Abstract
Hepatitis C virus (HCV) affects 130-210 million people worldwide and is one of the major risk factors for hepatocellular carcinoma. Globally, at least one third of hepatocellular carcinoma cases are attributed to HCV infection, and 350000 people died from HCV related diseases per year. There is a great geographical variation of HCV infection globally, with risk factors for the HCV infection differing in various countries. The progression of chronic hepatitis C to end-stage liver disease also varies in different study populations. A long-term follow-up cohort enrolling participants with asymptomatic HCV infection is essential for elucidating the natural history of HCV-caused hepatocellular carcinoma, and for exploring potential seromarkers that have high predictability for risk of hepatocellular carcinoma. However, prospective cohorts comprising individuals with HCV infection are still uncommon. The risk evaluation of viral load elevation and associated liver disease/cancer in HCV (REVEAL-HCV) study has followed a cohort of 1095 residents seropositive for antibodies against hepatitis C virus living in seven townships in Taiwan for more than fifteen years. Most of them have acquired HCV infection through iatrogenic transmission routes. As the participants in the REVEAL-HCV study rarely receive antiviral therapies, it provides a unique opportunity to study the natural history of chronic HCV infection. In this review, the prevalence, risk factors and natural history of HCV infection are comprehensively reviewed. The study cohort, data collection, and findings on liver disease progression of the REVEAL-HCV study are described.
Keywords: Hepatitis C virus, Epidemiology, Risk evaluation of viral load elevation and associated liver disease/cancer, Long-term liver progression
Core tip: This review includes summary tables describing the epidemiology of hepatitis C virus (HCV) infection in previous studies and recent findings from the risk evaluation of viral load elevation and associated liver disease/cancer in HCV study.
INTRODUCTION
Hepatitis C virus (HCV) is recognized as a major cause of chronic liver disease. Liver cirrhosis generally occurs in 20%-30% patients with chronic infection after 2 to 3 decades[1]. Once cirrhosis occurs, hepatocellular carcinoma develops in 1%-4% of these patients per year[2]. It was estimated that HCV was attributable to one thirds of hepatocellular carcinoma cases globally[3], representing a significant public health burden. In this review article, we summarize the prevalence, risk factors and the natural history of hepatitis C virus infection. In addition, we describe the study population, data collection, and findings of liver disease progression of the Risk Evaluation of Viral Load Elevation and Associated Liver Diseases/Cancers in HCV (REVEAL-HCV) study.
PREVALENCE OF HCV INFECTION
According to WHO reports, 3% of the world’s population has been infected with HCV, representing 170 million people at risk of developing chronic liver diseases[4,5]. The HCV-estimated prevalence in economically developed countries is relatively low with 1%-2% of the adult population whereas 5%-10% in less developed countries[4-6]. The countries with higher reported prevalence were located in Africa, Eastern Mediterranean, South-East Asia and the West Pacific[5,6]; areas with lower prevalence included North America, northern and western Europe and Australia.
The HCV seroprevalence studies provide useful descriptive data to understand global HCV epidemiology. A large number surveys were conducted to estimate the distributions of HCV. However, most studies mainly enrolled specific populations, such as blood donors and clinical patients, which are not representative of the population of the regions in which they reside. Moreover, the estimated prevalence might be underestimated or overestimated because blood donors are healthier than the general population and clinical patients already had symptoms.
The community-based studies were relatively limited and not available in most countries. The seroprevalence of HCV has a considerable geographical variation, which may be explained by different distributions and different contributions of risk factors in different study regions. The community-based HCV seroprevalence in different countries is displayed in Table 1. It has a striking geographical variation in anti-HCV seroprevalence, ranging from 0.5%-24.3%[7-18].
Table 1.
Seroprevalence of antibodies against hepatitis C virus in community-based studies
| Study site | Study period | Study population | Population size | % with HCV antibody | % with positive HCV RNA among anti-HCV seropositives |
| America | |||||
| United States[7] | 1988-1994 | National Health and Nutrition Examination Survey, subjects aged ≥ 6 yr | 21241 | 1.8 | 73.9 |
| Puerto Rico[15] | 2001-2002 | Individuals aged 21-64 yr residing in the municipality of San Juan | 964 | 6.3 | Not done |
| Europe | |||||
| Greece[11] | 1997-1998 | Individuals over 15 yr in south western Greece | 1500 | 0.5 | Not done |
| Norway[9] | 2000-2001 | A subset of Oslo Health Study, subjects older than 30 yr | 11456 | 0.7 | 79.5 |
| France[10] | 1994 | Individuals aged 20-59 undergoing routine medical checkup in social security medical centers | 6283 | 1.2 | 81.0 |
| Spain[16] | 1997-1998 | Random sample of all ages in northern Spain | 1170 | 1.6 | 63.0 |
| Italy[12] | 1996 | Individuals of all ages which representative to southern Italy | 1352 | 12.6 | 84.7 |
| Asia | |||||
| India[8] | 1999 | Individuals of all ages living in rural area and engaged in agriculture-related occupations | 2973 | 0.9 | 80.8 |
| China[18] | 1992 | Individuals aged 1-59 yr in 30 provinces | 68000 | 3.2 | Not done |
| Taiwan[17] | 1991-1992 | Males aged 30-65 yr participated a cancer screening project | 11904 | 4.9 | Not done |
| Japan[14] | 1984-1995 | Residents aged 20-89 yr in southern Miyazaki Prefecture | 973 | 23 | Not done |
| Africa | |||||
| Egypt[13] | 1997 | Adults and children aged older than 10 yr residing in Nile Delta | 3999 | 24.3 | Non done |
HCV: Hepatitis C virus.
RISK FACTORS FOR HCV INFECTION
The most important transmission modes of HCV are through blood or blood-related products. It was noticed that the supply of blood in the world was contaminated with an unidentified agent causing post-transfusion non-A, non-B hepatitis[19]. In developed countries post-transfusion hepatitis C has become relatively rare. Incidence of transfusion associated hepatitis, traced from 1970 to 1998, demonstrated a decrease from 33% to nearly eliminated HCV transmission caused by the effectiveness of a series of donor screening intervention[20]. In developing countries where HCV testing in blood donation has not been feasible, receiving blood products remains a dominant source of HCV infection. Most of these countries are located in Africa and Asia, where blood safety is threatened by poverty, insufficient instruments and laboratory reagents, limited supply of trained professionals, traditional cultural barriers, and difficulties in mobilizing volunteer donors[21,22].
