Abstract
Considering the recommended indications for Helicobacter pylori (H. pylori) eradication therapy and the broad spectrum of available diagnostic methods, a reliable diagnosis is mandatory both before and after eradication therapy. Only highly accurate tests should be used in clinical practice, and the sensitivity and specificity of an adequate test should exceed 90%. The choice of tests should take into account clinical circumstances, the likelihood ratio of positive and negative tests, the cost-effectiveness of the testing strategy and the availability of the tests. This review concerns some of the most recent developments in diagnostic methods of H. pylori infection, namely the contribution of novel endoscopic evaluation methodologies for the diagnosis of H. pylori infection, such as magnifying endoscopy techniques and chromoendoscopy. In addition, the diagnostic contribution of histology and the urea breath test was explored recently in specific clinical settings and patient groups. Recent studies recommend enhancing the number of biopsy fragments for the rapid urease test. Bacterial culture from the gastric biopsy is the gold standard technique, and is recommended for antibiotic susceptibility test. Serology is used for initial screening and the stool antigen test is particularly used when the urea breath test is not available, while molecular methods have gained attention mostly for detecting antibiotic resistance.
Keywords: Helicobacter pylori, Diagnosis, Endoscopy, Histology, Culture, Urea breath test, Stool antigen test, Serology, Molecular methods
Core tip: Considering the importance of a reliable diagnosis in the setting of current recommendations for Helicobacter pylori (H. pylori) eradication therapy, recent developments in both invasive and non-invasive methods may further contribute to improving H. pylori detection. The manuscript presents an extensive overview of the major advances in endoscopy, histology, culture, urea breath test, serology, stool tests and molecular methods, emphasizing their major contributions and potential shortcomings.
INTRODUCTION
A reliable primary diagnosis and control of treatment success of Helicobacter pylori (H. pylori) infection is crucial for patients with a wide spectrum of H. pylori-related conditions, including uncomplicated or complicated ulcer disease, mucosa associated lymphoid tissue (MALT) lymphoma, atrophic gastritis and previous partial gastric resection for gastric cancer. Accurate diagnosis of H. pylori infection involves the combined knowledge, effort and research of laboratories, gastroenterologists and pathologists. Traditional diagnosis is made using a combination of tests, both invasive and noninvasive. Considering the broad spectrum of diagnostic methods, only highly accurate tests should be used in clinical practice under specific circumstances and currently, the sensitivity and specificity of such tests should exceed 90%. The choice of tests usually depends on clinical circumstances, the likelihood ratio of positive and negative tests, the cost-effectiveness of the testing strategy and of the availability of the tests. The present paper aimed to present an overview of the most recent advances in both biopsy- and non-biopsy-based diagnostic methods for H. pylori infection (Table 1).
Table 1.
Summary of diagnostic methods
| Invasive/noninvasive | Reference method | Antibiotic resistance detection | |
| Endoscopy | Invasive | Yes | No |
| Histology | Invasive | Yes | No |
| Rapid urease test | Invasive | No | No |
| Culture | Invasive | Yes | Yes |
| Molecular methods | Both | No | Yes |
| Serology | Noninvasive | No | No |
| Urea breath test | Noninvasive | No | No |
| Stool antigen test | Noninvasive | No | No |
ENDOSCOPY
Considering that accurate prediction of H. pylori infection status on endoscopic images can improve early detection of gastric cancer, especially in some geographic areas, the contribution of both conventional and novel endoscopic evaluation methodologies has received increased attention, particularly in specific clinical settings. A summary of the latest endoscopic studies is presented below. Watanabe et al[1] studied the diagnostic yield of endoscopy for H. pylori infection at three endoscopist career levels - beginner, intermediate and advanced. For this study, 77 consecutive patients who underwent endoscopy were analyzed for H. pylori infection status by histology, serology and urea breath test (UBT). The diagnostic yield was 88.9% for H. pylori-uninfected, 62.1% for H. pylori-infected, and 55.8% for H. pylori-eradicated. Intra-observer agreement for H. pylori infection status was good (k > 0.6) for all physicians, while inter-observer agreement was lower (k = 0.46) for beginners than for intermediate and advanced (k > 0.6). For all physicians, good inter-observer agreement in endoscopic findings was seen for atrophic change (k = 0.69), but the accuracy was lower for beginners.
In 496 asymptomatic Japanese middle-aged men, a prospective evaluation (mean follow-up period of 54 years), of gastric cancer development was performed in non-atrophic stomachs with highly active inflammation identified by serum levels of pepsinogen and H. pylori antibody, together with a specific endoscopic feature: endoscopic rugal hyperplastic gastritis (RHG) (reflecting localized highly active inflammation)[2]. Cancer incidence was significantly higher in patients with RHG, high H. pylori antibody titers and low PG I/II ratio than in patients without. Significantly, no cancer development was observed in these high-risk subjects after H. pylori eradication. This study emphasizes the high risk of cancer development in subjects with H. pylori-associated highly active non-atrophic gastritis and the utility of the two serological tests and endoscopic RHG for their identification.
Considering that H. pylori eradication is essential for metachronous gastric cancer prevention in patients undergoing endoscopic mucosectomy (EMR) for early gastric cancer, as reported by Fukase et al[3], Lee et al[4] aimed to determine the optimal biopsy site for H. pylori detection in the atrophic remnant mucosa of 91 EMR patients. Three paired biopsies for histology were taken at the antrum, corpus lesser (CLC), and greater curve (CGC). Additional specimens were obtained at the antrum and CGC for a rapid urease test (RUT). H. pylori infection was defined as at least two positive specimens on histology and/or RUT. Pepsinogen levels were used to determine serological atrophy. The authors concluded that CGC is the optimal biopsy site for H. pylori diagnosis in EMR patients with extensive atrophy and that an antral biopsy should be avoided, especially in serologically atrophic patients.
Although gastroscopic biopsy-based tests such as the RUT, histological examination, and culture have been widely used to diagnose H. pylori infection, many investigators have attempted to categorize the endoscopic findings characteristic of an H. pylori-infected stomach.
In 2002, Japanese endoscopists[5] found that collecting venules, seen as numerous minute red dots in the gastric corpus, were a characteristic finding in the normal stomach without H. pylori infection, using both standard and magnifying endoscopy (identification of micro mucosal patterns). This finding was termed “regular arrangement of collecting venules” (RAC). However, these findings are not a reliable method of diagnosis because of their low sensitivity and specificity.
Although magnifying endoscopy provides more precise information concerning abnormal mucosal patterns[6,7], it is not available in all endoscopy units. Moreover, its use requires training under an experienced supervisor and expertise. In addition, magnifying endoscopy is not necessarily appropriate for routine clinical practice because it is time-consuming and only a few facilities carry out this technique on a routine basis. On the other hand, endoscopic features corresponding to Sydney System pathological findings have not yet been identified, and the diagnosis of H. pylori infection in the gastric mucosa by endoscopic features has not yet been established (Figure 1). In this setting, the Study Group of Japan Gastroenterological Endoscopy Society for Establishing Endoscopic Diagnosis of Chronic Gastritis performed a prospective multicenter study enrolling 275 patients[8], investigating the association between endoscopic findings (conventional findings and indigo carmine contrast) and histological diagnosis of H. pylori (antrum and corpus). It was shown that specific endoscopic findings, such as diffuse redness, spotty redness and mucosal swelling assessed by conventional endoscopy and swelling of areae gastricae by the indigo carmine contrast method, were useful for diagnosing H. pylori infection.
Figure 1.
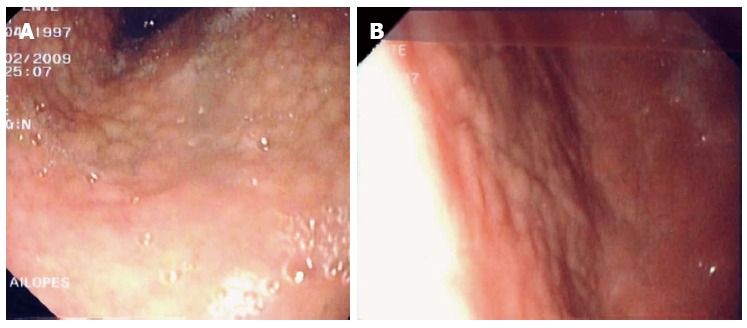
Endoscopic features of Helicobacter pylori infection (antral nodularity).
Cho et al[9] aimed to establish a new classification for predicting H. pylori-infected stomachs by non-magnifying standard endoscopy alone. A total of 617 participants who underwent gastroscopy were enrolled prospectively and a careful close-up examination of the corpus at the greater curvature was performed, maintaining a distance of 10 mm between the endoscope tip and the mucosal surface. Despite being a monocenter study in which standard endoscopy was not directly compared with magnifying endoscopy, these results suggest two important contributions for prediction of H. pylori infection status: (1) the observation of gastric mucosal patterns using standard endoscopy and proposal of a new endoscopic classification including a normal RAC and three abnormal mucosal patterns; and (2) an accuracy of prediction of H. pylori positivity at least similar to that reported in magnifying endoscopy studies (sensitivity of 95.2% and specificity of 82.2%)[10]. In the future, multicenter trials comparing standard endoscopy against magnifying endoscopy, including changes in mucosal patterns after H. pylori eradication, and including endoscopists with different levels of expertise, are needed to confirm the reliability of these data.
Chromoendoscopy has also regained attention recently as an additional methodology to detect H. pylori in the gastric mucosa. A multicenter Japanese study involving 275 patients evaluated the possibility of diagnosing H. pylori by conventional endoscopy and chromoendoscopy using indigo carmine compared with histology performed according to the Sydney System[7]. Based on several indices, the authors obtained a sensitivity of 94% in the corpus and 88% in the antrum. However, the specificities in the corpus and in the antrum were low (62% and 52%, respectively). Another study using a Cuban adult population[11] also aimed to evaluate the diagnostic yield of chromoendoscopy with red phenol at 0.1% for the detection of H. pylori infection against histology. This study reported a sensitivity of 72.6% (95%CI: 64.9-79.2) and a specificity of 75.5% (95%CI: 61.9-85.4). The authors concluded that it might be a useful method to diagnose H. pylori infection in the gastric mucosa, potentially with some specific advantages (topographic localization, avoidance of contamination and fast and immediate reading).
