Abstract
Background
Maintenance of ovarian blood flow (OBF) is suggested to be important for regular ovulation in women with polycystic ovaries (PCO). The purpose of the present study was to investigate whether electro-acupuncture (EA) of different frequencies and intensities can improve the OBF of anaesthetized rat in the animal model of PCO.
Methods
PCO was experimentally induced by a single intramuscular (i.m.) injection of estradiol valerate (EV) in rats. Control rats were given i.m. injection of oil. The involvement of the two ovarian sympathetic nerves; superior ovarian nerve (SON) and plexus ovarian nerve (OPN), in OBF responses was elucidated by severance of SON and OPN in both control and PCO rats. How systemic circulatory changes affect OBF was evaluated by continuous recording of the blood pressure. OBF was measured on the surface of the ovary-using laser Doppler flowmetry. Acupuncture needles were inserted bilaterally into the abdominal and hind limb muscles and connected to an electrical stimulator. Two frequencies – 2 Hz (low) and 80 Hz (high) – with three different intensities – 1.5, 3, and 6 mA – were applied for 35 s.
Results
Low-frequency EA at intensities of 3 and 6 mA elicited significant increases in OBF in the Control group compared to baseline. In the PCO group the increases in OBF were significant only when stimulating with low-frequency EA at 6 mA. After severance of the ovarian sympathetic nerves, the increased response of OBF that had been induced by low-frequency EA in both the Control and PCO group was abolished, indicating that the OBF response is mediated via the ovarian sympathetic nerves. High-frequency EA at 6 mA significantly decreased OBF and mean arterial blood pressure (MAP) in the Control group compared to baseline. In the PCO group, the same stimulation produced similar decreases in MAP, but not in OBF.
Conclusion
Low-frequency EA stimulation with a strong intensity (6 mA) increases OBF in rats with steroid-induced PCO whereas less strong intensity (3 mA) induces similar changes in control rats. Severance of the ovarian sympathetic nerves, abolish this OBF increase in both study groups, which suggests that the responses of OBF to EA are mediated via the ovarian sympathetic nerves.
Background
Polycystic ovary syndrome (PCOS) is a complex endocrinological disorder, associated with ovulatory dysfunction and infertility [1], hyperandrogenism, hyperinsulinemia and insulin resistance [2-4], and progression to type II diabetes [4,5]. The underlying mechanisms are still unclear, but there are indications that human PCOS is associated with hyperactivity in the sympathetic nervous system. For example, women with PCOS develop cardiovascular abnormalities such as hypertension [5]. That the sympathetic nervous system is involved in the pathophysiology of PCOS is supported by the fact that the innervation of the catecholaminergic nerve fibers in the polycystic ovaries of women with PCOS is more dense than in normal ovaries [6,7]. In addition, the effectiveness of ovarian wedge resection [8] or laparoscopic laser cauterization [9] to increase ovulatory response in women with PCOS raises the possibility that increased sympathetic input to the ovary may play a role in the development of polycystic ovaries observed in women with PCOS.
It has recently been shown that polycystic ovaries (PCO) in rats, induced by a single i.m. injection of estradiol valerate (EV), develop an anovulatory state that shares many endocrinological and morphological characteristics of human PCOS, and it is assumed that the activity of the ovarian sympathetic nerves in PCO rats is higher than in normal rats [10-12]. This is evidenced by an increase in tyrosine hydroxylase enzyme activity, an increase in norepinephrine (NE) content and down regulation of β2-adrenoceptors in the ovaries of rats with PCO [10-12]. The innervation of the rat ovary involves two sources; superior ovarian nerve (SON) in the suspensory ligament, and via the ovarian plexus nerve (OPN) along the ovarian artery. The restoration of estrous cyclicity and ovulatory capacity after transection of the SON confirms the involvement of sympathetic nerves in the development of PCO [10]. It seems that the higher activity of the ovarian sympathetic nerves in rats with steroid-induced PCO is related to an augmented production of ovarian nerve growth factor (NGF) [12]. Blocking ovarian NGF action with an antiserum to NGF and an antisense oligodeoxynucleotide to NGF receptors restores estrus cyclicity and ovulatory capacity. Even if it is impossible to exactly reproduce the human PCOS using a rat model, the present model has many similarities with the human PCOS and may provide important leads about the disease.
