Abstract
Background
Repeated electroacupuncture (EA) stimulation is known to stimulate the activity of the hypothalamus-pituitary-adrenal axis, and to enhance the circulation level of estrogen in the ovariectomized rats. To explore the source of the increased circulation estrogen, the extragonadal aromatization was detected.
Methods
Female rats were divided into five groups: 1) intact (INT), 2) intact with EA in specific points (INT+EA), 3) ovariectomized (OVX), 4) ovariectomized with EA in specific points (OVX+EA) and 5) ovariectomized with EA in non-specific points (OVX+C). Radiometric assay, Western blot and RT-PCR were adopted to determine the extragonadal aromatization in subcutaneous abdominal (SA) adipose and liver tissues of rats. The blood concentrations of estrogen, testosterone and corticosterone were measured by radioimmunoassay.
Results
The aromatase activities of the SA adipose and liver tissues in the OVX+EA rats increased significantly (p < 0.01) compared with those in the INT, INT+EA and OVX rats. The 58-kDa aromatase protein and aromatase mRNA expressions normalized to β-actin in the OVX+EA rats' SA adipose tissues showed higher levels than those from corresponding tissues in the INT and INT+EA rats (p < 0.05). And the ratios of aromatase mRNA and protein to β-actin in the OVX+EA rats' liver tissues increased significantly compared with those in the OVX rats (p < 0.05). Furthermore, blood estrogen and corticosterone concentrations showed significant increase in the OVX+EA rats compared with the concentrations in the OVX and OVX+C rats (p < 0.05), but no statistical disparity occurred on the blood testosterone concentrations between the OVX+EA rats and the OVX ones.
Conclusion
Both the subcutaneous abdominal adipose and the liver tissues contributed to the effects of electroacupuncture on the extragonadal aromatization to promote the blood concentrations of estrogen in the ovariectomized rats.
Background
Acupuncture has long been associated with homeostatic (Yin/Yang) regulation, and is known to possess effects such as buffering hormonal disturbance, modulating ovulation, as well as improving psychological or behavioural abnormity [1-3]. Our laboratory has been studying the mechanism of acupuncture using reproductive disorders as the model system for the past decade. We have observed that repeated electroacupuncture (EA) in specific acupoints significantly increased blood concentrations of estradiol (E2) in the OVX rats [4], while reducing the elevated plasma luteinizing hormone (LH) due to OVX [5]; in addition, EA also restored the number of gonadotropin-releasing hormone (GnRH) neurons in the OVX rats [4]. These results suggest that EA effectively enhance the function of the hypothalamus-pituitary-ovary axis (HPOA).
Ovaries are the primary source of estradiol, a circulating hormone acting on distal target tissues. In postmenopausal and ovariectomized women, the production of estrogen is shifted from the ovary to a number of extragonadal sites [6]. Simpson developed the intriguing concept of extragonadal aromatization, i.e. androgens, particularly androstenedione produced primarily in the adrenal glands, can be converted (aromatised) into estrogens at extraglandular sites, including the mesenchymal cells of the adipose tissue and skin, osteoblasts and chondrocytes in bone, vascular endothelial, aortic smooth muscle cells as well as numerous sites in the brain [6]. Estrogen synthesised within these sites is biologically active in a paracrine or intracrine fashion, although it may enter the circulation and increase the circulating levels of estrogen [6].
We have observed that repeated EA treatments significantly increased the number of corticotropin-releasing hormone (CRH) cells and the releasing of CRH in the hypothalamic paraventricular nucleus of the OVX rats [7]. As a consequence, blood concentrations of ACTH (unpublished data), the activity of adrenal cortex [5] and blood concentration of corticosterone [5] are all elevated. Meanwhile there is a significant increase of the blood E2 concentration in the same OVX+EA rats [4]. These observations lead us to hypothesize that more androgens secretion from adrenal cortex might be converted into E2 by extragonadal aromatase in the ovariectomized rats with repeated EA stimulations; they escape the local metabolism and enter circulation. To test the hypothesis, we detected the aromatase activity as well as its expression in adipose and liver tissues in the OVX rats that received EA. The data provide a comprehensive evidence for the acupuncture therapy in reproductive disorders.