HISTOLOGY
Although histology has been considered to be the gold standard for H. pylori detection, the influence of clinical practice on the histopathological detection of H. pylori infection has been insufficiently explored. Recognizing that the number and distribution of H. pylori organisms vary in patients taking proton pump inhibitors (PPIs), it has been recommended to discontinue PPIs two weeks before endoscopy and to take biopsies from both the body and the antrum.
In a representative study, Lash et al[12] aimed to evaluate the yield of different gastric sampling strategies and to determine the adherence to the Sydney System guidelines in a nationwide sample of endoscopists in United States. Using a database of biopsy records diagnosed at a single pathology laboratory, the results of gastric biopsies taken to evaluate gastric inflammatory conditions in patients with no endoscopic lesions were reviewed. The incisura angularis, rarely sampled, yielded minimal additional diagnostic information and the acquisition of at least two biopsy specimens from the antrum and corpus, essentially following the Sydney System recommendations, was confirmed as a sensible strategy that guarantees the maximum diagnostic yield for the most common gastric inflammatory conditions.
In a Canadian study[13], electronic patient records were evaluated for the sites of gastric sampling and PPI use at endoscopy, collecting 150 cases with biopsies taken from both the antrum and body, which were randomly selected for pathological re-review with special stains. The gastric regions sampled, H. pylori distribution and influence of clinical factors on pathological interpretation were assessed. This study confirmed that, despite national and international guidelines for managing H. pylori infection, these guidelines are infrequently adhered to, with PPIs frequently contributing to false diagnosis, and sampling only one region increases the likelihood of missing active infection by at least 15%.
Considering that atrophy of the stomach mucosa develops in about 50% of H. pylori infected individuals by the age of 65, and is considered a pre-malignant lesion for gastric cancer[14-16], H. pylori eradication is recommended in the presence of atrophy[17], because atrophy may reverse after successful eradication therapy. It is critically important and challenging, therefore, to determine the presence or absence of H. pylori in patients with atrophic gastritis. During atrophy progression, however, the density of H. pylori in the stomach mucosa decreases, and may disappear completely during the late stages of atrophy[14,16]. This may explain the markedly lower sensitivity of biopsy-based tests (RUT, histology, culture) in the presence of atrophy. Similarly, UBT and antigen stool detection can also give false-negative results in these circumstances. In contrast, serology is not influenced to such an extent by a lower density of the microorganism, and is reliable even in advanced gastric body atrophy[14,16]. Maastricht guidelines updates have reserved serology for special situations, including extensive atrophy of the stomach mucosa on the basis that other tests might be misleading at a low bacterial density. Thus, the debate continues regarding the most appropriate H. pylori diagnostic method in atrophic gastritis.
Lan et al[18] aimed to evaluate the site and sensitivity of biopsy-based tests in terms of degree of gastritis with atrophy. Biopsy-based tests (i.e., culture, histology Giemsa stain and RUT) and non-invasive tests (anti-H. pylori IgG) were performed in 164 uninvestigated dyspepsia patients. The sensitivity of biopsy-based tests decreased when the degree of gastritis with atrophy increased, regardless of biopsy site. In moderate to severe antrum or body gastritis with atrophy, additional corpus biopsy increased the sensitivity to 16.67%, as compared with single antrum biopsy. These results confirm that in moderate to severe gastritis with atrophy, biopsy-based test should include the corpus for avoiding false negative results in H. pylori detection.
Since the discovery of H. pylori, pathologists have used different diagnostic techniques, including immunohistochemical (IHC) methods and special stains, such as Giemsa and Warthin-Starry, on an institution- and laboratory-dependent basis (with variable sensitivities and specificities for identifying H. pylori). On the other hand, it is clear that IHC staining is highly sensitive and specific for H. pylori, with the lowest rate of inter observer variation and is much faster than conventional histology[19]. However, the necessity for routine special stains and/or IHC stains has been debated in recent years. A recent study by Wang et al[20] confirmed what many pathologists assume: routine special stains, specifically IHC stains, are not cost-effective or necessary. Recently, Smith et al[21], in a retrospective study involving 200 consecutive gastric biopsy specimens, further confirmed that H. pylori is easily observed in the majority of cases with HE (sensitivity 91% and specificity 100%), remaining the most expedient and least expensive test for identifying H. pylori in gastric biopsies.
An institutional quality assurance study of a conventional method for the diagnosis of H. pylori - associated gastritis was performed by Hartman et al[22] in the United States, based on head-to-head evaluation by four methods, HE stain, Giemsa stain, Warthin-Starry stain, and H. pylori immunostaining of 356 gastric biopsy specimens. About 83% of H. pylori gastritis identified were diagnosed on the initial HE-stained slides, further supporting the use of routine ancillary stains to diagnose H. pylori infection in gastric biopsy specimens. Usually, the use of special stains is only recommended for biopsy specimens with moderate to severe chronic active or inactive gastritis in which H. pylori is not identified by HE staining, for post-treatment biopsy specimens and in cases in which structures “suspicious”, but not definitive, for H. pylori are observed by HE staining[23].
Both routine conventional histology-based methods and novel methods for H. pylori detection have increasingly focused on specific clinical settings and patient groups (bleeding peptic ulcer, gastric cancer). False-negative results may occur when using histological and RUT to detect H. pylori in biopsy specimens obtained during peptic ulcer bleeding episodes (PUB). Choi et al[24] evaluated different diagnostic methods in the specific setting of peptic ulcer, concluding that histology was the most accurate test, regardless of bleeding, compared with culture, serology and RUT. Ramirez-Lazaro et al[25] found that IHC and real-time PCR methods might improve the sensitivity of biopsy-based diagnosis in this specific setting (PUB).
In patients submitted to a subtotal gastrectomy due to gastric cancer, the identification and treatment of H. pylori are the key points in the prevention of cancer recurrence. Xu et al[26] evaluated the predictive value of neutrophil infiltration, a hallmark of active inflammation (updated Sydney system), as a histological marker of H. pylori infection, in 315 dyspeptic patients undergoing upper gastrointestinal endoscopy, including patients with a subtotal gastrectomy. The diagnosis of H. pylori infection was based on UBT and on anti-H. pylori immunoglobulin G (IgG) antibody in patient with a subtotal gastrectomy. Although neutrophil infiltration of gastric mucosa was strongly associated with overall H. pylori infection, in patients with a subtotal gastrectomy, the diagnostic accuracy of neutrophil infiltration in H. pylori infection was low.
De Martel et al[27], using data from a large Venezuelan cohort of 1948 adults, compared the gastric detection of H. pylori by polymerase chain reaction (PCR) of the vacA gene in one antral biopsy, to the detection of H. pylori by histopathology (HE and Giemsa staining) in five biopsies (antrum and corpus). Overall, H. pylori was detected in 85% and 95% of the subjects by PCR and histopathology, respectively, thus confirming that histopathology on five biopsies is an accurate tool for H. pylori detection in most subjects, compared with the PCR method on one biopsy. However, in subjects with the most severe precancerous lesions (intestinal metaplasia type III and dysplasia), PCR displayed elevated sensitivity for detecting the bacteria (significantly more often than histopathology on a single biopsy), thus suggesting its potential usefulness in this setting.
Tian et al[28] reported a meta-analysis evaluating H. pylori diagnostic methods in patients with a partial gastrectomy. The pooled sensitivity and specificity were 93 and 85% for histology, 77 and 89% for UBT, and 79 and 94% for RUT, respectively, thus leading to the conclusion that histology was the most reliable test in this setting. Lee et al[4] evaluated 91 patients requiring endoscopic mucosal resection for early gastric cancer (GC), obtaining three pairs of biopsies from the antrum, CLC and CGC. The sensitivity of histology in detecting H. pylori was significantly higher in the CGC than that in the antrum or CLC, suggesting that the CGC might be the optimal biopsy site for H. pylori in patients with extensive atrophy.
The utility of routine biopsy of the gastric ulcer margin (currently performed to exclude malignancy) in diagnosing H. pylori infection, has recently been re-assessed by Lin et al[29], by examining prospectively a cohort of 50 patients with gastric ulcer (54% uninfected). Histology, RUT and UBT were compared; six biopsied specimens from the margin of the gastric ulcer and one specimen each from the antrum and body of non-ulcerous parts were obtained for histology using HE staining. The diagnostic accuracy of the histological examination of the ulcer margin was quite good and importantly, the addition of one specimen from the antrum or body did not increase its diagnostic yield, thus emphasizing its accuracy and usefulness for diagnosing H. pylori infection in these patients.
An increasing body of evidence supports H. pylori colonization in the esophageal mucosa of dyspeptic patients. Contreras et al[30] have further contributed to the field, with a study examining the presence of H. pylori in the gastroesophageal mucosa by histology, fluorescence in situ hybridization (FISH) and PCR analysis of DNA (using genus- and species-specific PCR primers) extracted from gastric and esophageal biopsies of 82 symptomatic Venezuelan patients. H. pylori in the stomach was detected by PCR and FISH, respectively, in 61% and 90% of dyspeptic patients, and in the esophagus in 70% and 73%. By combining the results of both methods, H. pylori was observed in the gastroesophageal mucosa in 86% of patients. These findings deserve specific attention and further elucidation.
Finally, the histology reporting of gastritis of the staging system OLGA (Operative Link on Gastritis Assessment) has also been re-examined, considering its relevance to the prediction of the gastric cancer risk[31,32]. Carrasco et al[33] reviewed the histology of the normal gastric mucosa, overviewing the role of H. pylori in the multistep cascade of GC. The role of the OLGA staging system in assessing the risk of GC was emphasized; specifically, the epigenetic bases of chronic gastritis, mainly DNA methylation of the promoter region of E-cadherin in H. pylori - induced chronic gastritis and its reversion after H. pylori eradication. In addition, the authors discussed the finding of circulating cell-free DNA, offering the opportunity for non-invasive risk assessment of GC.
RAPID UREASE TEST
The RUT is based on the production of large amounts of urease enzyme by H. pylori, which splits the urea test reagent to form ammonia, enabling its detection by a rapid indirect test. Many commercial RUTs are available, including gel-based tests, paper-based tests and liquid-based tests, providing a result in 1-24 h, depending on the format of the test and the bacterial density in the biopsy specimen. Typically, commercial RUTs have specificities above 95%-100%; however, the sensitivity is slightly less, ranging from 85%-95%[34].