We recently showed that treatment with electro-acupuncture (EA) of low frequency (2 Hz) induced regular ovulation in more than one-third of the women with PCOS anovulation [13]. It is possible that the EA effects on ovulation in women with PCOS are mediated via inhibition of the ovarian sympathetic nerve activity since EA is known to modulate various autonomic functions [14]. In line with these findings, we have recently reported that repeated treatment with low-frequency EA in rats with PCO significantly reduced elevated concentrations of ovarian NGF [15,16]. The results suggest that low-frequency EA has an inhibitory effect on the activity of the ovarian sympathetic nerves in rats with PCO. However, the exact impact of different stimulation frequency and intensities on sympathetic activity in rats with PCO remains to be elucidated.
The ovary is a highly vascularized organ and maintenance of high blood flow is important for the ovulatory process in both humans and animals [17,18]. The sympathetic nerves appear to be distinctly involved in the control of ovarian secretory activity and are important for the regulation of ovarian function [19]. Furthermore, ovarian sympathetic nerves are important regulators for ovarian blood flow (OBF) [20].
In this investigation, we aimed to study the effect of EA on OBF in order to elucidate the role of ovarian sympathetic nerves and the most optimal stimulation parameters in the rat model of PCO. We employed high and low frequencies of EA at different intensities and compared the OBF responses between rats with normal ovaries and rats with steroid-induced PCO ovaries. The involvement of the ovarian sympathetic nerves was elucidated by severance of the ovarian sympathetic nerves. In addition, the contribution of systemic circulatory changes to OBF was evaluated by continuous recording of the blood pressure.
This study highlights the crucial role of the sympathetic nervous system on OBF responses in rats with EV induced PCO.
Materials and methods
The experiments were carried out according to the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the animal ethics committee of International University of Health and Welfare, Otawara, Tochigi, Japan. Fourteen female Sprague-Dawley rats (235 – 281 g) were obtained from Japan SLC Inc., Shizuoka, Japan. The rats were housed two per cage and fed ad libitum. The ambient room temperature was 23 ± 1°C with illumination (12-h light: 12-h dark cycle). Thirty days before the experimental procedure, seven rats were each given a single intra muscular (i.m.) injection of 4 mg EV (Riedeldehaen, Germany) in 0.2 ml oil in order to induce PCO (PCO group) [15]. Seven control rats were each given a single i.m. injection of 0.2 ml oil only (Control group). Estrous cyclicity was monitored daily by vaginal smear obtained between 9.00–11.00 am. In the Control group, experiments were performed during estrus in 2 rats, during met estrus in 3 rats and during diestrus in 2 rats. In the PCO group experiments was performed during estrus in 3 rats and in a pseudoestrus (as described by Barria et al. [10]) state in 4 rats. In the analysis on OBF responses, no consideration was taken to cycle day.
Experimental conditions
Rats were anaesthetized with intra peritoneal (i.p.) injection of urethane (1.1 g·kg-1, Wako, Tokyo, Japan) and placed on a heating pad under an infrared lamp (ATB-110, Nihon-Kohden, Tokyo) to maintain constant body temperature at 37.0 ± 0.1°C, monitored in the rectum. The trachea was cannulated and respiration was artificially maintained using a ventilator (Model SN-480-7, Shinano, Tokyo, Japan). Mean arterial blood pressure (MAP) was continuously recorded from a catheter in the right femoral artery and was maintained above 90 mm Hg (systolic) by administration of 4% Ficoll 70 (Pharmacia Fine Chemicals, Uppsala, Sweden). The depth of anesthesia was continuously monitored by observing movements, blood pressure and heart rate. When needed, additional urethane (0.1 g·g-1) was administered through a catheter in the femoral vein.