In developed countries, HCV is mainly transmitted by drug abusers sharing injection equipment[23]. The prevalence of anti-HCV among intravenous drug users ranged from 31% to 98%[24]. It has been reported that injection drug use accounts for 60% and 80% of HCV infection in United States[7] and Australia[25], respectively. In developing countries, HCV transmission is mainly by unsafe therapeutic injections[12,17]. Unsafe injections, defined as reuse of syringes or needles from patient to patient without sterilization, resulted in 2.3-4.7 million HCV infections every year approximately[26]. Transmission of HCV through contaminated injection equipment has been recognized in most developing countries[8,27-29]. The results indicated that the injection equipment were contaminated or non-disposable, which resulted in the spread of HCV. People with increased frequencies of injections for therapeutic purposes had elevated cumulative risks of HCV infection. The evidence suggested that it is important to reduce injection reuse and overuse in the prevention aspect of HCV control, especially in areas with limited disposable injection equipment and health professionals.
Other sources of HCV transmission include activities involving the potential for percutaneous exposure to blood or blood-derived body fluids, such as tattooing[15], acupuncture[17,30], sharing cottons[31], and other biologically plausible modes of transmission, like body-piercing, cosmetic procedures, and commercial barbering[32]. Vertical transmission from mother to neonate is rare[33] and intra-household and within-couples spread of HCV infections is possible[34,35]. It was also indicated that lower education, poverty[7,23], and residing in highly deprived area[36] are risk factors for positive anti-HCV.
HCV INFECTION AND LIVER-RELATED DISEASES
HCV infection is infrequently diagnosed during the acute phase because majority of persons have either no symptoms or only mild symptoms. The asymptomatic infection becomes chronic in most cases, and people are unaware of the infections until end-stage liver diseases occur.
Several follow-up studies[37-44] were conducted to evaluate the clinical outcomes related to HCV infection, which are summarized in Table 2. These studies mainly enrolled specific populations such as patients in liver clinics[42], patients with post-transfusion hepatitis[38], blood donors[40], drug abusers[41] and women vaccinated with contaminated immunoglobulin[37,44]. The Japanese studies recruited residents in a community where HCV is endemic (prevalence approximately 25%), and most of the participants were of advanced age[39,43]. Most of the studies had difficulties in defining the source of HCV infection[38-43], particularly for the studies that enrolled the general population[39,43]. Although the time of HCV infection was reported in some studies, it might be biased by asking participants if they recalled their transfusion time or first injection use[38,41,42].
Table 2.
Follow-up studies to evaluate liver-related morbidities and mortality associated with hepatitis C virus infection
| Ref. | Study population | Mean follow-up (yr) | Identification of infection |
Liver-related disease |
Mortality |
Comments | ||
| LC | HCC | All-cause | Liver-related | |||||
| Tong et al[42], 1995United States | 213 patients | 3.9 | Patients recalled the time of transfusion | 51.1% | 5.3% | 15.3% | 14.5% | Most participants had symptoms |
| Patients from tertiary care center with a history of transfusion | ||||||||
| Recall bias | ||||||||
| Seeff et al[38], 2001United States | 222 patients | 25 | Time of transfusion | 67.1% | 4.1% | 70% were males | ||
| Wiese et al[44], 2005Germany | 683 CHC women | 25 | Vaccinated in 1978-1979 | 1.3% | 0.1% | Relatively young and healthy | ||
| CHC defined as HCV RNA (+) | ||||||||
| Kenny-Walsh[37], 1999Ireland | 376 CHC women | 17 | Vaccinated in 1977-1978 | 2% | Relatively young and healthy | |||
| CHC defined as HCV RNA (+) | ||||||||
| Thomas et al[41], 2000United States | 1667 drug abusers | 8.8 | First injection use | ESLD incidence:3101 | 1/3 with HIV (+) | |||
| Recall bias | ||||||||
| Tanaka et al[40], 2004Japan | 1927 blood donors | 8.3 | Unknown | 3341 | Participants from Osaka Red Cross Blood Center | |||
| relatively healthy | ||||||||
| Suruki et al[39], 2006Japan | 667 CHC adults | 7.9 | Unknown | 9831 | Community-based | |||
| CHC defined by at least 1 HCV RNA/core antigen result | ||||||||
| 70% participants of age older than 60 yr | ||||||||
| Serial tests for serum ALT | ||||||||
| Uto et al[43], 2009Japan | 1125 adults | 8.2 | Unknown | 25001 | Community-based | |||
| Tested for HCV RNA/core antigen | ||||||||
Per 100000 person-year. LC: Liver cirrhosis; HCC: Hepatocellular carcinoma; CHC: Chronic hepatitis C.
The clinical outcomes after HCV infection were highly variable. There were around 1.3%-51% of individuals with HCV infection who developed liver cirrhosis and 0.1%-5.3% developed hepatocellular carcinoma during 3.9-25 years[42,44]. The incidence of hepatocellular carcinoma was lower in blood donors than in the general population because the blood donors were relatively healthy and young[40]. Liver-related mortality ranged from 15.3%-67.1%[38,42]. Discrepancies resulted not only from the risk factors associated with progression distributed differently between these studies but also heterogeneity in study populations. Study participants from tertiary care facilities[42] might suffer from more severe conditions and the referral bias might lead to overestimations of serious liver diseases after HCV infection. Vaccinated women of childbearing age and blood donors were relatively young and healthy[37,40,44]. The estimations of progressions of liver disease were strongly influenced by study population samplings.
