, IL-18, CXCL12, HGF, and ghrelin tends to increase in the progression from LC to HCC. On the other hand, levels of adiponectin and adipsin decreased (Table 3). Serum levels of these biochemical markers have different significance during the progression of a disease; therefore, they can characterize the degree of the carcinogenic state in the liver of patients with HCV and LC. Furthermore, the serum levels of the significant proteins in LC and HCC patients were correlated with clinical/biochemical data by Pearson correlation. In short, sIL-6Ra showed a significant correlation with the Child-Pugh score in patients with LC, whereas ghrelin serum levels correlated with body mass index (BMI) in HCC patients. These results confirmed that sIL-6Ra could be used as a predictor of liver damage and of inflammatory processes leading to LC and, subsequently, to cancer. High levels of ghrelin are often correlated with malnutrition, which is a common problem in LC and HCC, and may readily deteriorate the clinical liver functions resulting in poor prognosis[37]. The aforementioned data were analyzed using the Ingenuity Pathway Analysis Version 7.1 (Ingenuity Systems, Inc., Redwood City, CA, United States). This computational analysis showed that IL-18, ADIPOQ, GHRL, HGF, CXCL12, and IL-2RA were associated in a network named “Cell-To-Cell Signaling and Interaction, Hematological System Development and Function, Immune Cell Trafficking” based on associated functions and data mining from experimental studies reported in the literature (Figure 3). The two subunits of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB1 and NFκB2), signal transducer and activator of transcription (STAT3), Fork-head box protein O1 (FOXO1), nuclear receptor subfamily 3 group C member 1 (NR3C1), and MET proto-oncogene are involved in the development, progression, and metastasis of HCC. The interaction of these six hub genes goes as follows: (1) STAT3 is associated with NFκB2, IL-6R, ERbB-2, ADIPOQ, and FOXO1; (2) FOXO1 is associated with NR3C1, STAT3 and PRL; (3) two subunits of NFκB correlate with CXCL12 and IL-2R; and (4) NR3C1 is associated with NFκB2, STAT3, NFκB1, and IL-18. Overall, the interactome approach showed that all the significant cytokines are strictly correlated and connected with interesting functional hub nodes.
Table 3.
Comparison of serum levels of significant cytokines between patients with live cirrhosis and patients with hepatocellular carcinoma
| Cytokines | LC vs HCC |
| Adiponectin | b↓ |
| Adipsin | a↓ |
| CXCL12 | a↑ |
| Ghrelin | b↑ |
| HGF | a↑ |
| IL-16 | b↑ |
| IL-18 | a↑ |
| IL-2R | b↑ |
| PECAM-1 | a↑ |
| Prolactin | a↑ |
| sHER-2/neu | a↑ |
| sIL-6Ra | a↑ |
P value was calculated using Mann-Whitney U test. aP value < 0.05, bP < 0.01, Up- and down-arrows indicate an increase/decrease in the serum concentrations in patient groups vs healthy controls. HCC:Hepatocellular carcinoma; LC: Liver cirrhosis; IL: Interleukin; HGF: Hepatocyte growth factor.
Figure 3.
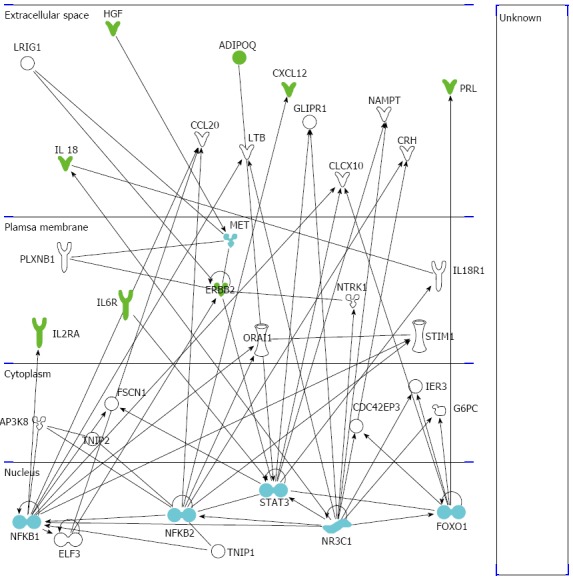
Ingenuity pathway analysis of the significant molecules. The illustration shows the network of significant cytokines (green symbols), hub nodes (light blue symbols), and other involved molecules (white symbols) obtained by the ingenuity pathway analysis software. IL: Interleukin;HGF: Hepatocyte growth factor.