Recording of OBF
Animals were placed in supine position. Midline incision (~40 mm in length) was then performed and the left ovary was identified and care was taken not to directly manipulate the ovary [21]. To avoid movement of the muscles in response to EA, the abdominal wall was fixed by several rods and the ovary itself was gently placed on a small plate. OBF was measured using a laser Doppler flow meter. The optical output power of the He – Ne laser beam in the laser Doppler flow meter used in this study (ALF 21, Advance, Tokyo, Japan) was 2 mW at the probe at a wavelength of 780 nm. For measurement of OBF the probe (Type H, 6-mm diameter, Advance, Tokyo, Japan) was gently placed on the left ovarian surface devoid of any larger vessels. The OBF and MAP signals were continuously recorded in a computer, and the data were analyzed by measuring them once a minute (1 s record) between 1 min before and 10 min after the onset of EA. The response magnitude is expressed as percentage of the pre-stimulus control value (1 s before the onset of stimulation). A stable blood flow signal was recorded, at least 2 min prior to the stimulation. Absolute basal value of OBF cannot be reliably determined with the present laser Doppler flowmetry method. For that reason, the responses of OBF to EA were expressed as percentage of the pre-stimulus value, i.e. baseline, and compared the response between the PCO and Control groups. For comparison with the responses of OBF, responses of MAP were also expressed as percentage of the pre-stimulus value.
Severance of the sympathetic nerves innervating the ovary
Denervation of the left ovary was performed under a binocular microscope, in order to determine contribution of the ovarian sympathetic nerves, the superior ovarian nerve (SON) and ovarian plexus nerve (OPN), to OBF responses. The left SON was denervated by severing the entire bundle of the suspensory ligament of the ovary and the left OPN was cut about 2 cm from the ovary after careful dissection from the ovarian artery and vein. The severance procedure took about 60 minutes to perform.
EA stimulation
Acupuncture needles of 0.3 mm diameter stainless steel (Xeno Hegu, Svenska AB, Landsbro, Sweden), were inserted about 10–12 mm perpendicularly into the bilateral muscle biceps femoris and about 6–8 mm obliquely into the bilateral muscle oblique externa and interna. Needles from each side were then connected to an electrical stimulator (CEFAR ACUS 4, Cefar, Lund, Sweden) and were electrically stimulated with square wave pulses (0.18 ms duration) with alternating polarity. The effect of two different stimulation frequencies were evaluated in both the Control group and the PCO group in the following order; 1) low frequency of 2 Hz with burst pulses (a burst length of 0.1 s and a burst frequency of 80 Hz), 2) high frequency of 80 Hz. Three different intensities, 1.5, 3, and 6 mA, were applied in each frequency before severance of the ovarian sympathetic nerves. After severance of the ovarian sympathetic nerves, the stimulation at 3 and 6 mA was repeated at each frequency. The duration of the stimulation was 35 s, but it took 1.5 s to reach 1.5 mA, 3 s to reach 3 mA and 6 s to reach 6 mA, giving the accurate stimulus period for 1.5 mA was 33.5 s, 3 mA was 32 s and 6 mA was 29 s.
Statistical analysis
Data were expressed as mean ± SEM. All group comparisons were made using two-way analysis of variance (ANOVA) followed by Dunnett's multiple post hoc comparison tests when appropriate. The changes over time within each group were analyzed by repeated measures with one-way ANOVA followed by Dunnett's multiple range tests for post hoc comparisons. P values less than 0.05 was considered significant.
Results
MAP and OBF in the resting state in PCO and Control rats
In a resting state, MAP was higher in the PCO group (95 ± 6 mmHg) than in the Control group (84 ± 4 mmHg), however, the difference did not reach statistical significance (p = 0.1290).
Effects of low-frequency (2 Hz) EA stimulation on OBF and MAP in PCO and Control rats
Responses of OBF to low-frequency (2 Hz) EA were investigated in control rats as well as in rats with steroid induced PCO. Fig. 1 summarizes the OBF and MAP responses to low-frequency EA at different stimulus intensities. Values during stimulus were excluded because of muscle contractions in response to EA sometimes caused artifacts that would lead to misinterpret the OBF recordings.