HCV INFECTION MARKERS AND RISK FOR LIVER DISEASES
There have been a number of studies that attempted to examine the relationship of serum concentration of HCV RNA with liver disease severity by relating it to histopathological abnormality[45-50] (Table 3). Some studies identified that the HCV RNA load correlated with hepatic inflammation[47,49,50]; however, others indicated that serum levels of HCV RNA were not associated with hepatic inflammation[45] nor fibrosis[46,48-50]. These studies were conducted to elucidate serum HCV RNA in the prediction of severity of liver diseases used cross-sectional design, resulting in the limitations of causal temporality. A report followed 6570 drug users for around eight years with a case-cohort design and showed that the HCV RNA level was a predictor of end-stage liver disease death, with adjusted hazard ratio and 95%CI 2.3 (1.5-5.9)-fold higher per log10 IU/mL increase in HCV load[51]. However, some of the drug users were co-infected with HIV and some had human lymphotropic virus type II; thus, the generalizability to the population in the community was limited.
Table 3.
Serum levels of hepatitis C virus RNA and liver-related diseases
| Ref. | Study population | Study design | Serum RNA measurements | Findings | Comments |
| Naito et al[50] | 22 HCV carriers with detectable RNA and persistently normal serum ALT levels in Japan | Cross-sectional | Competitive RT-PCR | Serum viral load were correlated with HAI score (r = 0.68, P < 0.01) | Limited number of study participants |
| Temporality | |||||
| No control for confounders | |||||
| De Moliner et al[45] | 96 patients without antiviral treatments in Italy | Cross-sectional | First-generation bDNA assay (QuantiplexTM HCV RNA 1.0) | Serum viral load was not correlated with liver histological diagnosis (r = 0.58) | Temporality |
| No control for confounders | |||||
| Fanning et al[47] | 77 women infected HCV genotype 1b through vaccination in Ireland | Cross-sectional | RT-PCR | Serum viral load was weakly (r = 0.26, P < 0.05) correlated with HAI score | Temporality |
| Not correlated with the degree of fibrosis (r = 0.22, P > 0.05) | Homogeneous participants with defined source of infection and the same duration of infection | ||||
| Lagging et al[48] | 98 patients without antiviral treatments in Sweden | Cross-sectional | RT-PCR with Cobas Amplicor HCV monitor test | Serum viral load was not associated with the degree of inflammation or fibrosis | Temporality |
| Hisada et al[51] | 385 drug users with detectable HCV RNA in United States | Case-cohort | Third-generation of bDNA assay (QuantiplexTM HCV RNA 3.0) | Elevated serum levels of HCV RNA increased the risk of ESLD death (relative hazard = 2.3 per log10 IU/mL, 95%CI: 1.5-5.9) | Coinfected with HIV or HTLV-II |
| Large population with eight yr of follow-up |
RT-PCR: Reverse-transcription polymerase chain reaction; HAI: Histological activity index; HCV: Hepatitis C virus; ESLD: End-stage liver disease; HTLV: Human T lymphotropic virus; HIV: Human immunodeficiency virus.
There are six major HCV genotypes[52]. Table 4 shows the associations of HCV genotype and liver related diseases. Most of the studies were limited to cross-sectional design[53] or enrolled clinical patients[53-57]. A prospective study followed 163 liver cirrhosis patients for seventeen years and indicated that HCV genotype 1b was a major risk factor with a three times higher risk of developing hepatocellular carcinoma compared with participants infected with other types[54]. However, most patients in the study had other liver-related co-morbidities and had been treated with interferon. Since patients infected with HCV genotype-1 had lower likelihood of sustained virological response and were recommended for longer duration of therapy[58], it was possible that the lower response rate resulted from the HCV genotype-1b infected patients with higher hepatocellular carcinoma incidence observed in the study. Moreover, the findings only indicated that infected HCV genotypes had an impact on the prognosis of late clinical stage. Whether HCV genotypes could predict progression of liver disease, especially for healthy carriers of chronic hepatitis C searching for clinical consultation, still lacked sufficient evidence.
Table 4.
Hepatitis C virus genotypes and liver-related diseases
| Ref. | Study population | Study design | HCV genotypes determination | Findings | Comments |
| Martinot-Peignoux et al[53] | 1872 HCV infected patients from 14 tertiary referral centers in France | Cross-sectional | Reverse hybridization with line probe assay (LiPA) | LC in genotype 1b and 4 (13% and 13%) were found more frequently than in genotype 1a, 2, or 3 (8%, 9%, and 7%), P = 0.03 | Clinical patients |
| temporality | |||||
| Only proportions provided, not control for other confounders | |||||
| Silini et al[57] | 162 LC and 162 HCC cases in Italy | Case-control | Polymerase chain reaction | Genotype 1b vs others: OR = 1.7 (1.1-2.9) | Clinical patients |
| Temporality | |||||
| Matched with age, gender, child’s class | |||||
| Kobayashi et al[56] | 140 untreated CHC patients in Japan | Retrospective follow-up | Enzyme-linked immunosorbent assay | Deterioration of the stage of liver histology:Genotype 1, 63%Genotype 2, 39%P < 0.05 | Clinical patients |
| Only proportions calculated and time not taken into analytical consideration | |||||
| Fattovich et al[55] | 292 biopsy-proven LC patients form 7 referral centers in Europe | Prospective follow-up | Nested polymerase chain reaction | HCC risk | Clinical patients and more than 1/2 were treated with interferon |
| Genotype 1b vs others | |||||
| HR = 1.0 (0.5-2.3) | |||||
| Bruno et al[54] | 163 liver cirrhosis patients in Italy | Prospective follow-up | INNO-LiPA HCV II (Bayer Corp., Tarrytown, NY) | HCC risk | Interferon treated patients |
| Genotype 1b vs 2a/c | Incidence of HCC calculated | ||||
| HR = 3.0 (1.4-6.5) |
CHC: Chronic hepatitis C; LC: Liver cirrhosis; HCC: Hepatocellular carcinoma; OR: Odds ratio; HR: Hazard ratio.
REVEAL-HCV STUDY COHORT
The REVEAL-HCV study cohort[59,60] is a community-based study that recruited subjects from seven townships in Taiwan during 1991-1992. The study areas included two northern townships (Sanchi and Chutung) and two southern townships (Potzu and Kaohsu) on the main Taiwan Island, and three townships (Makung, Huhsi, and Paihsa) on the Penghu Islets.