Methods
Animals
Sixty female Sprague-Dawley rats (180–200 g), with regular 4-day estrus cycles were purchased from Medical Experimental Animals Center of Fudan University (Shanghai, China). The animals were housed under laminar flow in an isolated room with controlled temperature and at a 12 /12 (light /dark) schedule. Thirty-six of them underwent ovariectomy with ether anaesthesia, which were then divided randomly into three groups: ovariectomized (OVX), ovariectomized with EA in specific points (OVX+EA) and ovariectomized with EA in non-specific points (OVX+C). The rest were treated as controls, which were divided into two groups: intact (INT) and intact with EA in specific points (INT+EA). Four weeks later, OVX+EA (n = 12), OVX+C (n = 12) and INT+EA (n = 12) received EA treatment. Thirty minutes before the EA treatment, all the animals were bound as comfortably as possible, and during the EA procedure, the rats were conscious without anaesthesia. Electrical stimulation was administered via three stainless steel needles of 0.3 mm diameter inserted 5 mm in four acupoints in the belly: "Guanyuan" acupoint (RN4), in the midline of abdomen (15 mm bellow the umbilicus); "Zhongji" acupoint (RN3), 5 mm bellow "Guanyuan" acupoints (one needle was flatly punctured in the RN4 which penetrated into the RN3); bilateral "Zigongxue" acupoints (EXTRA22), 7.5 mm lateral to the "Zhongji" acupoint, and one needle inserted 3 mm at one acupoint in the hind leg, "Sanyinjiao" (SP6), near ankle joint (at the concentration of the superior border of the medial melleolus, between the posterior border of the tibia and anterior border of the Achilles tendon). These acupoints are widely applied in Oriental medicine for the treatment of gynaecological disease in women [8] and for the acupuncture mechanism research in the ovariectomized rats [4,5,7,9]. The control acupoints were "Waiguan" (SJ5), between the radius and ulna (5 mm above the dorsal transverse crease of the wrist) and "Huatuojiaji" (EXTRA15), in the back (5 mm lateral to the lower border of each spinous process from the first thoracic vertebra to the fifth lumbar vertebra (Fig. 1). The stimulation was generated by an EA apparatus (Model G6805-2, SMIF, Shanghai, China) and lasted for 30 min (8:00–10:00 AM), Q.D, for 3 days altogether. The stimulation parameters were 2 mA of density and a low-burst frequency of 3 Hz. Individual pulses within the burst frequency were square wave pulses with alternating polarities and pulse duration of 0.2 ms, 80 pulse per second. The intensity was adjusted to produce a slight twitch of the limbs. All experimental procedures involving the use of animals were conducted in accordance with NIH Guidelines and were reviewed and approved by the Animal Use and Care Committee for the Fudan University.
Figure 1.
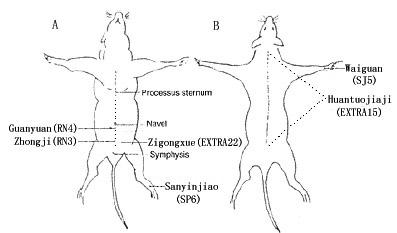
Sketch of ventral view (A) and dorsal view (B) of rat shows the acupoints we used.
Tissue collection, preparation of microsomal pellet and total tissue RNA
At the time of sacrifice (6 hours after the last EA), the vaginal cytology of each OVX+EA rat was first examined. The animals whose epithelial cells reappeared were adopted for the following experiments, which could be a validity indicator of the EA. The tissues of the OVX, OVX+EA and INT+EA rats were collected respectively, and those of the INT animals, during the period of proestrus. All the operations were carried out at 4°C. The rats were sacrificed by decapitation; the subcutaneous abdominal (SA) adipose and liver tissues were excised; and then snap-frozen in liquid nitrogen, stored at -80°C. The preparation of the microsomal pellet was accordance with the report by Hiroshi [10]. Total tissue RNA was extracted using 'TRIzol Regent' (Biobasic Inc, Canada), and the purity and integrity of the RNA were checked spectroscopically and by gel electrophoresis before analytical procedures.