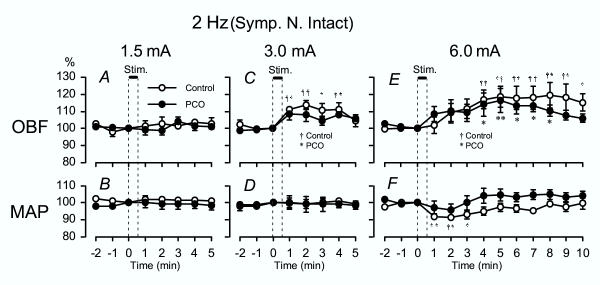
Figure 1.
2 Hz EA – OBF and MAP Summarized OBF (A, C, E) and MAP (B, D, F) responses in both the control rats and the PCO rats when stimulating with low-frequency (2 Hz) EA. Changes in OBF and MAP were calculated every minute (1 s record), and response magnitude is expressed as percentage of the pre-stimulus value at the time point 0 min. The vertical lines and thick horizontal bar indicate the time of stimulation. The onset of stimulation is indicated as zero. The data is expressed as mean ± SEM. †p < 0.05, ††p < 0.01 (control rats) * p < 0.05, **p < 0.01 (PCO rats), compared with the pre-stimulus control value.
Low-frequency EA at 1.5 mA had no significant influence on either OBF or MAP in both the Control group and the PCO group compared to baseline (Fig. 1A and 1B).
In the Control group, low-frequency EA at 3 mA elicited a significant increase in OBF 1–4 min after the onset of stimulation and return to pre-stimulus control levels 5 min after the stimulation, without affecting MAP (Fig. 1C and 1D). The PCO group showed a similar pattern in OBF response, but the increase was not significant (Fig. 1C and 1D).
In the Control group, low-frequency EA at 6 mA caused a longer lasting increase in OBF, i.e., OBF significantly increased 4–10 min after onset of stimulation (Fig. 1E). When stimulating with the same intensity in the PCO group, the OBF showed similar increases as in the Control group with significance at 4–8 min after onset of stimulation and returned to pre-stimulus control levels 9 min after onset of stimulation (Fig. 1E). In the Control group, MAP responded with a significant short-lasting decrease, and returned to the pre-stimulus control level 4 min after the stimulation (Fig. 1F). On the other hand, MAP had no significant responses to EA at 6 mA in the PCO group. There were no significant group differences between PCO and control rats in any parameters.
OBF and MAP responses to low-frequency (2 Hz) EA stimulation after severance of the ovarian sympathetic nerves in PCO and Control rats
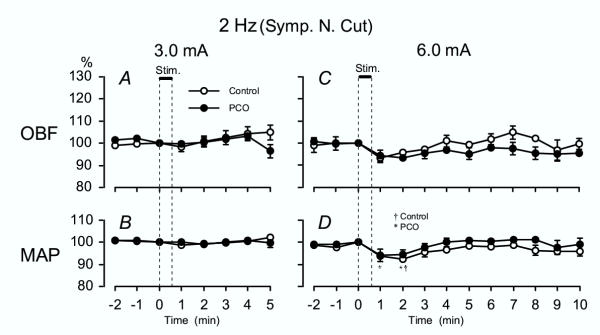
The OBF responses to low-frequency EA stimulation were investigated after severance of the ovarian sympathetic nerves, the SON and OPN, to determine contribution of the ovarian sympathetic nerves to OBF responses in the Control group and the PCO group. After severance of the SON and OPN, the increased responses of OBF to low-frequency EA at 3 and 6 mA were totally abolished in both the Control and the PCO group compared to baseline (Fig. 2A and 2C).
Figure 2.
2 Hz EA – OBF and MAP after sympathetic denervation Summarized OBF (A, C) and MAP (B, D) responses after severance of the ovarian sympathetic nerves in both the control rats and the PCO rats when stimulating with low-frequency (2 Hz) EA. See Fig. 1 for other details.