At the beginning, 89293 inhabitants aged 30-65 years old in the seven study townships were invited to participate in the study. Among them, 23820 (11973 males and 11847 females) were enrolled after giving written informed consent. At enrollment, well-trained public health nurses personally interviewed the participants using structured questionnaires. The collected information included sociodemographic characteristics (age, sex, educational levels, occupation, etc.), lifestyle (cigarette smoking, alcohol consumption, and betel nut chewing), and personal and family history of major diseases. Anthropometric measurements including weight and height were also performed. The vital statuses of the study participants were followed by the computerized linkage with the national cancer registration and death certification profiles. The national identification number, date at birth, and sex were used as the linking variables to double-check the vital status and causes of death of study participants.
In addition to the questionnaire interview, 10 mL blood samples were collected from each participant at study entry and during follow-up. We invited the participants to undergo health examinations every six to twelve months. The blood samples were obtained using disposable needles and heparinized vacuum syringes. They were fractioned on the day of collection and stored at -70 °C until assayed. Serum samples of all participants were tested for hepatitis B surface antigen (HBsAg) by radioimmunoassay (Abbott Laboratories, North Chicago, IL, United States), anti-HCV by enzyme immunoassay (Abbott Laboratories), and serum levels of aspirate aminotransferase and alanine aminotransferase by a serum chemistry autoanalyzer (Model 736, Hitachi, Tokyo, Japan) using commercial reagents (Biomerieux, Marcy L’Etoile, France).
Participants who were seropositive for anti-HCV were further examined for serum HCV RNA levels by polymerase chain reaction using the COBAS TaqMan HCV test, v2.0 (Roche Diagnostics, Indianapolis, NJ, United States), and an in vitro nucleic acid amplification test for the quantification of HCV RNA. The quantification method used the high pure system viral nucleic acid kit for manual specimen preparation and the COBAS TaqMan 48 Analyzer for automated amplification and detection. The manufacturer’s procedures for sample preparation to extract HCV RNA, automated reverse transcription of the target RNA to generate complementary DNA, and amplification of target cDNA, were followed. In any test procedure, a replicate of negative, low-positive, and high-positive controls were included in each run for HCV RNA quantification. The HCV RNA titer was expressed in International Units (IU)/mL, according to the WHO International Standard for HCV RNA NAT assays, and the linear range for the COBAS TaqMan HCV test was from 25 to 3.9 × 108 IU/mL. Moreover, those with positive serum HCV RNA levels were examined for HCV genotypes by melting curve analysis, which could effectively differentiate different HCV genotypes by showing different melting temperatures[61,62].
AGE AND SEX SEROPREVALENCE OF ANTI-HCV IN REVEAL-HCV STUDY COHORT
Among the 23820 participants who agreed to be enrolled in this study, there were 1,313 seropositive for anti-HCV. The overall anti-HCV seroprevalence in the community was 5.5%. Seroprevalence increased with advancing age in the population. For females, the seroprevalences of HCV were 3.0%, 3.6%. 4.2%, 6.8%, 7.3%, 9.7% and 9.8%, respectively, for the age groups 30-34, 35-39, 40-44, 45-49, 50-54, 55-59, and 60-65 years old. The corresponding seroprevalences for males were 2.7%, 3.7%, 3.2%, 5.2%, 5.6%, 6.4%, and 6.1%, respectively. Females had higher age-specific anti-HCV seroprevalences than males, with an overall seroprevalence of 6.2% vs 4.8%, respectively[63].
There were 1095 participants who were seropositive for anti-HCV but seronegative for HBsAg. Among them, 975 (89%) had adequate retrievable serum samples for the HCV RNA test. Comparing those who had adequate serum samples (n = 975) and those without adequate serum samples for an HCV RNA test (n = 120), there were no significant differences in the distributions of baseline characteristics, except for gender. However, for the 975 anti-HCV seropositives with adequate samples for HCV RNA test, the gender proportion was similar to that of all 1095 anti-HCV seropositives.
CUMULATIVE LIFETIME INCIDENCE OF HEPATOCELLULAR CARCINOMA IN REVEAL-HCV STUDY COHORT
The participants were followed from 1991 to the end of 2008. One hundred and one newly developed hepatocellular carcinoma cases occurred after 17944 person-years of follow-up, giving the incidence rate of 562.9 per 100000 person-years. Figure 1 shows the cumulative lifetime incidence of hepatocellular carcinoma, using age as a follow-up scale. The cumulative lifetime incidence (from 30 to 80 years old) of HCC was 18.6% for the participants in the REVEAL-HCV cohort. In previous reports of the REVEAL-HCV study, the risk of developing hepatocellular carcinoma was significantly associated with increasing age, positive HCV RNA, elevated serum ALT levels and HCV genotype 1[59]. Figure 2 shows the cumulative lifetime incidence of hepatocellular carcinoma by sex, HCV RNA, serum levels of ALT, HCV genotype. The cumulative lifetime risk was 19.7% and 17.15% for males and females (P = 0.18), respectively. The lifetime risk was 3.63% and 24.77% for those with undetectable and detectable serum HCV RNA levels, respectively. There was a biological gradient of cumulative lifetime incidence of hepatocellular carcinoma across the serum ALT levels. For those with serum ALT levels ≤ 15 U/L, 16-45 U/L, > 45 U/L, the cumulative lifetime risks were 11.62%, 18.45% and 34.3%, respectively. In addition, the cumulative risks for HCV genotype non-1 and HCV genotype 1 were 20.1% and 25.85%, respectively. In Taiwan, the most prevalent HCV genotypes were 1b and 2a[64]. Thus, the results implied that HCV genotype 1b infection increased the risk of hepatocellular carcinoma[65].
Figure 1.
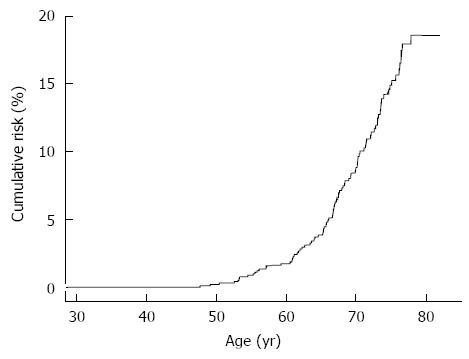
Cumulative life-time risk (30-80 years old) of hepatocellular carcinoma.