Compared with histology and culture, urease tests are faster, cheaper and have comparable sensitivity and specificity in normal clinical settings. The sensitivity can, however, decrease in patients with bleeding peptic ulcers (67%-85%), as well as in patients with partial gastrectomy (79%)[24,28,34,35]. Formalin contamination of forceps used to collect the biopsy may also contribute to reduced sensitivity[24,36].
An important conclusion of several studies is that enhancing the number of biopsy fragments and/or collecting them from various regions of the stomach (antrum and body, from example), achieves a higher sensibility of the RUT[37]. Moreover, it was shown recently that combining tissues prior to RUT increased the detection of H. pylori, compared with testing separate specimens, and produced faster results[38].
CULTURE
Since the discovery of H. pylori, bacterial culture has been used as routine diagnostic test, being considered the gold standard. Currently, the Maastricht-4 Consensus Report recommends H. pylori culture for performing antibiotic susceptibility testing if primary resistance to clarithromycin is higher than 20% or after failure of second-line treatment[17].
Despite its long use, culture tests remain a challenge because of the fastidious nature of the bacterium, with particular growth requirements of medium and atmosphere. The most commonly used media include Brucella, Columbia Wilkins-Chalgren, brain-heart infusion or trypticase agar bases, supplemented with sheep or horse blood[39]. An alternative to blood is supplementation of the agar base with β-cyclodextrin or yolk emulsion[40,41].
The most recent advances on H. pylori culture concern growth medium composition, besides the usual serum or blood additives. A recent study showed that supplementation of media with cholesterol instead of serum was a viable option for H. pylori growth[42]. Another original approach used liquid culture medium for the rapid cultivation and subsequent antibiotics susceptibility testing of H. pylori directly from biopsy specimens, with a final detection step by an enzyme linked immunosorbent assay (ELISA)[43].
Concerning the growth atmosphere, H. pylori is a capnophilic organism that requires an atmosphere enriched with CO2 (varying from 5%-10%). In addition, it has been considered a microaerophile, but there is no general consensus about its specific O2 requirements[44]. A recent advance on this topic was made by Park et al[45], who showed that unlike previous reports, H. pylori may be a capnophilic aerobe whose growth is promoted by atmospheric oxygen levels in the presence of 10% CO2.
Typically, culture of H. pylori is performed on gastric biopsy samples, and because bacteria display an irregular distribution in the gastric mucosa, culture of more than one biopsy, from the antrum and corpus, is sometimes mandatory, especially after antibiotic treatment. Another important issue to bear in mind are factors that may affect the outcome of H. pylori culture from endoscopic gastric mucosal specimens. Besides the issue concerning bleeding peptic ulcers, for which culture has a lower sensitivity than in nonbleeding cases, other host-related factors, such as high activity of gastritis, low bacterial load, drinking alcohol and the use of histamine H2 receptor blockers, have been recently described as the cause of failed H. pylori culture from gastric mucosa in the infected subjects[24,46].
Culturing from stools has been shown to be extremely difficult because of the complex nature of the sample regarding microbiota composition and shedding of unviable H. pylori cells, and this technique has been successful in the setting of rapid gastrointestinal tract transit[47]. In a recent study, the authors were able to culture H. pylori in nine and 12 rectal and ileal fluids, respectively, after polyethylene glycol (colyte) ingestion in 20 healthy adults with positive UBT[48]. Other studies have looked for the role of the oral cavity as a reservoir of H. pylori. A recent work evaluated the occurrence of the organism in subgingival plaque and was able, by culture, to recover H. pylori in nine of 30 studied patients that were H. pylori positive with RUT and histopathological examination. Thus, they concluded that detection of H. pylori in dental plaque of dyspeptic patients cannot be neglected and might represent a risk factor for recolonization of the stomach after systemic eradication therapy[49]. The same conclusion was reached by another study in which H. pylori was detected in subgingival dental plaque of children and their families, possibly acting as a “reservoir” contributing to the intra-familial spread[50].
MOLECULAR METHODS
Diagnostics tests rely more and more on molecular tests, which can provide faster, more accurate and sensitive detection of the bacterium than conventional methods, with the possibility of extension to other purposes, such as detection of antibiotic resistance and virulence determinants, and bacterial quantification. Moreover, biological samples other than gastric biopsies can be used, obtained using less invasive methods, such as stool or oral cavity samples. Whatever the case, amplification of the nucleic acids by PCR is almost always present, either conventional PCR or, increasingly, by real-time PCR.
H. pylori, like a few other bacteria, acquires resistance by mutation, which has enabled the development of numerous assays, in several formats, to detect mutations leading to resistance, especially to macrolides and fluoroquinolones. To detect H. pylori and resistances to fluoroquinolones and clarithromycin, there is a multiplex PCR followed by a hybridization and alkaline phosphatase reaction on a membrane strip (the Genotype® HelicoDR kit), that uses as a starting material biopsy specimens, as well as culture material extracted from it. The test shows a high sensitivity and permits detecting infection with multiple strains. The performance in detecting fluoroquinolone-resistance strains was, however, lower than culture, emphasizing the need to expand the range of gyrA mutations included in the kit[51,52]. Several real-time PCR based assays, using either TaqMan or FRET (Fluorescence Resonance Energy Transfer) are available, as in-house assays or commercial kits, for clarithromycin resistance, performed on cultured strains, directly on biopsies[53-55] or in stool samples. The latter is particularly useful as a non-invasive test in pediatric populations, where a high prevalence of clarithromycin-resistant strains is suspected, as well as for tracking the emergence of clarithromycin resistance following eradication treatment[57,58].
Recently, a dual-priming oligonucleotide (DPO)-based multiplex PCR was developed to detect both H. pylori infection and the most common point mutations conferring resistance to clarithomycin, directly on gastric biopsy specimens. This assay proved to be fast and does not require expensive instrumentation, making it valuable in countries with a high prevalence of clarithromycin resistance[59,60].
The detection of clarithromycin-resistance from formalin-fixed, paraffin-embedded gastric biopsies has also been described, and is useful mostly before treatment when culture and susceptibility testing is not available, or to detect primary resistance to clarithromycin in the case of failure of an empirical therapy based on this antibiotic. Real-time PCR assays, as well as a peptide nucleic acid-fluorescence in situ hybridization (PNA-FISH) method, have been described recently[61-63].
Another area of particular interest is the detection of virulence determinants, such as the cagA (cytotoxin-associated gene A) and the vacA (vacuolating cytotoxin) major toxins. Several studies showed that the risk of progression of gastric preneoplastic lesions is higher in patients infected with strains harboring the most virulent cagA and vacA genotypes than in patients infected with the least virulent strains. Therefore, H. pylori genotyping may be useful to identify patients at high risk of progression of gastric preneoplastic lesions and who need more intensive surveillance[64]. Concerning vacA, a novel method for genotyping the vacA intermediate gene region was reported recently, using a novel primer combination allowing the amplification of smaller DNA fragments than the original PCR, which can therefore be applied to paraffin-embedded biopsies. Patients infected with vacA i1 strains showed an increased risk of gastric atrophy and gastric carcinoma, with odds ratios of 8.0 (95%CI: 2.3-27) and of 22 (95%CI: 7.9-63)[65].
CagA undergoes phosphorylation on tyrosines within the Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs at the polymorphic C-terminus[66]. Several studies suggest a role for the polymorphic CagA EPIYA-containing region in the pathogenicity of H. pylori, although conflicting results have been reported[67,68]. The in vivo role of this region was emphasized recently in a study showing that infection with strains harboring two or more CagA EPIYA C motifs was associated with the presence of surface epithelial damage, and with atrophic gastritis and gastric carcinoma. Moreover, the presence of two or more CagA EPIYA C motifs increased the risk of atrophic gastritis from 7.3 (95%CI: 2.1-25) to 12 (95%CI: 2.5-58) and of gastric carcinoma from 17 (95%CI: 5.4-55) to 51 (95%CI: 13-198), when compared with one EPIYA C motif. Therefore, genotyping H. pylori virulence determinants could represent a useful approach in defining severe gastric-disease risk.
Bacterial quantification can also be important for clinical management of the infection; for example, for monitoring the treatment outcome or in particular settings, such as upper gastrointestinal bleeding[69].
A recently developed real-time quantitative PCR assay based on H. pylori ureC (single copy gene) copy number proved to be around 10 times more sensitive than the conventional PCR method. Moreover, the copy number of ureC was significantly increased when overall gastritis, bacterial density, chronic inflammation and intestinal metaplasia were present[70]. Nevertheless, further studies are necessary to determine the optimum cut-off point, making it possible to differentiate between asymptomatic colonization and infection with clinical implications for patients. These highly sensitive real-time quantitative PCRs can have a large application on the study of environmental reservoirs as well[71,72].
By improving our knowledge of bacteria, at the molecular level, new strategies for treatment/prevention of bacterial-associated diseases, as well as diagnostic tests, can be developed. Proteomic approaches aimed at identifying gene products differentially expressed in association with a given pathology can provide an important input towards understanding the pathways that are associated with the respective disease, contributing to the identification of novel therapeutic or diagnostic targets.
Our current knowledge on the proteome of this organism is largely based on data obtained for the soluble proteome[73], membrane proteome[74,75] and secreted proteome[76] of strain 26695, the first isolate to be sequenced. More recently, relevant contributions have made been through this approach, such as novel biomarkers for gastric cancer and for peptic ulcer disease[77,78].
NONINVASIVE TESTS
Although the reliability of both the 13C-UBT and a monoclonal ELISA stool test (HpSA) to diagnose H. pylori infection in very young children has been confirmed, additional background information is warranted for epidemiological studies in infants and toddlers.