Responses of MAP after severance of the SON and OPN in both the Control and the PCO groups were similar to those in rats with intact ovarian sympathetic nerves, when stimulating with an intensity of both 3 and 6 mA (Fig. 2B and 2D). Stimulation with an intensity of 6 mA in the Control group resulted in a significant decrease in MAP at 1 and 2 min after the onset of stimulation (Fig. 2D). The response of MAP returned to the pre-stimulus control level after 3 min.
Effects of high-frequency (80 Hz) EA stimulation on OBF and MAP in PCO and Control rats
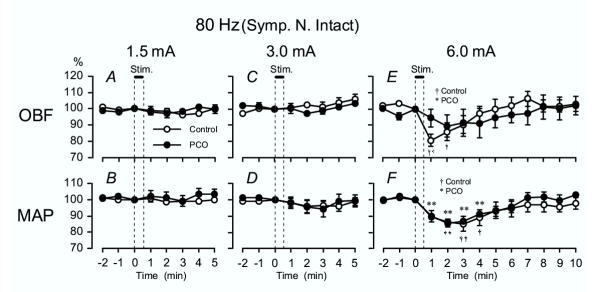
Responses of OBF to high-frequency (80 Hz) EA were investigated in control rats as well as in rats with steroid induced PCO. Fig. 3 summarizes the OBF and MAP responses to high-frequency EA at different stimulus intensities.
Figure 3.
80 Hz EA – OBF and MAP Summarized OBF (A, C, E) and MAP (B, D, F) responses in both the control rats and the PCO rats when stimulating with high-frequency (80 Hz) EA. See Fig. 1 for other details.
At 1.5 and 3 mA, there was no significant influence on either OBF or MAP in any of the study groups (Fig. 3A,3B,3C,3D). In the Control group, at 6 mA OBF responded with a significant decrease 1 and 2 min after the onset of stimulation and gradually returned to pre-stimulus control level (Fig. 3E). In the PCO group OBF showed a similar pattern, but the decrease was not significant (Fig. 3E). MAP significantly decreased 1–4 min after the onset of stimulation in the PCO group and at 2–4 min in the Control group compared to baseline (Fig. 3F). There were no significant group differences between the PCO and the Control group in any parameters.
OBF and MAP responses to high-frequency (80 Hz) EA stimulation after severance of the ovarian sympathetic nerves in PCO and Control rats
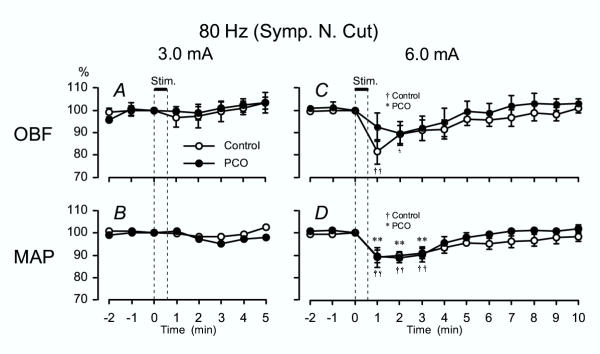
The OBF responses to high-frequency EA stimulation were also investigated after severance of the ovarian sympathetic nerves, the SON and OPN, in the Control group and the PCO group.
OBF and MAP response to high-frequency EA at 3 mA did not show any significant response, same as those rats with intact sympathetic nerves (Fig. 4A and 4B). The OBF responses to high-frequency EA at 6 mA after severance of the sympathetic nerves did not differ from those in rats with intact sympathetic nerves in both the Control and the PCO group. In the Control group OBF significantly decreased 1 and 2 min after the onset of stimulation and gradually returned to pre-stimulus control levels, whereas there were no significant decreases of OBF in the PCO groups (Fig. 4C). In both Control and the PCO group, MAP responses to EA with high frequency at 6 mA also showed similar decreases as those in rats with intact sympathetic nerves. Significant decreases were observed at 1, 2 and 3 min after onset of stimulation in both the Control and PCO groups (Fig. 4D).
Figure 4.