Figure 2.
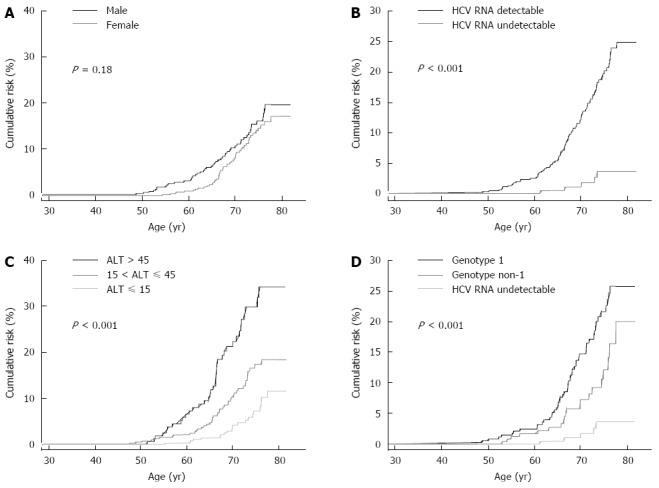
Cumulative life-time risk. A: Cumulative lifetime risk (30-80 years old) of hepatocellular carcinoma by gender; B: Cumulative lifetime risk (30-80 years old) of hepatocellular carcinoma by hepatitis C virus (HCV) RNA; C: Cumulative lifetime risk (30-80 years old) of hepatocellular carcinoma by serum levels of alanine aminotransferase; D: Cumulative lifetime risk (30-80 years old) of hepatocellular carcinoma by HCV genotype. ALT: Alanine aminotransferase.
LONG-TERM PREDICTORS OF HEPATOCELLULAR CARCINOMA IN REVEAL-HCV STUDY COHORT
In the multivariate analysis, the seromarkers, including serum levels of HCV RNA and ALT and HCV genotype, remained significantly associated with hepatocellular carcinoma after adjustment for age, sex, cigarette smoking, alcohol consumption, obesity and history of diabetes[59]. The seromarkers were mutually independent risk predictors of hepatocellular carcinoma among patients with chronic hepatitis C virus infection. It will be interesting to integrate the relevant seromarkers for the development of prediction models for hepatocellular carcinoma among chronic hepatitis C patients. Furthermore, the new predictors, such as host genetic markers, will increase the accuracy of long-term prediction of end-stage liver diseases.
ADVANTAGES AND LIMITATIONS OF REVEAL-HCV STUDY
The REVEAL-HCV can be considered as a natural history cohort. Most of the participants in this cohort had no experience of antiviral treatment. In Taiwan, chronic hepatitis C patients rarely received antiviral treatment with interferon because of its high cost and adverse effects, until November 2003, when patients with abnormal serum ALT levels (> 82 U/L) and moderate fibrosis proven by liver biopsy could be reimbursed for treatment by the National Health Insurance. To ensure study participants received standard care, those who had abnormal serum levels of ALT and α-fetoprotein or abnormal ultrasound findings were referred to medical centers for further clinical managements in this study. This cohort, comprising 1000 anti-HCV seropositives, provided an exceptional opportunity to examine the seromarker changes and liver disease occurrence of anti-HCV seropositives during the natural course of HCV infection.
Unlike other cohorts that enrolled patients with experiences of drug injections[51] or HCV-contaminated vaccinations[44], the REVEAL-HCV cohort enrolled participants living in the community. Thus, the exact time of HCV infection was not obtainable for our study participants. The major risk factors of HCV infection in the REVEAL-HCV cohort were iatrogenic factors[17]; therefore, it was difficult to obtain the exact time of HCV infection. In addition, it was not practical to have the asymptomatic participants examined by liver biopsy, thus the information on advanced fibrosis or mild cirrhosis was not available in this community-based cohort.
CONCLUSION
Individuals with HCV infection are often asymptomatic and unaware of their illness until severe liver diseases present; therefore, it is necessary to understand the natural history of chronic hepatitis C virus infection from a prospective viewpoint. Residents living in the same community as clinical patients represent the general population. Based on the findings by including this population, prevention strategies could precede clinical stages. Individuals seropositive for anti-HCV should be monitored regularly and tested for their serum HCV RNA by sensitive assays. Those who have high serum HCV RNA levels and ALT levels, and HCV genotype 1 infection, should be consulted for their high risk for the liver diseases and intensive care options discussed.
In the near future, several issues could be addressed. The seromarkers, serum HCV RNA and ALT levels and HCV genotype, could be used to predict subsequent risk of HCV related hepatocellular carcinoma, indicating that the seromarkers have potential to be used as pretreatment markers in clinical decisions to classify high-risk patient who need intensive care. However, a risk assessment calculator, which incorporates several patients’ characteristics, is more convenient and comprehensive for clinical consultations. It is helpful for communications between clinicians and patients to discuss treatment options based on patients’ individual risk profiles. Therefore, to develop a risk calculator including the seromarkers found in our study will be informative.
In addition, it is probable that the host genetic background affects HCV infection outcomes. IL28B gene variants were found to be associated with sustained virological response among chronic hepatitis C patients receiving antiviral therapy[66-68]. Moreover, the individuals who carried the variants with favorable treatment response had increased probability of experiencing spontaneous HCV RNA clearance[69,70]. Patients without experiencing spontaneous HCV resolution, who were considered to have active HCV infection and with detectable HCV RNA, had an increased risk of hepatocellular carcinoma and liver-related mortality[59,60]. Thus, the associations between IL28B variants and the hepatocarcinogenesis deserve to be investigated. However, most individuals in Asia carry the favorable genotype[71]. In other worlds, the minor allele frequency of IL28B in the Asian population is rare. It is essential to carry out a large-scale study to elucidate the associations between IL28B variants and the risk of hepatocellular carcinoma. Recently, the development of high-throughput technology has enabled researchers to test hundreds of thousands of single nucleotide polymorphisms distributed throughout the human genome. Comparing hepatocellular carcinoma cases and controls to evaluate the differences in their genetic variants will help to identify genetic markers that could be utilized as predictive tools.