Measurement of aromatase activity
A modified version of the radiometric assay of Charles and William [11] was applied to determine the aromatase activity. The microsomal pellets were sonicated in 1:30 (w/v) phosphate buffers (10 mM KPO4, 100 mM KCl and 1 mM EDTA, pH 7.4). The incubation mixture, made of NADPH-generating system and 0.13 μM (1β-3H) androstenedione (Amersham Pharmacia Biotech, UK, 24 Ci/mmol) in 100 μl phosphate buffer (10 mM KPO4, 100 mM KCl, 10 mM dithiothreitol, 1 mM EDTA and 2.5 mM glucose-6-phosphate dehydrogenase/ml, pH 7.4), was pre-incubated at 37°C for 30 min to generate sufficient NADPH for aromatase reaction. Aliquots (100 μl) of microsome samples and ovarian microsome that were treated as positive controls, were then added for an incubation in a Dubnoff shaking water bath for 1 h at 37°C. The reaction was terminated by addition of 0.4 ml ice-cold 15% trichloroacetic acid containing 40 mg charcoal/ml, and then the labelled aqueous phases were separated from the unmetabolized substrate, and from the steroid products formed during the incubation, by chloroform extraction and 5% charcoal/0.5% dextran precipitation. After a rapid passage through a cotton filter, the amount of the tritiated water formed during the aromatization process was measured in a liquid scintillation spectrometer. A calibration standard curve helped obtain quench corrected dpm of the isotope. The amount of protein in each tube was evaluated using the Bradford method (Bradford, 1976). To validate the 3H2O assay, aliquots of microsomes were incubated with increasing amounts of (1β-3H) androstenedione (e.g. 10–200 nM), and the amount of 3H2O generated was measured and corrected for counts in buffer blanks. The data were plotted as a saturation plot; the maximal velocity (Vmax) and Michaelis constant (Km), derived by non-linear regression analysis.
Western blotting
A 50 μg sample of the microsomal protein was loaded into each lane along with a prestained protein size marker (Bio-Rad Laboratories, Inc., Hercules, CA), electrophoresed on a 10% SDS-polyacrylamide gel at 18 V/cm, and electroblotted onto a polyvinylidene difluoride membrane (Micron Separations, Westboro, MA) using a wet electroblotter. After blocking in fat-free milk, incubation was conducted with the antiaromatase antibody (1:200; Boster Biological Technology LTD., China) and β-actin antibody (1:3000) at room temperature for 4 h in TBS-T solution (20 mM Tris, 137 mM NaCl, and 0.1% Tween-20, pH 7.6). After extensive washing, blots were incubated with AP-labelled goat antirabbit antiserum (Sino-American Biotechnology Co., China) for 60 min at room temperature and developed using NBT/BCIP detection system (Amersham Pharmacia Biotech). The intensities of the bands were evaluated using the Image Master Software (SYDR-1990, SYNGENE, U.S.A.), and values were normalized to β-actin immunoreactivity in each sample and expressed as percent of the control. Specificity of the aromatase immuno-staining was determined by preincubation of antiserum for 24 h at 4°C with varying concentrations of aromatase, with the primary antibody omitted to identify non-specific staining as well.
RNA analysis
Total RNA (2 μg) was transcribed in reverse, in a final volume of 20 μl, using 200 IU M-MLV reverse transcriptase in the presence of 25 pmol downstream primer (Sangon Inc), 0.5 mM deoxy-NTP and 20 IU Rnasin (from Promega) for 60 min at 42°C before heat denatured for 5 min at 95°C. The cDNAs obtained were further amplified by PCR using 25 pmol of upstream primer (Sangon Inc) designed to amplify P450 arom highly conserved sequence (289 bp length) including helical and aromatic region, (upstream Aro-Ex8, 5'GCT TCT CAT CGC AGA GTA TCC GG-3' located in the exon 8; downstream Aro-Ex9, 5'-CAA GGG TAA ATT CAT TGG GCT TGG-3' located in the exon 9; amplified product 289 bp) [12]. We first determined the linear range of amplification of cDNA using each of the primer sets, and then chose an appropriate amplification cycle within this range for each cDNA species. For P450 arom, we used 35 PCR amplification cycles, and 20 cycles for β-actin gene expression. P450 arom PCR was performed (1 min at 94°C, 1 min at 60°C, 2 min at 72°C) with Taq DNA polymerase (3 U per tube) and 2.2 mM magnesium chloride (from Promega) in a final volume of 50 μl. To check the presence of DNA contamination RT-PCR was performed on 2 μg of total RNA without M-MLV reverse transcriptase (negative control). An internal control (water instead of RNA) for each RT-PCR was performed to investigate RNA contamination of the mixture. For each sample 5 μl of the PCR amplification products were analysed on 2% agarose gels and stained with ethidium bromide. The intensities of the bands were evaluated using the Image Master Software (SYDR-1990, SYNGENE, U.S.A.). The RT-PCR products were extracted and purified from agarose gel by Golden Beads Gel Extraction kit (Sangon Inc., China) and sequenced using radioactive dideoxychain terminating method (Sangon Inc., China).