80 Hz EA – OBF and MAP after sympathetic denervation Summarized OBF (A, C) and MAP (B, D) responses after severance of the ovarian sympathetic nerves in both the control rats and the PCO rats when stimulating with high-frequency (80 Hz) EA. See Fig. 1 for other details.
Discussion
The present study demonstrates that anaesthetized rats with steroid-induced PCO have similar OBF responses to EA stimulation as control rats when measured 30 days after EV injection. The OBF responses in both Control and PCO rats were dependent on the frequency of the EA stimulation; that is, stimulation with a low frequency increased OBF, while stimulation with a high frequency decreased OBF in control rats. The increase of OBF in response to low-frequency EA was abolished after severance of the ovarian sympathetic nerves in both study groups, which suggests that the responses of OBF to EA are mediated via the ovarian sympathetic nerves. These results confirmed the previous findings that EA of low-frequency, 2 Hz, increased OBF as a reflex response via ovarian sympathetic nerves in rats with normal ovaries [22].
Fibers innervating the ovary through SON appear to be more related with the regulation of steroidogenic activity of the ovary, whereas the fibers coming from the plexus (OPN) appear to be more involved in the control of the vascular tone [19,20]. In the present study, all experiments were conducted interrupting all sympathetic innervation by severance of both SON and OPN. Therefore, the relative contribution of each of the nerves to OBF remains to be investigated by severance of each one of the nerve sources separately.
The OBF response to low-frequency EA was less pronounced in the PCO rats, compared with the control rats. The increase of OBF in response to low-frequency EA in the PCO rats was only significant when stimulations with a strong intensity, 6 mA, were used, whereas stimulation at 3 mA produced a significant increase in OBF in the control rats. This indicates that PCO rats are less sensitive to the ability of low-frequency EA to elicit sympathetically mediated OBF increases than control rats. It is well known that ovarian sympathetic nerves take part in the regulation of OBF [20]. One plausible explanation to the resistance to sensory stimulation might be that rats with EV induced PCO have increased ovarian sympathetic nerve input [10-12].
Recently, electrical stimulation of the splanchnic nerve was shown to decrease OBF via activation of α adrenoceptors of the ovarian blood vessels [23]. Increase in ovarian sympathetic tone in rats with PCO has been evidenced by an increase in tyrosin hydroxylase enzyme activity, an increase in NE content, and down regulation of β2-adrenoreceptors in the ovaries [10-12]. Although the number of β- adrenoceptors in rats with PCO has not been investigated, these receptors can be assumed to be down or up regulated because of the high sympathetic activity in the ovaries of PCO rats. A disturbed number of β- adrenoceptors might be one cause of the resistance in the OBF response following EA stimulation in rats with PCO. It is also possible that the less pronounced effect of low-frequency EA on OBF might depend on the stronger excitatory drive of the sympathetic nerves to the ovary in rats with PCO.
After undergoing strong stimulation with high-frequency EA at 6 mA, control rats experienced a significant decrease in OBF. The OBF in the PCO rats, on the other hand, tended to decrease, but the decrease was non-significant, despite significant decreases in MAP. These results further indicate that OBF in PCO rats is resistant to systemic circulatory changes.
Interestingly, basal MAP were higher in the PCO rats than the control rats which support an general higher sympathetic tone in PCO animals [24]. Women with PCOS are known to be candidates for increased cardiovascular risk, and it has recently been shown that they have diastolic dysfunction, an early indicator of hypertension [25]. Furthermore, enhanced sympathetic and adrenal medullar activities are important links between development of hypertension and also defects in insulin action [24].