UREA BREATH TESTS
The 13 C-urea breath test (13C-UBT) is one of the most reliable tests for diagnosing H. pylori infection. It is a non-invasive, simple and safe test that provides excellent accuracy both for the initial diagnosis of H. pylori infection and for the confirmation of its eradication after treatment. The simplicity, good tolerance and economy of the citric acid test meal probably make its systematic use advisable. The UBT protocol may be performed with relatively low doses (< 100 mg) of urea: 75 mg or even 50 mg seem to be sufficient. With the most widely used protocol (with citric acid and 75 mg of urea), excellent accuracy is obtained when breath samples are collected as early as 10-15 min after urea ingestion. A unique and generally proposed cut-off level is not possible, because it has to be adapted to different factors, such as the test meal, the dose and type of urea, or the pre-/post-treatment setting. As positive and negative UBT results tend to cluster outside of the range between 2 and 5, a change in cut-off value within this range would be expected to have little effect on the clinical accuracy of the test[79,80]. UBT is now marketed for use with a nondispersive, isotope-selective infrared spectroscope or laser-assisted ratio analysis, which are reliable and valid alternatives to isotope ratio mass spectrometry (IRMS) of potential interest for epidemiologic studies of children, for screening symptomatic children before endoscopy or assessment of treatment efficacy. These devices are far smaller and cheaper, and they allow for in-office, near-immediate reading of results. Validation studies to establish the cut-off value for this test were preliminarily performed in Japan[81]; however, further data are needed[82,83].
The 13C-UBT in adults has a high sensitivity (88%-95%) and specificity (95%-100%)[17]. However, the test has shown heterogeneous accuracy in the pediatric population, especially in young children, with values of sensitivity and specificity ranging from 75% to 100%, before and after treatment (using several protocols), despite being a simple and safe non-invasive test in children older than 6 years old[84]. Although several modifications have been proposed since the original description by Graham of the 13C-UBT to diagnose H. pylori infection[85], in children, performance criteria are not yet sufficiently established[86]. In the specific age group of younger children, accurate non-invasive tests for diagnosing H. pylori infection are required, as they may avoid invasive and painful procedures, such as endoscopy and blood sampling, and to overcoming the false negative results observed with gold standard tests (histology, culture, and RUT), where colonization of the stomach may be weak and patchy.
Potential explanations for UBT performance variability in children might include: (1) urease activity from the oral bacterial flora[87]; (2) differences in delta time (decrease in specificity if samples obtained at 15 min instead of 30 min); and (3) variability in cut-off values. The administration of 13C-urea in capsules to avoid activity of oral bacteria, though effective in adults, is not feasible in infants or toddlers[88]. Finally, the cut-off value (usually determined by a ROC curve) represents a crucial factor for the accuracy of the test, where low cut-off values might increase sensitivity but reduce specificity, and vice versa[81]. Additionally, the individual’s CO2 production is influenced by anthropometric characteristics, as well as by age and sex (lower in young children with relatively low weight and height)[89].
Leal et al[90] performed an informative systematic review and meta-analysis (31 articles and 135 studies from January 1998 to May 2009), aiming to evaluate the performance of the 13C-UBT diagnostic test for H. pylori infection in children. Studies with at least 30 children and reporting the comparison of 13C-UBT against a gold standard for H. pylori diagnosis (H. pylori culture, histologic examination, or RUT) were included for analysis. Children were stratified in subgroups of < 6 and ≥ 6 years of age. The 13C-UBT performance meta-analyses showed: (1) good accuracy in all ages combined [sensitivity 95.9%, specificity 95.7%, diagnostic odds ratio (DOR) 424.9]; (2) high accuracy in children > 6 years (sensitivity 96.6%, specificity 97.7%, DOR 1042.7); and (3) greater variability in accuracy estimates and a lower specificity in children ≤ 6 years (sensitivity 95%, specificity 93.5%, DOR 224.8). The authors identified as potentially important sources of heterogeneity: (1) tracer dose; (2) pretest meal; and (3) cut-off value, observing that a unique tracer dose of 50 mg of 13C-urea showed greater accuracy when it was adjusted to body weight (50-75 mg were used between studies). Accordingly, Mégraud[91] previously reported that reducing the dose from 75 to 45 mg in younger children resulted in improved specificity. Although citric acid has demonstrated good performance in adults, it is not well accepted by children, and apple, orange, or grape juice seem to be good alternatives. Finally, a cut-off value of 6.0‰ improved overall performance in children younger than 6 years, as compared to a cut-off of 4.0 ‰ for children older than 6 years.
Pacheco et al[92] evaluated the diagnostic accuracy of detecting H. pylori infection of low dose 13C-UBT with early sampling at pediatric age (129 patients between the ages of 2.1 and 19 years old, median = 11.6 years) submitted to upper gastrointestinal endoscopy. The 13C-UBT was performed after a 4-h fasting period with four points of collection: baseline (T0, at 10, 20 and 30 min) after ingestion of 25 mg 13C-urea diluted in 100 mL of apple juice; analysis of exhaled breath samples was performed with an isotope-selective infrared spectrometer. The sensitivity and specificity were similar at T10, T20 and T30 (94.7%/96.8%; 96.2%/96.1% and 96.2%/94.7%, respectively).
Recently, Queiroz et al[93] investigated the agreement between the 13C-UBT and a monoclonal ELISA (HpSA) to detect H. pylori antigen in stool in a prospective study enrolling 414 South-American infants (123 from Brazil and 291 from Peru) aged 6-30 mo. Breath and stool samples were obtained at intervals of at least three-months. 13C-UBT and stool test results concurred with each other in 94.86% cases (kappa coefficient = 0.90, 95%CI: 87-92). In the H. pylori-positive group, DOB and OD values were positively correlated (r = 0.62, P < 0.001, suggesting that both 13C-UBT and stool monoclonal test are reliable to diagnose H. pylori infection in very young children.
In contrast to pediatric studies, where attention has been focused on methodological issues, in adult studies, the validity and usefulness of UBT have increasingly been evaluated in a wide spectrum of specific clinic settings. Olafsson et al[94] evaluated 620 UBT in 595 subjects at a gastroenterology clinic. UBT was negative in 526 patients, but: (1) 45% patients were tested < 4 wk before the end of treatment; and (2) 23% of negative results occurred in patients recently treated. The authors emphasized the need for strict protocol adherence in clinical practice for a fully reliable UBT assessment. Velayos et al[95] investigated the accuracy of UBT performed immediately after emergency endoscopy in 74 patients with peptic ulcer bleeding by comparing the results with those of UBT performed after hospital discharge in a subset of 53 patients (gold standard). Although UBT carried out immediately after emergency endoscopy in peptic ulcer bleeding is an effective, safe and easy-to-perform procedure, the relatively low sensitivity and specificity suggested the requirement of a subsequent control, in accordance with recommendations concerning peptic ulcer bleeding[96].
Few studies using UBT have been performed in patients subjected to a partial gastrectomy, a specific group in which the identification of H. pylori infection is mostly relevant. Wardi et al[97] evaluated the sensitivity and specificity of the continuous UBT (BreathID) in 76 post gastrectomized patients (older than 18 years) (lowering the gastric pH by the addition of citric acid), against RUT and histology as gold standards. H. pylori was positive in 14/76 (18.4%) patients when histology was considered as the gold standard method. The positive predictive values of the continuous UBT and the RUT were 0.64 and 0.35, respectively. The negative predictive value was high by both the methods, 0.92 and 0.95, respectively, supporting the view that BreathID might have some reliability to exclude H. pylori after partial gastrectomy.
STOOL ANTIGEN TESTS
The stool antigen test is a non-invasive method to detect H. pylori, usually recommended when the UBT is not available[98]. Besides being non-invasive, the advantages of using this method include the unneeded requirement of expensive equipment and medical personnel, and the collection of the sample at home without a visit to the hospital. This method is especially relevant for children’s access to a safe diagnosis and also for its low cost[99,100].
A meta-analysis revealed that the global sensitivity and specificity of stool antigen tests are 94% (95%CI: 93-95) and 97% (95%CI: 96-98), respectively[101]. A prospective study to evaluate the efficacy of a new EZ-STEP H. pylori polyclonal enzyme immunoassay (EIA) stool antigen test enrolled 555 patients undergoing routine checkups. At the optimal cut-off value (optical density 0.160), this test presented high level of sensitivity (93.1%), specificity (94.6%) and accuracy (93.8%)[99].
There are two types of stool antigen tests used for H. pylori detection, the EIA and an assay based on immunochromatography. Two new stool tests were developed recently[102]. These tests are the Testmate pylori antigen EIA, in which plastic 96-well EIA microtiter plates are coated with monoclonal antibody (Mab) 21G2[103], and the Testmate rapid pylori antigen, which is based in immunochromatography and is presented as a test strip. For the EIA test, a drop of the suspended stool sample or a sample of the diluted bacterial antigen sample is mixed with the peroxidase-conjugated MAb 21G2. After proper incubation and washing, the optical density is measured and considered positive if greater than 0.100. For the test strip, a drop of stool sample is applied in the specimen application of the test strip. When H. pylori antigens are present, they form immune complexes with the red latex-labeled MAb 21Ge and migrate by capillarity action until captured by the solid phase anti-mouse rabbit polyclonal antibodies and form a visible red test line. A control line is also present. After application of these tests to 111 stool samples, both new tests provide 100% specificity, sensibility and accuracy[102], which is very promising. However, not all studies report these high values for sensitivity and specificity. For example, the report of Chehter et al[100] analyzed the stools of 75 patients and determined a lower sensitivity (87.2%) and specificity (44%); Iranikhah et al[104] analyzed the stools of 103 children and obtained similar values for sensitivity (85%), but improved specificity (83%).
Recently, five different stool antigen tests were compared: the Premier Platinum HpSA Plus test (based on monoclonal EIA; Meridian Bioscience, Inc, Cincinnati, OH, United States); the Hp Ag test (based on monoclonal EIA; Dia.Pro Diagnostic Bioprobes Srl, Milano, Italy); the ImmunoCard STAT! HpSA test (based on monoclonal lateral flow chromatography (LFC); Meridian Bioscience, Europe Srl Milano, Italy); the H. pylori fecal antigen test (based on monoclonal LFC; Vegal Farmaceutica, Madrid, Spain) and the one-step H. pylori antigen (based on LFC with polyclonal antibodies; IHP-602, ACON Laboratories, Inc, San Diego, United States). Data comparison showed an uneven performance, favoring the Premier Platinum HpSA Plus test (sensitivity 92.2%; specificity 94.4%). The selection of the stool antigen assay is very important to achieve accurate results.
Stool antigen tests are also useful to detect H. pylori in infected animal models, such as C57BL/6 mice[105].