The substitution of amino acids 70 and 91 in the HCV core region was associated with hepatocarcinogenesis among clinical patients with HCV genotype 1b infection and antiviral treatment[72]. In addition, the amino acid substitution had predictability for early and sustained virological responses in treated patients[73,74]. Even considering the genetic variation of IL28B gene, the association between the amino acid substitutions in HCV core region and antiviral treatment response remained[73]. Interestingly, the amino acid substitution in HCV core region had impact on the risk of hepatocellular carcinoma and the survival of HCV-infected patients without treatment[75]. Both host and virus factors are important determinants of liver diseases. The elucidation of the interactive effects of host and virus factors on hepatocarcinogenesis will help prevent severe HCV-related liver diseases.
The expression of HCV core protein in the transgenic mice was directly responsible for the insulin resistance[76]. Among non-diabetic patients infected with HCV genotype 3, insulin resistance was associated with an increased risk of liver fibrosis[77]. Metabolic factors, including obesity and diabetes, were found to be predictors for the development of hepatocellular carcinoma among hepatitis C patients[78]. The relationship between diabetes and hepatocellular carcinoma were observed in a large scale community-based study conducted in the United States[79]. Therefore, it was suspected that the lipid metabolism might represent one of the pathways leading to hepatocarcinogenesis. The association between HCV infection and the development of diabetes remains controversial[80,81]. A large follow-up study will help the elucidation of the relationship between HCV infection and the incidence of diabetes. Other than diabetes, HCV infection has been reported to be responsible for extrahepatic diseases[60,82]. In addition, to investigate the associations and mechanisms of HCV infection and extrahepatic diseases, it is worth evaluating the reductions of hepatic and extrahepatic diseases after implementation of HCV antiviral therapy.
In conclusion, chronic hepatitis C patients have increased risk for hepatocellular carcinoma and need intensive care. Determining host and virus genetic variants, and their interactions, will aid the development of predictive biomarkers and therapeutic strategies.
Footnotes
P- Reviewer: Chuang WL, Chiu KW, Franceschi F, Kawaguchi T, Lakatos PL, Xia HHX S- Editor: Gou SX L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation. Hepatitis C: global prevalence. Weekly Epidemiological Record. 1999;74:421–428. [Google Scholar]
- 5.Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 6.Madhava V, Burgess C, Drucker E. Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. Lancet Infect Dis. 2002;2:293–302. doi: 10.1016/s1473-3099(02)00264-5. [DOI] [PubMed] [Google Scholar]
- 7.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Chaudhuri S, Maity SG, Naik TN, Bhattacharya SK, Mazumder DN. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37:802–809. doi: 10.1053/jhep.2003.50157. [DOI] [PubMed] [Google Scholar]
- 9.Dalgard O, Jeansson S, Skaug K, Raknerud N, Bell H. Hepatitis C in the general adult population of Oslo: prevalence and clinical spectrum. Scand J Gastroenterol. 2003;38:864–870. doi: 10.1080/00365520310004542. [DOI] [PubMed] [Google Scholar]
- 10.Dubois F, Desenclos JC, Mariotte N, Goudeau A. Hepatitis C in a French population-based survey, 1994: seroprevalence, frequency of viremia, genotype distribution, and risk factors. The Collaborative Study Group. Hepatology. 1997;25:1490–1496. doi: 10.1002/hep.510250630. [DOI] [PubMed] [Google Scholar]
- 11.Gogos CA, Fouka KP, Nikiforidis G, Avgeridis K, Sakellaropoulos G, Bassaris H, Maniatis A, Skoutelis A. Prevalence of hepatitis B and C virus infection in the general population and selected groups in South-Western Greece. Eur J Epidemiol. 2003;18:551–557. doi: 10.1023/a:1024698715741. [DOI] [PubMed] [Google Scholar]
- 12.Guadagnino V, Stroffolini T, Rapicetta M, Costantino A, Kondili LA, Menniti-Ippolito F, Caroleo B, Costa C, Griffo G, Loiacono L, et al. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology. 1997;26:1006–1011. doi: 10.1002/hep.510260431. [DOI] [PubMed] [Google Scholar]
- 13.Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT, et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248–253. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 14.Okayama A, Stuver SO, Tabor E, Tachibana N, Kohara M, Mueller NE, Tsubouchi H. Incident hepatitis C virus infection in a community-based population in Japan. J Viral Hepat. 2002;9:43–51. doi: 10.1046/j.1365-2893.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 15.Pérez CM, Suárez E, Torres EA, Román K, Colón V. Seroprevalence of hepatitis C virus and associated risk behaviours: a population-based study in San Juan, Puerto Rico. Int J Epidemiol. 2005;34:593–599. doi: 10.1093/ije/dyi059. [DOI] [PubMed] [Google Scholar]
- 16.Riestra S, Fernández E, Leiva P, García S, Ocio G, Rodrigo L. Prevalence of hepatitis C virus infection in the general population of northern Spain. Eur J Gastroenterol Hepatol. 2001;13:477–481. doi: 10.1097/00042737-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sun CA, Chen HC, Lu CF, You SL, Mau YC, Ho MS, Lin SH, Chen CJ. Transmission of hepatitis C virus in Taiwan: prevalence and risk factors based on a nationwide survey. J Med Virol. 1999;59:290–296. [PubMed] [Google Scholar]
- 18.Xia GL, Liu CB, Cao HL, Bi SL, Zhan MY, Su CA, Nan JH, Qi XQ. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int Hepatol Comm. 1996;5:62–73. [Google Scholar]
- 19.Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med. 1975;292:767–770. doi: 10.1056/NEJM197504102921502. [DOI] [PubMed] [Google Scholar]
- 20.Alter HJ, Houghton M. Clinical Medical Research Award. Hepatitis C virus and eliminating post-transfusion hepatitis. Nat Med. 2000;6:1082–1086. doi: 10.1038/80394. [DOI] [PubMed] [Google Scholar]