It is important to underline that the functional role of cytokines depends on those signal transduction pathways that they are able to activate, and on liver cells that have receptors to these cytokines to initiate signal transduction. Using Ingenuity Pathway Analysis, the metabolic pathways, in which the significant cytokines are involved, were analyzed (Table 4). Results showed these molecules being present in pathways such as IL-6 signaling, HGF signaling, and ErbB2-ErbB3 signaling, which are important in the progression of chronic inflammation processes leading to cancer[38-40].
Table 4.
List of cytokines with significantly different serum levels between liver cirrhosis and hepatocellular carcinoma patients, and the pathways in which they play important roles
| Ingenuity canonical pathways | Molecules |
| T helper cell differentiation | IL-18, IL-6R, IL-2RA |
| Role of macrophages, fibroblasts, and endothelial | IL16, IL-18, IL-6R, CXCL12 |
| Hepatic fibrosis/hepatic stellate cell activation | LEP, HGF, IL-6R |
| Leptin signaling in obesity | LEP, GHRL |
| IL-6 Signaling | IL-18, IL-6R |
| AMPK Signaling | LEP, ADIPOQ |
| Hepatic cholestasis | IL-18, GCG |
| Acute phase response signaling | IL-18, IL-6R |
| Role of cytokines in mediating communication between immune cells | IL-18 |
| IL-2 signaling | IL-2RA |
| ErbB2-ErbB3 signaling | ERBB2 |
| Role of JAK1 and JAK3 in cytokine signaling | IL-2RA |
| Chemokine signaling | CXCL12 |
| HGF Signaling | HGF |
| Type II diabetes mellitus signaling | ADIPOQ |
| p38 MAPK signaling | IL-18 |
| LXR/RXR activation | IL-18 |
| CXCR4 signaling | CXCL12 |
| NF-κB signaling | IL-18 |
LC: Liver cirrhosis; HCC: Hepatocellular carcinoma; HGF: Hepatocyte growth factor.
CONCLUSION
Over the past several years, there has been a renaissance of research into connecting inflammation and cancer. Inflammation plays a role in tumor suppression by stimulating an antitumor immune response, but more often, under certain conditions, it appears to stimulate tumor development[41]. The intensity and nature of the inflammation can explain this apparent contradiction. In fact, in the presence of persistent inflammatory stimuli, inflammation may become chronic. However, it has been suggested that cancer-associated inflammation is similar to that of chronic inflammation. In both cases, growth and angiogenic factors are produced to stimulate tissue repair. In some cases, though, these factors can also promote cancer-cell survival, implantation, and growth[7]. Thus, it is now clear that the immune response can promote anticancer effects as well as carcinogenesis and tumor growth[41].
Considering that cytokines are expressed before and during the inflammatory processes, they play a pivotal role in cancer developmental stages[27,30]. Recently, the serum levels of many cytokines have been evaluated by a broad-spectrum bead-based multiplex immunoassay in patients with (1) chronic HCV or with LC; (2) with LC in the presence and absence of type 2 diabetes; and (3) with LC in the presence and absence of HCC. Certain interleukins and chemokines were identified as putative markers of the progression of HCV to LC by increasing fibrosis, and can be used as platforms for designing new drugs able to block the progression of the inflammatory processes[27,30]. Further research, with a larger sample size, is necessary before specific cytokines are used in the clinical diagnosis of different stages in liver injury. Thus far, we have learned that HGF is up-regulated only in patients with HCV-related LC, showing a negative correlation coefficient with albumin values, and can be used as an index of the progression from HCV to LC and HCC. Glucagon, on the other hand, is higher in LCD patients than in LC patients, showing a significant positive correlation with glycemia and BMI and a negative correlation with albumin values, and can be used as index of the co-presence of type 2 diabetes and cirrhosis in HCV patients. And last, sIL-6Ra has higher levels in LC patients than in HCC patients, showing a significant correlation with the Child-Pugh score in LC patients, and can be used as an index of progression from LC to HCC.
All the data related to the cytokine evaluations have to be computationally modeled, using graphs or metabolic networks, to connect the various data groups in terms of probabilistic dynamic maps of biochemical and/or physiological activities and/or pathogenetic pathways. This approach would enable the use of the human cytokinome as a valuable tool for the analysis of the cytokine interaction network in both healthy subjects and HCC patients[16]. Further studies may open the data sets to other diseases and implement other statistical tools and classification methods to improve or to discover new predictive relationships among data groups. It is necessary to point out that the usefulness of the cytokinome, in the evaluation of human diseases, can only be achieved through frequent and thorough repopulation of the related databases[42,43].