ANTIBODY - BASED TESTS
Serology was one of the first methods used for diagnosis of H. pylori infection[106]. Currently, serology is recommended for initial screening, requiring further confirmation by histology and/or culture before treatment[107]. Detection of antibodies is useful for detecting past or present exposure. In fact, a limitation of serology tests is the failure to distinguish between past and current H. pylori infection[99]. Moreover, the antibody levels to H. pylori are significantly heritable. Thus, individual genetic differences of the human host contribute substantially to antibody levels to H. pylori[108].
Serological tests have several advantages, namely they are non-invasive and they do not produce false negative results in patients receiving treatment (proton pump inhibitors and antibiotics) or presenting acute bleeding[109].
Blood samples are used for serology testing, detecting anti-H. pylori antibodies (IgG) by ELISA. Recently, the performance of 29 different serological tests kits was compared, revealing sensitivities ranging from 55.6% to 100%, specificities ranging from 59.6% to 97.9 %, positive predictive values ranging from 69.8% and 100%, and negative predictive values ranging from 68.3% and 100%[106]. According to the goal, such as screening, initial diagnosis and confirmation of another test, the most appropriate kit should be chosen. Antibody-based tests for the detection of H. pylori are easily available, but present high negative predictive value[110]. The heterogeneity of H. pylori strains has been well documented, with considerable variation in the prevalence of specific strains, especially from different geographical areas[111-113]; thus, the success of a serology test depends on the use of antigens that are present in H. pylori strains from a given population. Moreover, kits developed using H. pylori strains from the west are not suitable for detecting H. pylori infection in the East[114]. The use of high-molecular-weight cell-associated antigens that are conserved in H. pylori strains overcomes this limitation[115]. Several H. pylori immunogenic proteins have been presented as candidates to detect infection, such as the FlidD protein[116]; multiple recombinant (CagA, VacA, GroEL, gGT, HcpC and UreA) proteins[116]; CagA[115] or Omp18[117].
Modifications to serology tests have been suggested, such as the automated immunoaffinity assay for H. pylori IgG detection using purified antigen of H. pylori immobilized on magnetic nanobeads, which is faster than ELISA and requires a smaller volume of serum[118]. The lateral flow immunoassay, an immunochromatographic assay, maintains the serological approach with the advantage of being fast, economic and requiring no additional equipment or experience[119].
Detection of gastrin and the serum PG I/II ratio combined with H. pylori serology is useful to predict gastric preneoplastic conditions[110]. The PG I/II ratio decreases with advancing extensive atrophic gastritis, since PG I is produced by chief and mucous neck cells in the fundus glands, which are impaired in case of gastritis of the fundus; while PG II is produced by the former cells and also by cardiac, pyloric and duodenal Brunner’s glands[120].
DETECTION OF H. PYLORI IN OTHER SPECIMENS
Other specimens have been evaluated to determine their usefulness to detect H. pylori infection. These include saliva[121,122], subgingival biofilm[123], dental plaque[124], gastric juice, gastroesophageal biopsies[125] and adenotonsillar tissue[126]. Contradictory results have been reported regarding H. pylori detection in adenotonsillar tissue, either favoring[127] or against[126] adenotonsillar tissue as an extra-gastric reservoir of H. pylori. The ability to detect H. pylori antibodies in saliva is lower than in blood-based serology. However, the use of molecular techniques for the detection of H. pylori infection in saliva or dental plaque may make these specimens attractive because they are easier to collect[114]. The molecular techniques include PCR[122,123] and PCR-denaturing gradient gel electrophoresis (PCR-DGGE)[128]. Other techniques used to analyze these specimens are the RUT, immunohistochemistry and PNA-FISH[126].
The enterotest or string test was designed decades ago specially for children. The string test consists of a gelatin capsule attached to a 90-140 cm long nylon string that unwinds during ingestion. Upon reaching the stomach, the gelatin capsule dissolves and the string absorbs gastric secretions. The extraction of the string occurs 30-180 min later and should avoid contact with teeth and tongue to prevent contamination. The string may be used for culture (sensitivity 65% and specificity 99%) or PCR (sensitivity 79% and specificity 99%) for H. pylori detection[129].
CONCLUSION
Recent developments in both biopsy- and non-biopsy-based diagnostic methods for H. pylori infection will further contribute to improving current clinical approach and management of H. pylori-associated diseases.
We predict that in the future, standard and newer methods will evolve to improve the diagnostic yield of H. pylori infection detection in specific age groups (children versus adults) and clinical conditions, such as peptic ulcer bleeding, atrophic gastritis, post-gastrectomy status, as well as for wider application in epidemiological studies. The specific contribution of each method to the evolving strategies and algorithms for evaluation and management of H. pylori infection (test and treat) will remain of paramount relevance.
Footnotes
P- Reviewer: Assem M, Kodama M, Pandya S, Ozcan C, Yucel O S- Editor: Zhai HH L- Editor: Stewart GJ E- Editor: Zhang DN
References
- 1.Watanabe K, Nagata N, Shimbo T, Nakashima R, Furuhata E, Sakurai T, Akazawa N, Yokoi C, Kobayakawa M, Akiyama J, et al. Accuracy of endoscopic diagnosis of Helicobacter pylori infection according to level of endoscopic experience and the effect of training. BMC Gastroenterol. 2013;13:128. doi: 10.1186/1471-230X-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T, et al. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632–2642. doi: 10.1002/ijc.27514. [DOI] [PubMed] [Google Scholar]
- 3.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Park YS, Choi KS, Kim do H, Choi KD, Song HJ, Lee GH, Jang SJ, Jung HY, Kim JH. Optimal biopsy site for Helicobacter pylori detection during endoscopic mucosectomy in patients with extensive gastric atrophy. Helicobacter. 2012;17:405–410. doi: 10.1111/j.1523-5378.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 5.Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39–45. doi: 10.1046/j.1440-1746.2002.02665.x. [DOI] [PubMed] [Google Scholar]
- 6.Yagi K, Honda H, Yang JM, Nakagawa S. Magnifying endoscopy in gastritis of the corpus. Endoscopy. 2005;37:660–666. doi: 10.1055/s-2005-861423. [DOI] [PubMed] [Google Scholar]
- 7.Gonen C, Simsek I, Sarioglu S, Akpinar H. Comparison of high resolution magnifying endoscopy and standard videoendoscopy for the diagnosis of Helicobacter pylori gastritis in routine clinical practice: a prospective study. Helicobacter. 2009;14:12–21. doi: 10.1111/j.1523-5378.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Yagi N, Kamada T, Shimbo T, Watanabe H, Ida K. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: a multicenter prospective study. Dig Endosc. 2013;25:508–518. doi: 10.1111/den.12031. [DOI] [PubMed] [Google Scholar]
- 9.Cho JH, Chang YW, Jang JY, Shim JJ, Lee CK, Dong SH, Kim HJ, Kim BH, Lee TH, Cho JY. Close observation of gastric mucosal pattern by standard endoscopy can predict Helicobacter pylori infection status. J Gastroenterol Hepatol. 2013;28:279–284. doi: 10.1111/jgh.12046. [DOI] [PubMed] [Google Scholar]
- 10.Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246–253. doi: 10.1016/j.gie.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Garcés HR, Castellanos-González VV, González-Fabián L, Infante-Velázquez M, Peña K, Andrain-Sierra Y. Chromoendoscopy with red phenol in the diagnosis of Helicobacter pylori infection. Rev Esp Enferm Dig. 2012;104:4–9. doi: 10.4321/s1130-01082012000100002. [DOI] [PubMed] [Google Scholar]
- 12.Lash JG, Genta RM. Adherence to the Sydney System guidelines increases the detection of Helicobacter gastritis and intestinal metaplasia in 400738 sets of gastric biopsies. Aliment Pharmacol Ther. 2013;38:424–431. doi: 10.1111/apt.12383. [DOI] [PubMed] [Google Scholar]
- 13.El-Zimaity H, Serra S, Szentgyorgyi E, Vajpeyi R, Samani A. Gastric biopsies: the gap between evidence-based medicine and daily practice in the management of gastric Helicobacter pylori infection. Can J Gastroenterol. 2013;27:e25–e30. doi: 10.1155/2013/897423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvet X, Lehours P, Lario S, Mégraud F. Diagnosis of Helicobacter pylori infection. Helicobacter. 2010;15 Suppl 1:7–13. doi: 10.1111/j.1523-5378.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin CM, Kim N, Lee HS, Lee HE, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, et al. Validation of diagnostic tests for Helicobacter pylori with regard to grade of atrophic gastritis and/or intestinal metaplasia. Helicobacter. 2009;14:512–519. doi: 10.1111/j.1523-5378.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 16.Lahner E, Vaira D, Figura N, Pilozzi E, Pasquali A, Severi C, Perna F, Delle Fave G, Annibale B. Role of noninvasive tests (C-urea breath test and stool antigen test) as additional tools in diagnosis of Helicobacter pylori infection in patients with atrophic body gastritis. Helicobacter. 2004;9:436–442. doi: 10.1111/j.1083-4389.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 18.Lan HC, Chen TS, Li AF, Chang FY, Lin HC. Additional corpus biopsy enhances the detection of Helicobacter pylori infection in a background of gastritis with atrophy. BMC Gastroenterol. 2012;12:182. doi: 10.1186/1471-230X-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anim JT, Al-Sobkie N, Prasad A, John B, Sharma PN, Al-Hamar I. Assessment of different methods for staining Helicobacter pylori in endoscopic gastric biopsies. Acta Histochem. 2000;102:129–137. doi: 10.1078/S0065-1281(04)70022-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang XI, Zhang S, Abreo F, Thomas J. The role of routine immunohistochemistry for Helicobacter pylori in gastric biopsy. Ann Diagn Pathol. 2010;14:256–259. doi: 10.1016/j.anndiagpath.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Smith SB, Snow AN, Perry RL, Qasem SA. Helicobacter pylori: to stain or not to stain? Am J Clin Pathol. 2012;137:733–738. doi: 10.1309/AJCP8DGTAVG7MBMT. [DOI] [PubMed] [Google Scholar]