The findings in the present study are of importance in future studies evaluating the effect of EA in EV induced PCO. The fact that the present rat PCO model has a high sympathetic activity in the ovaries, render support that low-frequency EA (2 Hz) with stimulation intensity over 3 mA should be used in future experiments. In our previous EA studies on the present rat PCO model we used repeated low-frequency EA with an intensity of 1.5 mA. That stimulation protocol resulted in reduction of high ovarian NGF, corticotrophin releasing factor as well as endothelin concentrations, indicating a decreased ovarian sympathetic activity [15,16,26]. Furthermore, repeated low-frequency EA treatments significantly increase low hypothalamic β-endorphin concentrations [27]. However, we have not been able to identify any substantial influence in ovarian morphology with that study protocol [15,16]. The results in the present study confirm our previous reports that low-frequency EA modulates sympathetic activity, but indicate that further studies have to be done with more intensive stimulation in order to determine if that would initiate estrus cyclicity and ovulation in rats with EV induced PCO.
Conclusion
The present study shows that low-frequency EA at strong intensities can increase OBF via the ovarian sympathetic nerves in rats with steroid-induced PCO. The response patterns of the control and the PCO rats were similar, but the OBF responses of PCO rats seem to be less sensitive to EA.
Authors' contributions
ES-V participated in the design of the study, carried out animal preparation and blood flow measurements, performed the statistical analysis and drafted the manuscript. RK participated in animal preparation, blood flow measurements and statistical analysis. OW participated in blood flow measurements. TL participated in the design of the study. MK participated in the design of the study, carried out the denervation of sympathetic nerves. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are thankful to Prof. Akio Sato, University of Human Arts and Sciences, Japan, for his encouragement to perform this work. This study was supported by the 7th Leica Award (to ES-V), Leica Microsystems Japan; grants from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), and The Royal Society of Art and Sciences in Göteborg (to ES-V); and a Grant-in-Aid for Scientific Research (No. 15590214) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to MK).
Contributor Information
Elisabet Stener-Victorin, Email: elsv@fhs.gu.se.
Rie Kobayashi, Email: kobari@alpha.ocn.ne.jp.
Orie Watanabe, Email: orie-w@f3.dion.ne.jp.
Thomas Lundeberg, Email: thomas.lundeberg@lidingo.mail.telia.com.
Mieko Kurosawa, Email: mieko-ku@iuhw.ac.jp.
References
- Yen SSC. Polycystic ovary syndrome (hyperandrogenic chronic anovulation) In: Yen SSC Jaffe RB, editor. Reproductive endocrinology. 4th. Philadelphia, WB Saunders Co; 1999. pp. 436–476. [Google Scholar]
- Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insuline sensitivity but persistently increased early insuline secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:2586–2593. doi: 10.1210/jc.80.9.2586. [DOI] [PubMed] [Google Scholar]
- Holte J. Polycystic ovary syndrome and insulin resistance: thrifty genes struggling with over-feeding and sedentary life style? J Endocrinol Invest. 1998;21:598–601. doi: 10.1007/BF03350784. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/er.18.6.774. [DOI] [PubMed] [Google Scholar]
- Dahlgren E, Janson PO, Johansson S, Lapidus L, Lindstedt G, Tengborn L. Hemostatic and metabolic variables in women with polycystic ovary syndrome. Fertil Steril. 1994;61:455–460. [PubMed] [Google Scholar]
- Semenova II. Adrenergic innervation of the ovaries in Stein-Leventhal syndrome. Vestn Akad Med Nauk SSSR (Abstract in english) 1969;24:58–62. [PubMed] [Google Scholar]
- Heider U, Pedal I, Spanel-Borowski K. Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertil Steril. 2001;75:1141–1147. doi: 10.1016/S0015-0282(01)01805-2. [DOI] [PubMed] [Google Scholar]
- Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: wedge resection revisited in the age of laparoscopy. Fertil Steril. 1995;63:439–463. doi: 10.1016/s0015-0282(16)57408-1. [DOI] [PubMed] [Google Scholar]