Footnotes
P- Reviewer: Bozdayi AM, Chuang WL, Imazeki F, Osna NA S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 2.Bondar T, Medzhitov R. The origins of tumor-promoting inflammation. Cancer Cell. 2013;24:143–144. doi: 10.1016/j.ccr.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Read SA, Douglas MW. Virus induced inflammation and cancer development. Cancer Lett. 2014;345:174–181. doi: 10.1016/j.canlet.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 6.Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol. 2013;49:887–892. doi: 10.1016/j.oraloncology.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Ueda T, Honda M, Horimoto K, Aburatani S, Saito S, Yamashita T, Sakai Y, Nakamura M, Takatori H, Sunagozaka H, et al. Gene expression profiling of hepatitis B- and hepatitis C-related hepatocellular carcinoma using graphical Gaussian modeling. Genomics. 2013;101:238–248. doi: 10.1016/j.ygeno.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Kuiken C, Yoon H, Abfalterer W, Gaschen B, Lo C, Korber B. Viral genome analysis and knowledge management. Methods Mol Biol. 2013;939:253–261. doi: 10.1007/978-1-62703-107-3_16. [DOI] [PubMed] [Google Scholar]
- 11.Estrabaud E, Vidaud M, Marcellin P, Asselah T. Genomics and HCV infection: progression of fibrosis and treatment response. J Hepatol. 2012;57:1110–1125. doi: 10.1016/j.jhep.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Sarvari J, Mojtahedi Z, Taghavi SA, Kuramitsu Y, Shamsi Shahrabadi M, Ghaderi A, Nakamura K. Differentially Expressed Proteins in Chronic Active Hepatitis, Cirrhosis, and HCC Related to HCV Infection in Comparison With HBV Infection: A proteomics study. Hepat Mon. 2013;13:e8351. doi: 10.5812/hepatmon.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tripathi LP, Mizuguchi K. A combined proteomics and computational approach provides a better understanding of HCV-induced liver disease. Expert Rev Proteomics. 2012;9:493–496. doi: 10.1586/epr.12.47. [DOI] [PubMed] [Google Scholar]
- 14.Csermely P, Korcsmáros T. Cancer-related networks: a help to understand, predict and change malignant transformation. Semin Cancer Biol. 2013;23:209–212. doi: 10.1016/j.semcancer.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Costantini S, Capone F, Guerriero E, Castello G. An approach for understanding the inflammation and cancer relationship. Immunol Lett. 2009;126:91–92. doi: 10.1016/j.imlet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Costantini S, Castello G, Colonna G. Human Cytokinome: a new challenge for systems biology. Bioinformation. 2010;5:166–167. doi: 10.6026/97320630005166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houser B. Bio-Rad’s Bio-Plex® suspension array system, xMAP technology overview. Arch Physiol Biochem. 2012;118:192–196. doi: 10.3109/13813455.2012.705301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris G, Chen W. Profiling of cytokine and chemokine responses using multiplex bead array technology. Methods Mol Biol. 2013;1061:265–278. doi: 10.1007/978-1-62703-589-7_16. [DOI] [PubMed] [Google Scholar]
- 19.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm. 2006;2006:28398. doi: 10.1155/MI/2006/28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasumi Y, Takikawa Y, Endo R, Suzuki K. Interleukin-17 as a new marker of severity of acute hepatic injury. Hepatol Res. 2007;37:248–254. doi: 10.1111/j.1872-034X.2007.00040.x. [DOI] [PubMed] [Google Scholar]
- 21.Matte I, Lane D, Laplante C, Rancourt C, Piché A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res. 2012;2:566–580. [PMC free article] [PubMed] [Google Scholar]
- 22.Miyahara K, Nouso K, Morimoto Y, Takeuchi Y, Hagihara H, Kuwaki K, Onishi H, Ikeda F, Miyake Y, Nakamura S, et al. Pro-angiogenic cytokines for prediction of outcomes in patients with advanced hepatocellular carcinoma. Br J Cancer. 2013;109:2072–2078. doi: 10.1038/bjc.2013.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costantini S, Autiero I, Colonna G. On new challenge for the Bioinformatics. Bioinformation. 2008;3:238–239. doi: 10.6026/97320630003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woller N, Kühnel F. Virus infection, inflammation and prevention of cancer. Recent Results Cancer Res. 2014;193:33–58. doi: 10.1007/978-3-642-38965-8_3. [DOI] [PubMed] [Google Scholar]