- 22.Hartman DJ, Owens SR. Are routine ancillary stains required to diagnose Helicobacter infection in gastric biopsy specimens? An institutional quality assurance review. Am J Clin Pathol. 2012;137:255–260. doi: 10.1309/AJCPD8FFBJ5LSLTE. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal N, Snyder P, Owens SR. Unusual Helicobacter pylori in gastric resection specimens: an old friend with a new look. Int J Surg Pathol. 2011;19:297–302. doi: 10.1177/1066896911398654. [DOI] [PubMed] [Google Scholar]
- 24.Choi YJ, Kim N, Lim J, Jo SY, Shin CM, Lee HS, Lee SH, Park YS, Hwang JH, Kim JW, et al. Accuracy of diagnostic tests for Helicobacter pylori in patients with peptic ulcer bleeding. Helicobacter. 2012;17:77–85. doi: 10.1111/j.1523-5378.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramírez-Lázaro MJ, Lario S, Casalots A, Sanfeliu E, Boix L, García-Iglesias P, Sánchez-Delgado J, Montserrat A, Bella-Cueto MR, Gallach M, et al. Real-time PCR improves Helicobacter pylori detection in patients with peptic ulcer bleeding. PLoS One. 2011;6:e20009. doi: 10.1371/journal.pone.0020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu XQ, Wang ZH, Liao JX, Chen XY, Liu WZ, Xiao SD, Lu H. Predictive value of neutrophil infiltration as a marker of Helicobacter pylori infection. World J Gastroenterol. 2012;18:5101–5105. doi: 10.3748/wjg.v18.i36.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Martel C, Plummer M, van Doorn LJ, Vivas J, Lopez G, Carillo E, Peraza S, Muñoz N, Franceschi S. Comparison of polymerase chain reaction and histopathology for the detection of Helicobacter pylori in gastric biopsies. Int J Cancer. 2010;126:1992–1996. doi: 10.1002/ijc.24898. [DOI] [PubMed] [Google Scholar]
- 28.Tian XY, Zhu H, Zhao J, She Q, Zhang GX. Diagnostic performance of urea breath test, rapid urea test, and histology for Helicobacter pylori infection in patients with partial gastrectomy: a meta-analysis. J Clin Gastroenterol. 2012;46:285–292. doi: 10.1097/MCG.0b013e318249c4cd. [DOI] [PubMed] [Google Scholar]
- 29.Lin MH, Cheng HT, Chuang WY, Yu LK, Tsou YK, Lee MS. Histological examination of ulcer margin for diagnosing Helicobacter pylori infection in patients with gastric ulcers. Ann Diagn Pathol. 2013;17:63–66. doi: 10.1016/j.anndiagpath.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Contreras M, Salazar V, García-Amado MA, Reyes N, Aparcero M, Silva O, Castro D, Romero R, Gueneau P, Michelangeli F. High frequency of Helicobacter pylori in the esophageal mucosa of dyspeptic patients and its possible association with histopathological alterations. Int J Infect Dis. 2012;16:e364–e370. doi: 10.1016/j.ijid.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, Di Mario F. Gastritis: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S373–S384. doi: 10.1016/S1590-8658(11)60593-8. [DOI] [PubMed] [Google Scholar]
- 32.Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fassan M, Basso D, Plebani M, Graham DY. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104–1111. doi: 10.1111/j.1365-2036.2010.04277.x. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco G, Corvalan AH. Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol Res Pract. 2013;2013:393015. doi: 10.1155/2013/393015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng CA, Wang WM, Wu DC. Comparison of the clinical feasibility of three rapid urease tests in the diagnosis of Helicobacter pylori infection. Dig Dis Sci. 2005;50:449–452. doi: 10.1007/s10620-005-2456-5. [DOI] [PubMed] [Google Scholar]
- 35.Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848–863. doi: 10.1111/j.1572-0241.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 36.Ozaslan E, Koseoglu T, Purnak T, Yildiz A. A forgotten cause of false negative rapid urease test: formalin contamination of the sample. Hepatogastroenterology. 2010;57:2 p. preceding table of contents. [PubMed] [Google Scholar]
- 37.Hsu WH, Wang SS, Kuo CH, Chen CY, Chang CW, Hu HM, Wang JY, Yang YC, Lin YC, Wang WM, et al. Dual specimens increase the diagnostic accuracy and reduce the reaction duration of rapid urease test. World J Gastroenterol. 2010;16:2926–2930. doi: 10.3748/wjg.v16.i23.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon SW, Kim TH, Kim HS, Ju JH, Ahn YJ, Jang HJ, Shim SG, Kim HJ, Jung WT, Lee OJ. United Rapid Urease Test Is Superior than Separate Test in Detecting Helicobacter pylori at the Gastric Antrum and Body Specimens. Clin Endosc. 2012;45:392–396. doi: 10.5946/ce.2012.45.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ndip RN, MacKay WG, Farthing MJ, Weaver LT. Culturing Helicobacter pylori from clinical specimens: review of microbiologic methods. J Pediatr Gastroenterol Nutr. 2003;36:616–622. doi: 10.1097/00005176-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Olivieri R, Bugnoli M, Armellini D, Bianciardi S, Rappuoli R, Bayeli PF, Abate L, Esposito E, de Gregorio L, Aziz J. Growth of Helicobacter pylori in media containing cyclodextrins. J Clin Microbiol. 1993;31:160–162. doi: 10.1128/jcm.31.1.160-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westblom TU, Madan E, Midkiff BR. Egg yolk emulsion agar, a new medium for the cultivation of Helicobacter pylori. J Clin Microbiol. 1991;29:819–821. doi: 10.1128/jcm.29.4.819-821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiménez-Soto LF, Rohrer S, Jain U, Ertl C, Sewald X, Haas R. Effects of cholesterol on Helicobacter pylori growth and virulence properties in vitro. Helicobacter. 2012;17:133–139. doi: 10.1111/j.1523-5378.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 43.Perna F, Vaira D. A new 24 h ELISA culture based method for Helicobacter pylori chemosusceptibility. J Clin Pathol. 2010;63:648–651. doi: 10.1136/jcp.2010.076844. [DOI] [PubMed] [Google Scholar]
- 44.Bury-Moné S, Kaakoush NO, Asencio C, Mégraud F, Thibonnier M, De Reuse H, Mendz GL. Is Helicobacter pylori a true microaerophile? Helicobacter. 2006;11:296–303. doi: 10.1111/j.1523-5378.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 45.Park SA, Ko A, Lee NG. Stimulation of growth of the human gastric pathogen Helicobacter pylori by atmospheric level of oxygen under high carbon dioxide tension. BMC Microbiol. 2011;11:96. doi: 10.1186/1471-2180-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leszczyńska K, Namiot A, Namiot Z, Leszczyńska JK, Jakoniuk P, Chilewicz M, Namiot DB, Kemona A, Milewski R, Bucki R. Patient factors affecting culture of Helicobacter pylori isolated from gastric mucosal specimens. Adv Med Sci. 2010;55:161–166. doi: 10.2478/v10039-010-0028-1. [DOI] [PubMed] [Google Scholar]
- 47.Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. 1999;282:2240–2245. doi: 10.1001/jama.282.23.2240. [DOI] [PubMed] [Google Scholar]
- 48.Kim do H, Jung HM, Hwang YJ, Ahn YS, Mun JS, Myoung BH, Park H, Jeong EJ, Im YM, Oh HM, et al. [Culture and polymerase chain reaction of Helicobacter pylori from rectal and terminal ileal fluid after polyethylene glycol (colyte) ingestion in healthy adults with positive urea breath test] Korean J Gastroenterol. 2010;56:27–32. doi: 10.4166/kjg.2010.56.1.27. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal S, Jithendra KD. Presence of Helicobacter pylori in subgingival plaque of periodontitis patients with and without dyspepsia, detected by polymerase chain reaction and culture. J Indian Soc Periodontol. 2012;16:398–403. doi: 10.4103/0972-124X.100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsami A, Petropoulou P, Kafritsa Y, Mentis YA, Roma-Giannikou E. The presence of Helicobacter pylori in dental plaque of children and their parents: is it related to their periodontal status and oral hygiene? Eur J Paediatr Dent. 2011;12:225–230. [PubMed] [Google Scholar]
- 51.Miendje Deyi VY, Burette A, Bentatou Z, Maaroufi Y, Bontems P, Lepage P, Reynders M. Practical use of GenoType® HelicoDR, a molecular test for Helicobacter pylori detection and susceptibility testing. Diagn Microbiol Infect Dis. 2011;70:557–560. doi: 10.1016/j.diagmicrobio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, Deforges L, Soussy CJ, Delchier JC, Megraud F. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol. 2009;47:3600–3607. doi: 10.1128/JCM.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oleastro M, Ménard A, Santos A, Lamouliatte H, Monteiro L, Barthélémy P, Mégraud F. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 2003;41:397–402. doi: 10.1128/JCM.41.1.397-402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burucoa C, Garnier M, Silvain C, Fauchère JL. Quadruplex real-time PCR assay using allele-specific scorpion primers for detection of mutations conferring clarithromycin resistance to Helicobacter pylori. J Clin Microbiol. 2008;46:2320–2326. doi: 10.1128/JCM.02352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scaletsky IC, Aranda KR, Garcia GT, Gonçalves ME, Cardoso SR, Iriya K, Silva NP. Application of real-time PCR stool assay for Helicobacter pylori detection and clarithromycin susceptibility testing in Brazilian children. Helicobacter. 2011;16:311–315. doi: 10.1111/j.1523-5378.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 56.Oleastro M, Cabral J, Ramalho PM, Lemos PS, Paixão E, Benoliel J, Santos A, Lopes AI. Primary antibiotic resistance of Helicobacter pylori strains isolated from Portuguese children: a prospective multicentre study over a 10 year period. J Antimicrob Chemother. 2011;66:2308–2311. doi: 10.1093/jac/dkr293. [DOI] [PubMed] [Google Scholar]