- 21.Shan H, Wang JX, Ren FR, Zhang YZ, Zhao HY, Gao GJ, Ji Y, Ness PM. Blood banking in China. Lancet. 2002;360:1770–1775. doi: 10.1016/S0140-6736(02)11669-2. [DOI] [PubMed] [Google Scholar]
- 22.Tagny CT, Mbanya D, Tapko JB, Lefrère JJ. Blood safety in Sub-Saharan Africa: a multi-factorial problem. Transfusion. 2008;48:1256–1261. doi: 10.1111/j.1537-2995.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 24.Memon MI, Memon MA. Hepatitis C: an epidemiological review. J Viral Hepat. 2002;9:84–100. doi: 10.1046/j.1365-2893.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 25.Garner JJ, Gaughwin M, Dodding J, Wilson K. Prevalence of hepatitis C infection in pregnant women in South Australia. Med J Aust. 1997;167:470–472. doi: 10.5694/j.1326-5377.1997.tb126673.x. [DOI] [PubMed] [Google Scholar]
- 26.Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ. 1999;77:801–807. [PMC free article] [PubMed] [Google Scholar]
- 27.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 28.Khan UR, Janjua NZ, Akhtar S, Hatcher J. Case-control study of risk factors associated with hepatitis C virus infection among pregnant women in hospitals of Karachi-Pakistan. Trop Med Int Health. 2008;13:754–761. doi: 10.1111/j.1365-3156.2008.02075.x. [DOI] [PubMed] [Google Scholar]
- 29.Stark K, Poggensee G, Höhne M, Bienzle U, Kiwelu I, Schreier E. Seroepidemiology of TT virus, GBC-C/HGV, and hepatitis viruses B, C, and E among women in a rural area of Tanzania. J Med Virol. 2000;62:524–530. doi: 10.1002/1096-9071(200012)62:4<524::aid-jmv19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Shin HR, Kim JY, Ohno T, Cao K, Mizokami M, Risch H, Kim SR. Prevalence and risk factors of hepatitis C virus infection among Koreans in rural area of Korea. Hepatol Res. 2000;17:185–196. doi: 10.1016/s1386-6346(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 31.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149:203–213. doi: 10.1093/oxfordjournals.aje.a009792. [DOI] [PubMed] [Google Scholar]
- 32.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 33.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 34.Akhtar S, Moatter T. Multilevel modeling of intra-household spread of hepatitis C virus infection, Karachi, Pakistan. Am J Trop Med Hyg. 2007;76:446–449. [PubMed] [Google Scholar]
- 35.McMahon JM, Pouget ER, Tortu S. Individual and couple-level risk factors for hepatitis C infection among heterosexual drug users: a multilevel dyadic analysis. J Infect Dis. 2007;195:1572–1581. doi: 10.1086/516785. [DOI] [PubMed] [Google Scholar]
- 36.Hutchinson SJ, Goldberg DJ, King M, Cameron SO, Shaw LE, Brown A, MacKenzie J, Wilson K, MacDonald L. Hepatitis C virus among childbearing women in Scotland: prevalence, deprivation, and diagnosis. Gut. 2004;53:593–598. doi: 10.1136/gut.2003.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 38.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, Ishak KG, Iber FL, Toro D, Samanta A, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 39.Suruki R, Hayashi K, Kusumoto K, Uto H, Ido A, Tsubouchi H, Stuver SO. Alanine aminotransferase level as a predictor of hepatitis C virus-associated hepatocellular carcinoma incidence in a community-based population in Japan. Int J Cancer. 2006;119:192–195. doi: 10.1002/ijc.21796. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Tsukuma H, Yamano H, Oshima A, Shibata H. Prospective study on the risk of hepatocellular carcinoma among hepatitis C virus-positive blood donors focusing on demographic factors, alanine aminotransferase level at donation and interaction with hepatitis B virus. Int J Cancer. 2004;112:1075–1080. doi: 10.1002/ijc.20507. [DOI] [PubMed] [Google Scholar]
- 41.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 42.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 43.Uto H, Stuver SO, Hayashi K, Kumagai K, Sasaki F, Kanmura S, Numata M, Moriuchi A, Hasegawa S, Oketani M, et al. Increased rate of death related to presence of viremia among hepatitis C virus antibody-positive subjects in a community-based cohort study. Hepatology. 2009;50:393–399. doi: 10.1002/hep.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiese M, Grüngreiff K, Güthoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany--a 25-year multicenter study. J Hepatol. 2005;43:590–598. doi: 10.1016/j.jhep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 45.De Moliner L, Pontisso P, De Salvo GL, Cavalletto L, Chemello L, Alberti A. Serum and liver HCV RNA levels in patients with chronic hepatitis C: correlation with clinical and histological features. Gut. 1998;42:856–860. doi: 10.1136/gut.42.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duvoux C, Pawlotsky JM, Bastie A, Cherqui D, Soussy CJ, Dhumeaux D. Low HCV replication levels in end-stage hepatitis C virus-related liver disease. J Hepatol. 1999;31:593–597. doi: 10.1016/s0168-8278(99)80336-5. [DOI] [PubMed] [Google Scholar]
- 47.Fanning L, Kenny E, Sheehan M, Cannon B, Whelton M, O’Connell J, Collins JK, Shanahan F. Viral load and clinicopathological features of chronic hepatitis C (1b) in a homogeneous patient population. Hepatology. 1999;29:904–907. doi: 10.1002/hep.510290310. [DOI] [PubMed] [Google Scholar]
- 48.Lagging LM, Garcia CE, Westin J, Wejstål R, Norkrans G, Dhillon AP, Lindh M. Comparison of serum hepatitis C virus RNA and core antigen concentrations and determination of whether levels are associated with liver histology or affected by specimen storage time. J Clin Microbiol. 2002;40:4224–4229. doi: 10.1128/JCM.40.11.4224-4229.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau JY, Davis GL, Kniffen J, Qian KP, Urdea MS, Chan CS, Mizokami M, Neuwald PD, Wilber JC. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993;341:1501–1504. doi: 10.1016/0140-6736(93)90635-t. [DOI] [PubMed] [Google Scholar]
- 50.Naito M, Hayashi N, Hagiwara H, Hiramatsu N, Kasahara A, Fusamoto H, Kamada T. Serum hepatitis C virus RNA quantity and histological features of hepatitis C virus carriers with persistently normal ALT levels. Hepatology. 1994;19:871–875. [PubMed] [Google Scholar]