- 25.Shrivastava S, Mukherjee A, Ray RB. Hepatitis C virus infection, microRNA and liver disease progression. World J Hepatol. 2013;5:479–486. doi: 10.4254/wjh.v5.i9.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzahrani B, Iseli TJ, Hebbard LW. Non-viral causes of liver cancer: does obesity led inflammation play a role? Cancer Lett. 2014;345:223–229. doi: 10.1016/j.canlet.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Costantini S, Capone F, Guerriero E, Maio P, Colonna G, Castello G. Serum cytokine levels as putative prognostic markers in the progression of chronic HCV hepatitis to cirrhosis. Eur Cytokine Netw. 2010;21:251–256. doi: 10.1684/ecn.2010.0214. [DOI] [PubMed] [Google Scholar]
- 28.Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27:85–94. doi: 10.1007/s10555-007-9107-6. [DOI] [PubMed] [Google Scholar]
- 29.Yamagamim H, Moriyama M, Matsumura H, Aoki H, Shimizu T, Saito T, Kaneko M, Shioda A, Tanaka N, Arakawa Y. Serum concentrations of human hepatocyte growth factor is a useful indicator for predicting the occurrence of hepatocellular carcinomas in C-viral chronic liver diseases. Cancer. 2002;95:824–834. doi: 10.1002/cncr.10732. [DOI] [PubMed] [Google Scholar]
- 30.Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, Scala S, Izzo F, Castello G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw. 2010;21:99–104. doi: 10.1684/ecn.2010.0192. [DOI] [PubMed] [Google Scholar]
- 31.Zekri AR, Alam El-Din HM, Bahnassy AA, Zayed NA, Mohamed WS, El-Masry SH, Gouda SK, Esmat G. Serum levels of soluble Fas, soluble tumor necrosis factor-receptor II, interleukin-2 receptor and interleukin-8 as early predictors of hepatocellular carcinoma in Egyptian patients with hepatitis C virus genotype-4. Comp Hepatol. 2010;9:1. doi: 10.1186/1476-5926-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324–G1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura K, Nagaki M, Nishihira J, Satake S, Kuwata K, Moriwaki H. Role of macrophage migration inhibitory factor in hepatitis B virus-specific cytotoxic-T-lymphocyte-induced liver injury. Clin Vaccine Immunol. 2006;13:415–419. doi: 10.1128/CVI.13.3.415-419.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung CH, Lee CM, Wang JH, Hu TH, Chen CH, Lin CY, Lu SN. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int J Cancer. 2011;128:2344–2352. doi: 10.1002/ijc.25585. [DOI] [PubMed] [Google Scholar]
- 35.Costantini S, Capone F, Guerriero E, Marfella R, Sorice A, Maio P, Di Stasio M, Paolisso G, Castello G, Colonna G. Cytokinome profile of patients with type 2 diabetes and/or chronic hepatitis C infection. PLoS One. 2012;7:e39486. doi: 10.1371/journal.pone.0039486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 37.Ataseven H, Bahcecioglu IH, Kuzu N, Yalniz M, Celebi S, Erensoy A, Ustundag B. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators Inflamm. 2006;2006:78380. doi: 10.1155/MI/2006/78380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 40.Arima N, Kuziel WA, Grdina TA, Greene WC. IL-2-induced signal transduction involves the activation of nuclear NF-kappa B expression. J Immunol. 1992;149:83–91. [PubMed] [Google Scholar]
- 41.Lee JM, Yang J, Newell P, Singh S, Parwani A, Friedman SL, Nejak-Bowen KN, Monga SP. β-Catenin signaling in hepatocellular cancer: Implications in inflammation, fibrosis, and proliferation. Cancer Lett. 2014;343:90–97. doi: 10.1016/j.canlet.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miele M, Sharma A, Capone F, Raucci R, Guerriero E, Colonna G, Castello G, Stasio MD, Costantini S. CytReD: A database collecting human cytokinome information. Bioinformation. 2011;6:207–208. doi: 10.6026/97320630006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evangelista D, Colonna G, Miele M, Cutugno F, Castello G, Desantis S, Costantini S. CDMS (Clinical Data Mining Software): a cytokinome data mining system for a predictive medicine of chronic inflammatory diseases. Protein Eng Des Sel. 2010;23:899–902. doi: 10.1093/protein/gzq068. [DOI] [PubMed] [Google Scholar]