- 57.Vécsei A, Innerhofer A, Graf U, Binder C, Giczi H, Hammer K, Bruckdorfer A, Hirschl AM, Makristathis A. Helicobacter pylori eradication rates in children upon susceptibility testing based on noninvasive stool polymerase chain reaction versus gastric tissue culture. J Pediatr Gastroenterol Nutr. 2011;53:65–70. doi: 10.1097/MPG.0b013e318210586d. [DOI] [PubMed] [Google Scholar]
- 58.Xiong LJ, Tong Y, Wang Z, Mao M. Detection of clarithromycin-resistant Helicobacter pylori by stool PCR in children: a comprehensive review of literature. Helicobacter. 2013;18:89–101. doi: 10.1111/hel.12016. [DOI] [PubMed] [Google Scholar]
- 59.Woo HY, Park DI, Park H, Kim MK, Kim DH, Kim IS, Kim YJ. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 2009;14:22–28. doi: 10.1111/j.1523-5378.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 60.Lehours P, Siffré E, Mégraud F. DPO multiplex PCR as an alternative to culture and susceptibility testing to detect Helicobacter pylori and its resistance to clarithromycin. BMC Gastroenterol. 2011;11:112. doi: 10.1186/1471-230X-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monno R, Giorgio F, Carmine P, Soleo L, Cinquepalmi V, Ierardi E. Helicobacter pylori clarithromycin resistance detected by Etest and TaqMan real-time polymerase chain reaction: a comparative study. APMIS. 2012;120:712–717. doi: 10.1111/j.1600-0463.2012.02896.x. [DOI] [PubMed] [Google Scholar]
- 62.Schmitt BH, Regner M, Mangold KA, Thomson RB, Kaul KL. PCR detection of clarithromycin-susceptible and -resistant Helicobacter pylori from formalin-fixed, paraffin-embedded gastric biopsies. Mod Pathol. 2013;26:1222–1227. doi: 10.1038/modpathol.2013.48. [DOI] [PubMed] [Google Scholar]
- 63.Cerqueira L, Fernandes RM, Ferreira RM, Oleastro M, Carneiro F, Brandão C, Pimentel-Nunes P, Dinis-Ribeiro M, Figueiredo C, Keevil CW, et al. Validation of a fluorescence in situ hybridization method using peptide nucleic acid probes for detection of Helicobacter pylori clarithromycin resistance in gastric biopsy specimens. J Clin Microbiol. 2013;51:1887–1893. doi: 10.1128/JCM.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.González CA, Figueiredo C, Lic CB, Ferreira RM, Pardo ML, Ruiz Liso JM, Alonso P, Sala N, Capella G, Sanz-Anquela JM. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Am J Gastroenterol. 2011;106:867–874. doi: 10.1038/ajg.2011.1. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira RM, Machado JC, Letley D, Atherton JC, Pardo ML, Gonzalez CA, Carneiro F, Figueiredo C. A novel method for genotyping the Helicobacter pylori vacA intermediate region directly in gastric biopsy specimens. J Clin Microbiol. 2012;50:3983–3989. doi: 10.1128/JCM.02087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36–43. doi: 10.1111/j.1349-7006.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batista SA, Rocha GA, Rocha AM, Saraiva IE, Cabral MM, Oliveira RC, Queiroz DM. Higher number of Helicobacter pylori CagA EPIYA C phosphorylation sites increases the risk of gastric cancer, but not duodenal ulcer. BMC Microbiol. 2011;11:61. doi: 10.1186/1471-2180-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acosta N, Quiroga A, Delgado P, Bravo MM, Jaramillo C. Helicobacter pylori CagA protein polymorphisms and their lack of association with pathogenesis. World J Gastroenterol. 2010;16:3936–3943. doi: 10.3748/wjg.v16.i31.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saez J, Belda S, Santibáñez M, Rodríguez JC, Sola-Vera J, Galiana A, Ruiz-García M, Brotons A, López-Girona E, Girona E, et al. Real-time PCR for diagnosing Helicobacter pylori infection in patients with upper gastrointestinal bleeding: comparison with other classical diagnostic methods. J Clin Microbiol. 2012;50:3233–3237. doi: 10.1128/JCM.01205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shukla SK, Prasad KN, Tripathi A, Ghoshal UC, Krishnani N, Nuzhat H. Quantitation of Helicobacter pylori ureC gene and its comparison with different diagnostic techniques and gastric histopathology. J Microbiol Methods. 2011;86:231–237. doi: 10.1016/j.mimet.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Nayak AK, Rose JB. Detection of Helicobacter pylori in sewage and water using a new quantitative PCR method with SYBR green. J Appl Microbiol. 2007;103:1931–1941. doi: 10.1111/j.1365-2672.2007.03435.x. [DOI] [PubMed] [Google Scholar]
- 72.Voytek MA, Ashen JB, Fogarty LR, Kirshtein JD, Landa ER. Detection of Helicobacter pylori and fecal indicator bacteria in five North American rivers. J Water Health. 2005;3:405–422. doi: 10.2166/wh.2005.054. [DOI] [PubMed] [Google Scholar]
- 73.Jungblut PR, Bumann D, Haas G, Zimny-Arndt U, Holland P, Lamer S, Siejak F, Aebischer A, Meyer TF. Comparative proteome analysis of Helicobacter pylori. Mol Microbiol. 2000;36:710–725. doi: 10.1046/j.1365-2958.2000.01896.x. [DOI] [PubMed] [Google Scholar]
- 74.Baik SC, Kim KM, Song SM, Kim DS, Jun JS, Lee SG, Song JY, Park JU, Kang HL, Lee WK, et al. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J Bacteriol. 2004;186:949–955. doi: 10.1128/JB.186.4.949-955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabarth N, Lamer S, Zimny-Arndt U, Jungblut PR, Meyer TF, Bumann D. Identification of surface proteins of Helicobacter pylori by selective biotinylation, affinity purification, and two-dimensional gel electrophoresis. J Biol Chem. 2002;277:27896–27902. doi: 10.1074/jbc.M204473200. [DOI] [PubMed] [Google Scholar]
- 76.Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, Sabarth N, Meyer TF, Jungblut PR. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 2002;70:3396–3403. doi: 10.1128/IAI.70.7.3396-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin LL, Huang HC, Juan HF. Discovery of biomarkers for gastric cancer: a proteomics approach. J Proteomics. 2012;75:3081–3097. doi: 10.1016/j.jprot.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 78.Vitoriano I, Saraiva-Pava KD, Rocha-Gonçalves A, Santos A, Lopes AI, Oleastro M, Roxo-Rosa M. Ulcerogenic Helicobacter pylori strains isolated from children: a contribution to get insight into the virulence of the bacteria. PLoS One. 2011;6:e26265. doi: 10.1371/journal.pone.0026265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graham DY, Klein PD. Accurate diagnosis of Helicobacter pylori. 13C-urea breath test. Gastroenterol Clin North Am. 2000;29:885–93, x. doi: 10.1016/s0889-8553(05)70156-4. [DOI] [PubMed] [Google Scholar]
- 80.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 2004;20:1001–1017. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 81.Kato S, Ozawa K, Konno M, Tajiri H, Yoshimura N, Shimizu T, Fujisawa T, Abukawa D, Minoura T, Iinuma K. Diagnostic accuracy of the 13C-urea breath test for childhood Helicobacter pylori infection: a multicenter Japanese study. Am J Gastroenterol. 2002;97:1668–1673. doi: 10.1111/j.1572-0241.2002.05825.x. [DOI] [PubMed] [Google Scholar]
- 82.Kawakami E, Machado RS, Reber M, Patrício FR. 13 C-urea breath test with infrared spectroscopy for diagnosing helicobacter pylori infection in children and adolescents. J Pediatr Gastroenterol Nutr. 2002;35:39–43. doi: 10.1097/00005176-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Parente F, Bianchi Porro G. The (13)C-urea breath test for non-invasive diagnosis of Helicobacter pylori infection: which procedure and which measuring equipment? Eur J Gastroenterol Hepatol. 2001;13:803–806. doi: 10.1097/00042737-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 84.Guarner J, Kalach N, Elitsur Y, Koletzko S. Helicobacter pylori diagnostic tests in children: review of the literature from 1999 to 2009. Eur J Pediatr. 2010;169:15–25. doi: 10.1007/s00431-009-1033-x. [DOI] [PubMed] [Google Scholar]
- 85.Graham DY, Klein PD, Evans DJ, Evans DG, Alpert LC, Opekun AR, Boutton TW. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987;1:1174–1177. doi: 10.1016/s0140-6736(87)92145-3. [DOI] [PubMed] [Google Scholar]
- 86.Machado RS, Patrício FR, Kawakami E. 13C-urea breath test to diagnose Helicobacter pylori infection in children aged up to 6 years. Helicobacter. 2004;9:39–45. doi: 10.1111/j.1083-4389.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 87.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaira D, Gatta L, Ricci C, Di Mario F, Lanzini A. Accuracy of urea breath tests tablets after 10 minutes compared with standard 30 minutes to diagnose and monitoring Helicobacter pylori infection: a randomized controlled trial. J Clin Gastroenterol. 2009;43:693–694. doi: 10.1097/MCG.0b013e318193e487. [DOI] [PubMed] [Google Scholar]
- 89.Slater C, Preston T, Weaver LT. Is there an advantage in normalising the results of the Helicobacter pylori [13C]urea breath test for CO2 production rate in children? Isotopes Environ Health Stud. 2004;40:89–98. doi: 10.1080/10256010310001621164. [DOI] [PubMed] [Google Scholar]
- 90.Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16:327–337. doi: 10.1111/j.1523-5378.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 91.Mégraud F. Comparison of non-invasive tests to detect Helicobacter pylori infection in children and adolescents: results of a multicenter European study. J Pediatr. 2005;146:198–203. doi: 10.1016/j.jpeds.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 92.Pacheco SL, Ogata SK, Machado RS, Patrício FR, Pardo ML, Kawakami E. Diagnosis of Helicobacter pylori infection by means of reduced-dose ¹³C-urea breath test and early sampling of exhaled breath. J Pediatr Gastroenterol Nutr. 2013;57:607–611. doi: 10.1097/MPG.0b013e3182a02608. [DOI] [PubMed] [Google Scholar]