- Balen AH, Jacobs HS. A prospective study comparing unilateral and bilateral laparoscopic ovarian diathermy in women with the polycystic ovary syndrome. Fertil Steril. 1994;62:921–925. doi: 10.1016/s0015-0282(16)57051-4. [DOI] [PubMed] [Google Scholar]
- Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: role of sympathetic innervation. Endocrinology. 1993;133:2696–2703. doi: 10.1210/en.133.6.2696. [DOI] [PubMed] [Google Scholar]
- Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology. 1993;133:2690–2695. doi: 10.1210/en.133.6.2690. [DOI] [PubMed] [Google Scholar]
- Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology. 2000;141:1059–1072. doi: 10.1210/en.141.3.1059. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Waldenstrom U, Tagnfors U, Lundeberg T, Lindstedt G, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2000;79:180–188. doi: 10.1034/j.1600-0412.2000.079003180.x. [DOI] [PubMed] [Google Scholar]
- Andersson S, Lundeberg T. Acupuncture - from empiricism to science: functional background to acupuncture effects in pain and disease. Med Hypotheses. 1995;45:271–281. doi: 10.1016/0306-9877(95)90117-5. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lundeberg T, Waldenstrom U, Manni L, Aloe L, Gunnarsson S, Janson PO. Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol Reprod. 2000;63:1497–1503. doi: 10.1095/biolreprod63.5.1497. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lundeberg T, Cajander S, Aloe L, Manni L, Waldenstrom U, Janson PO. Steroid-induced polycystic ovaries in rats: effect of electro-acupuncture on concentrations of endothelin-1 and nerve growth factor (NGF), and expression of NGF mRNA in the ovaries, the adrenal glands, and the central nervous system. Reprod Biol Endocrinol. 2003;1:33. doi: 10.1186/1477-7827-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännström M, Löfman C, Mikuni M. Morphological and vascular changes during ovulation in vivo in rat. Biol Reprod. 1997;Suppl.:178 (Abstract). [Google Scholar]
- Zackrisson U, Mikuni M, Peterson MC, Nilsson B, Janson PerOlof., Brännström M. Evidence for the involvement of blood flow-related mechanisms in the ovulatory process of the rat. Human Reproduction. 2000;5:264–272. doi: 10.1093/humrep/15.2.264. [DOI] [PubMed] [Google Scholar]
- Ahmed CE, Dees WL, Ojeda SR. The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology. 1986;118:1682–1689. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Owman Ch, Stjernquist M. Origin, distribution, and functional aspects of aminergic and peptidergic nerves in the male and female reproductive tracts. In: Björklund A Hökfelt T and Owman Ch (eds), editor. Handbook of Chemical Neuroanatomy. 6: The Peripheral Nervous System. Amsterdam, Elsevier; 1988. pp. 445–544. [Google Scholar]
- Janson PO, Albrecht I. Methodological aspects of blood flow measurements in ovaries containing corpora lutea. J Appl Physiol. 1975;38:288–293. doi: 10.1152/jappl.1975.38.2.288. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Kobayashi R, Kurosawa M. Ovarian blood flow responses to electro-acupuncture stimulation at different frequencies and intensities in anaesthetized rats. Auton Neurosci. 2003;108:50–56. doi: 10.1016/j.autneu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hotta H, Kagitani F, Aikawa Y. Ovarian blood flow is reflexively regulated by mechanical afferent stimulation of hindlimb in non-pregnant anesthetized rats. Autonomic Neuroscience: Basic and Clinical. 2003;106:91–97. doi: 10.1016/S1566-0702(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- Tíras MB, Yalcin R, Noyan V, Maral I, Yìldìrìm M, Dörtlemez O, Daya S. Alterations in cardiac outflow parameters in patients with polycystic ovarian syndrome. Human Reproduction. 1999;14:1949–1952. doi: 10.1093/humrep/14.8.1949. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lundeberg T, Waldenstrom U, Bileviciute-Ljungar I, Janson PO. Effects of electro-acupuncture on corticotropin-releasing factor in rats with experimentally-induced polycystic ovaries. Neuropeptides. 2001;35:227–231. doi: 10.1054/npep.2002.0878. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lindholm C. Immunity and beta-endorphin concentrations in hypothalamus and plasma in rats with steroid-induced polycystic ovaries; effect of low frequency electro- acupuncture. Biol Reprod. 2004;70:329–333. doi: 10.1095/biolreprod.103.022368. [DOI] [PubMed] [Google Scholar]