- 51.Hisada M, Chatterjee N, Kalaylioglu Z, Battjes RJ, Goedert JJ. Hepatitis C virus load and survival among injection drug users in the United States. Hepatology. 2005;42:1446–1452. doi: 10.1002/hep.20938. [DOI] [PubMed] [Google Scholar]
- 52.Simmonds P. Viral heterogeneity of the hepatitis C virus. J Hepatol. 1999;31 Suppl 1:54–60. doi: 10.1016/s0168-8278(99)80375-4. [DOI] [PubMed] [Google Scholar]
- 53.Martinot-Peignoux M, Roudot-Thoraval F, Mendel I, Coste J, Izopet J, Duverlie G, Payan C, Pawlotsky JM, Defer C, Bogard M, et al. Hepatitis C virus genotypes in France: relationship with epidemiology, pathogenicity and response to interferon therapy. The GEMHEP. J Viral Hepat. 1999;6:435–443. doi: 10.1046/j.1365-2893.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- 54.Bruno S, Crosignani A, Maisonneuve P, Rossi S, Silini E, Mondelli MU. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology. 2007;46:1350–1356. doi: 10.1002/hep.21826. [DOI] [PubMed] [Google Scholar]
- 55.Fattovich G, Ribero ML, Pantalena M, Diodati G, Almasio P, Nevens F, Tremolada F, Degos F, Rai J, Solinas A, et al. Hepatitis C virus genotypes: distribution and clinical significance in patients with cirrhosis type C seen at tertiary referral centres in Europe. J Viral Hepat. 2001;8:206–216. doi: 10.1046/j.1365-2893.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi M, Tanaka E, Sodeyama T, Urushihara A, Matsumoto A, Kiyosawa K. The natural course of chronic hepatitis C: a comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996;23:695–699. doi: 10.1053/jhep.1996.v23.pm0008666319. [DOI] [PubMed] [Google Scholar]
- 57.Silini E, Bottelli R, Asti M, Bruno S, Candusso ME, Brambilla S, Bono F, Iamoni G, Tinelli C, Mondelli MU, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a case-control study. Gastroenterology. 1996;111:199–205. doi: 10.1053/gast.1996.v111.pm8698200. [DOI] [PubMed] [Google Scholar]
- 58.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 59.Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–4593. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 60.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 61.Liu CJ, Chuang WL, Lee CM, Yu ML, Lu SN, Wu SS, Liao LY, Chen CL, Kuo HT, Chao YC, et al. Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology. 2009;136:496–504.e3. doi: 10.1053/j.gastro.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 62.Yeh SH, Tsai CY, Kao JH, Liu CJ, Kuo TJ, Lin MW, Huang WL, Lu SF, Jih J, Chen DS, et al. Quantification and genotyping of hepatitis B virus in a single reaction by real-time PCR and melting curve analysis. J Hepatol. 2004;41:659–666. doi: 10.1016/j.jhep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Lee MH, Yang HI, Jen CL, Lu SN, Yeh SH, Liu CJ, You SL, Sun CA, Wang LY, Chen WJ, et al. Community and personal risk factors for hepatitis C virus infection: a survey of 23,820 residents in Taiwan in 1991-2. Gut. 2011;60:688–694. doi: 10.1136/gut.2010.220889. [DOI] [PubMed] [Google Scholar]
- 64.Yu ML, Chuang WL, Chen SC, Dai CY, Hou C, Wang JH, Lu SN, Huang JF, Lin ZY, Hsieh MY, et al. Changing prevalence of hepatitis C virus genotypes: molecular epidemiology and clinical implications in the hepatitis C virus hyperendemic areas and a tertiary referral center in Taiwan. J Med Virol. 2001;65:58–65. [PubMed] [Google Scholar]
- 65.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, L’Italien G, Chen CJ, Yuan Y. Hepatitis C virus genotype 1b increases cumulative lifetime risk of hepatocellular carcinoma. Int J Cancer. 2014;135:1119–1126. doi: 10.1002/ijc.28753. [DOI] [PubMed] [Google Scholar]
- 66.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 68.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 69.Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–145, 1338-145. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 70.Duggal P, Thio CL, Wojcik GL, Goedert JJ, Mangia A, Latanich R, Kim AY, Lauer GM, Chung RT, Peters MG, et al. Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Ann Intern Med. 2013;158:235–245. doi: 10.7326/0003-4819-158-4-201302190-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, et al. Amino acid substitutions in the hepatitis C virus core region are the important predictor of hepatocarcinogenesis. Hepatology. 2007;46:1357–1364. doi: 10.1002/hep.21836. [DOI] [PubMed] [Google Scholar]
- 73.Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, et al. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421–429. doi: 10.1002/hep.23690. [DOI] [PubMed] [Google Scholar]
- 74.Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, et al. Predictive factors of early and sustained responses to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b: amino acid substitutions in the core region and low-density lipoprotein cholesterol levels. J Hepatol. 2007;46:403–410. doi: 10.1016/j.jhep.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 75.Akuta N, Suzuki F, Seko Y, Kawamura Y, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Hara T, Kobayashi M, et al. Complicated relationships of amino acid substitution in hepatitis C virus core region and IL28B genotype influencing hepatocarcinogenesis. Hepatology. 2012;56:2134–2141. doi: 10.1002/hep.25949. [DOI] [PubMed] [Google Scholar]
- 76.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 77.Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, Helbling B, Hadengue A, Gonvers JJ, et al. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41–46. doi: 10.1016/j.jhep.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 78.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 79.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruhl CE, Menke A, Cowie CC, Everhart JE. The Relationship of Hepatitis C Virus Infection with Diabetes in the United States Population. Hepatology. 2014:Epub ahead of print. doi: 10.1002/hep.27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 82.Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894–2900. doi: 10.1161/STROKEAHA.110.598136. [DOI] [PubMed] [Google Scholar]