- 93.Queiroz DM, Saito M, Rocha GA, Rocha AM, Melo FF, Checkley W, Braga LL, Silva IS, Gilman RH, Crabtree JE. Helicobacter pylori infection in infants and toddlers in South America: concordance between [13C]urea breath test and monoclonal H. pylori stool antigen test. J Clin Microbiol. 2013;51:3735–3740. doi: 10.1128/JCM.01752-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olafsson S, Patel B, Jackson C, Cai J. Helicobacter pylori breath testing in an open access system has a high rate of potentially false negative results due to protocol violations. Helicobacter. 2012;17:391–395. doi: 10.1111/j.1523-5378.2012.00964.x. [DOI] [PubMed] [Google Scholar]
- 95.Velayos B, Fernández-Salazar L, Pons-Renedo F, Muñoz MF, Almaraz A, Aller R, Ruíz L, Del Olmo L, Gisbert JP, González-Hernández JM. Accuracy of urea breath test performed immediately after emergency endoscopy in peptic ulcer bleeding. Dig Dis Sci. 2012;57:1880–1886. doi: 10.1007/s10620-012-2096-5. [DOI] [PubMed] [Google Scholar]
- 96.Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 97.Wardi J, Shalev T, Shevah O, Boaz M, Avni Y, Shirin H. A rapid continuous-real-time 13C-urea breath test for the detection of Helicobacter pylori in patients after partial gastrectomy. J Clin Gastroenterol. 2012;46:293–296. doi: 10.1097/MCG.0b013e31823eff09. [DOI] [PubMed] [Google Scholar]
- 98.Cirak MY, Akyön Y, Mégraud F. Diagnosis of Helicobacter pylori. Helicobacter. 2007;12 Suppl 1:4–9. doi: 10.1111/j.1523-5378.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 99.Choi J, Kim CH, Kim D, Chung SJ, Song JH, Kang JM, Yang JI, Park MJ, Kim YS, Yim JY, et al. Prospective evaluation of a new stool antigen test for the detection of Helicobacter pylori, in comparison with histology, rapid urease test, (13)C-urea breath test, and serology. J Gastroenterol Hepatol. 2011;26:1053–1059. doi: 10.1111/j.1440-1746.2011.06705.x. [DOI] [PubMed] [Google Scholar]
- 100.Chehter EZ, Bacci MR, Fonseca FL, Gonçalves JA, Buchalla G, Shiraichi SA, Mariano RC. Diagnosis of the infection by the Helicobacter pylori through stool examination: method standardization in adults. Clin Biochem. 2013;46:1622–1624. doi: 10.1016/j.clinbiochem.2013.05.071. [DOI] [PubMed] [Google Scholar]
- 101.Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:1921–1930. doi: 10.1111/j.1572-0241.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- 102.Sato M, Shimoyama T, Takahashi R, Kajiyama H, Sano Y, Sakaedani N, Kato A, Hirata H, Fukuda Y. Characterization and usefulness of stool antigen tests using a monoclonal antibody to Helicobacter pylori catalase. J Gastroenterol Hepatol. 2012;27 Suppl 3:23–28. doi: 10.1111/j.1440-1746.2012.07066.x. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki N, Wakasugi M, Nakaya S, Okada K, Mochida R, Sato M, Kajiyama H, Takahashi R, Hirata H, Ezure Y, et al. Production and application of new monoclonal antibodies specific for a fecal Helicobacter pylori antigen. Clin Diagn Lab Immunol. 2002;9:75–78. doi: 10.1128/CDLI.9.1.75-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iranikhah A, Ghadir MR, Sarkeshikian S, Saneian H, Heiari A, Mahvari M. Stool antigen tests for the detection of Helicobacter pylori in children. Iran J Pediatr. 2013;23:138–142. [PMC free article] [PubMed] [Google Scholar]
- 105.Moon DI, Shin EH, Oh HG, Oh JS, Hong S, Chung Y, Kim O. Usefulness of a Helicobacter pylori stool antigen test for diagnosing H. pylori infected C57BL/6 mice. Lab Anim Res. 2013;29:27–32. doi: 10.5625/lar.2013.29.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burucoa C, Delchier JC, Courillon-Mallet A, de Korwin JD, Mégraud F, Zerbib F, Raymond J, Fauchère JL. Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter. 2013;18:169–179. doi: 10.1111/hel.12030. [DOI] [PubMed] [Google Scholar]
- 107.Mégraud F. The most important diagnostic modalities for Helicobacter pylori, now and in the future. Eur J Gastroenterol Hepatol. 2012;9 Suppl 1:S13–S5; discussion S15. [PubMed] [Google Scholar]
- 108.Rubicz R, Leach CT, Kraig E, Dhurandhar NV, Duggirala R, Blangero J, Yolken R, Göring HH. Genetic factors influence serological measures of common infections. Hum Hered. 2011;72:133–141. doi: 10.1159/000331220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Braden B. Diagnosis of Helicobacter pylori infection. BMJ. 2012;344:e828. doi: 10.1136/bmj.e828. [DOI] [PubMed] [Google Scholar]
- 110.Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:1–8. doi: 10.1111/j.1523-5378.2012.00975.x. [DOI] [PubMed] [Google Scholar]
- 111.Vale FF, Encarnação P, Vítor JM. A new algorithm for cluster analysis of genomic methylation: the Helicobacter pylori case. Bioinformatics. 2008;24:383–388. doi: 10.1093/bioinformatics/btm621. [DOI] [PubMed] [Google Scholar]
- 112.Vale FF, Mégraud F, Vítor JM. Geographic distribution of methyltransferases of Helicobacter pylori: evidence of human host population isolation and migration. BMC Microbiol. 2009;9:193. doi: 10.1186/1471-2180-9-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vitoriano I, Rocha-Gonçalves A, Carvalho T, Oleastro M, Calado CR, Roxo-Rosa M. Antigenic diversity among Portuguese clinical isolates of Helicobacter pylori. Helicobacter. 2011;16:153–168. doi: 10.1111/j.1523-5378.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- 114.McNulty CA, Lehours P, Mégraud F. Diagnosis of Helicobacter pylori Infection. Helicobacter. 2011;16 Suppl 1:10–18. doi: 10.1111/j.1523-5378.2011.00875.x. [DOI] [PubMed] [Google Scholar]
- 115.Marchildon PA, Sugiyama T, Fukuda Y, Peacock JS, Asaka M, Shimoyama T, Graham DY. Evaluation of the effects of strain-specific antigen variation on the accuracy of serologic diagnosis of Helicobacter pylori infection. J Clin Microbiol. 2003;41:1480–1485. doi: 10.1128/JCM.41.4.1480-1485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khalifeh Gholi M, Kalali B, Formichella L, Göttner G, Shamsipour F, Zarnani AH, Hosseini M, Busch DH, Shirazi MH, Gerhard M. Helicobacter pylori FliD protein is a highly sensitive and specific marker for serologic diagnosis of H. pylori infection. Int J Med Microbiol. 2013;303:618–623. doi: 10.1016/j.ijmm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 117.Talebkhan Y, Ebrahimzadeh F, Esmaeili M, Zamaninia L, Nahvijoo A, Khedmat H, Fereidooni F, Mohagheghi MA, Mohammadi M. Helicobacter pylori Omp18 and its application in serologic screening of infection. Curr Microbiol. 2011;62:325–330. doi: 10.1007/s00284-010-9694-2. [DOI] [PubMed] [Google Scholar]
- 118.Stege PW, Raba J, Messina GA. Online immunoaffinity assay-CE using magnetic nanobeads for the determination of anti-Helicobacter pylori IgG in human serum. Electrophoresis. 2010;31:3475–3481. doi: 10.1002/elps.201000123. [DOI] [PubMed] [Google Scholar]
- 119.Karakus C, Salih BA. Comparison of the lateral flow immunoassays (LFIA) for the diagnosis of Helicobacter pylori infection. J Immunol Methods. 2013;396:8–14. doi: 10.1016/j.jim.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 120.Toyoda K, Furusyo N, Ihara T, Ikezaki H, Urita Y, Hayashi J. Serum pepsinogen and Helicobacter pylori infection--a Japanese population study. Eur J Clin Microbiol Infect Dis. 2012;31:2117–2124. doi: 10.1007/s10096-011-1543-0. [DOI] [PubMed] [Google Scholar]
- 121.Adamsson I, Edlund C, Nord CE. Microbial ecology and treatment of Helicobacter pylori infections: review. J Chemother. 2000;12:5–16. doi: 10.1179/joc.2000.12.1.5. [DOI] [PubMed] [Google Scholar]
- 122.Kabir S. Detection of Helicobacter pylori DNA in feces and saliva by polymerase chain reaction: a review. Helicobacter. 2004;9:115–123. doi: 10.1111/j.1083-4389.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 123.Souto R, Colombo AP. Detection of Helicobacter pylori by polymerase chain reaction in the subgingival biofilm and saliva of non-dyspeptic periodontal patients. J Periodontol. 2008;79:97–103. doi: 10.1902/jop.2008.070241. [DOI] [PubMed] [Google Scholar]
- 124.Chaudhry S, Idrees M, Izhar M, Butt AK, Khan AA. Simultaneous amplification of two bacterial genes: more reliable method of Helicobacter pylori detection in microbial rich dental plaque samples. Curr Microbiol. 2011;62:78–83. doi: 10.1007/s00284-010-9662-x. [DOI] [PubMed] [Google Scholar]
- 125.Abrante L, Reyes N, García-Amado MA, Suárez P, Romero R, Michelangeli F, Contreras M. [Diagnosis of Helicobacter pylori infection by PCR in gastric juice and gastroesophageal biopsies from dyspeptic patients] Invest Clin. 2012;53:168–177. [PubMed] [Google Scholar]
- 126.Vilarinho S, Guimarães NM, Ferreira RM, Gomes B, Wen X, Vieira MJ, Carneiro F, Godinho T, Figueiredo C. Helicobacter pylori colonization of the adenotonsillar tissue: fact or fiction? Int J Pediatr Otorhinolaryngol. 2010;74:807–811. doi: 10.1016/j.ijporl.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 127.Abdel-Monem MH, Magdy EA, Nour YA, Harfoush RA, Ibreak A. Detection of Helicobacter pylori in adenotonsillar tissue of children with chronic adenotonsillitis using rapid urease test, PCR and blood serology: a prospective study. Int J Pediatr Otorhinolaryngol. 2011;75:568–572. doi: 10.1016/j.ijporl.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 128.Petersen RF, Harrington CS, Kortegaard HE, On SL. A PCR-DGGE method for detection and identification of Campylobacter, Helicobacter, Arcobacter and related Epsilobacteria and its application to saliva samples from humans and domestic pets. J Appl Microbiol. 2007;103:2601–2615. doi: 10.1111/j.1365-2672.2007.03515.x. [DOI] [PubMed] [Google Scholar]
- 129.del Pozo García AJ, Gisbert JP. Is the string test a useful alternative to gastroscopy with biopsy for H. pylori identification? Rev Esp Enferm Dig. 2006;98:542–549. doi: 10.4321/s1130-01082006000700007. [DOI] [PubMed] [Google Scholar]