RIA of blood estrogen, testosterone and corticosterone concentrations
At the time of sacrifice, the blood samples (0.8 ml) of the OVX, OVX+C, OVX+EA and INT+EA rats were collected (6 hours after the last EA) from tail veins respectively, and those of the INT animals, during the period of proestrus. The plasma was separated by centrifugation and stored at -70#176;C until assayed. Concentration of blood hormones were determined by double-antibody RIA kits purchased from the National Atomic Energy Research Institute (Beijing, China.). The samples were assayed in duplicate, and all the subjects' samples were assayed together. The sensitivity of the kit was 0.8 pg/ml (testosterone); 1.4 pg/ml (estrogen) and 0.05 pg/ml (corticosterone), the intra- and interassay coefficients of variation, 3.7–8.0% and 4.74–7.7%.
Statistical analysis
All data are presented as means ± S.E.M. Statistical analysis was performed on raw data using one-way analysis of variance (ANOVA), with the significance concentrations of p < 0.05 and p < 0.01 in two-tailed testing chosen.
Results
Vaginal cytology of the animals
The epithelial cells were stained by haematoxylin-eosin (HE). The INT and INT+EA rats showed regular 4-day estrus cycle change, and the cyclic change disappeared in the OVX, OVX+EA and OVX+C rats. Few mature vaginal epithelia were observed in the smears of the OVX and OVX+C rats, and the percent of mature epithelia increased significantly in the OVX+EA rats (p < 0.05) (Table 1).
Table 1.
The percent of mature vaginal epithelia of the INT, INT+EA, OVX, OVX+C and OVX+EA rats
| INT (n = 12) | INT+EA (n = 12) | OVX (n = 12) | OVX+C (n = 12) | OVX+EA (n = 10) | |
| The percent of mature vaginal epithelia | (15.6 ± 0.88)% | (15.3 ± 0.51)% | (0.19 ± 0.019)%* | (0.18 ± 0.021)%* | (0.31 ± 0.018)%*# |
*p < 0.05 vs INT and INT+EA; # p < 0.05 vs OVX
Blood concentrations of estrogen, testosterone and corticosterone
The blood E2 concentration decreased significantly in the OVX (p < 0.01) compared with that in the INT and INT+EA groups and was higher in the OVX+EA (p < 0.05) than in the OVX. The blood testosterone concentration decreased significantly in the OVX (p < 0.01) compared with that in the INT and INT+EA, but there is no statistical disparity between the OVX and OVX+EA (Fig. 2). The corticosterone concentration in the OVX+EA increased significantly compared with that in the INT, INT+EA and OVX groups (p < 0.05) (Fig. 2). Moreover, there were no disparities between the OVX and OVX+C, the INT and the INT+EA as well.
Figure 2.
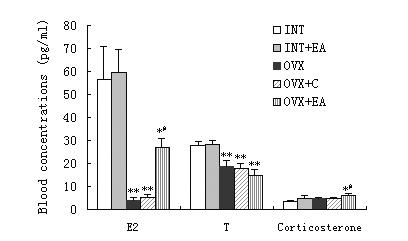
The blood concentrations of E2, testosterone and corticosterone of the INT (n = 12), INT+EA (n = 12), OVX (n = 12), OVX+C (n = 12) and OVX+EA (n = 10) rats. *p < 0.05 vs INT and INT+EA; ** p < 0.01 vs INT and INT+EA; # p < 0.05 vs OVX and OVX+C.
Effects of EA on aromatase activities
The tissues were incubated with (1β-3H) androstenedione for various periods of time, and the production of 3H2O (aromatase activity) was linear with time up to 1 h of incubation. The aromatase activity was also linear with increasing amounts of tissue up to 5 mg/incubation. The optimal pH for the aromatase was 7.4. The maximum rates were obtained with a substrate concentration of 0.2 μM, and the enzyme preparation exhibited an apparent Michaelis-Menten constant (Km) of 0.04 μM. Therefore, incubation conditions were optimised that tissue homogenates ~3 mg tissue/ incubation; and incubation for 1 h in 0.2 ml potassium phosphate buffer containing 0.13 μM substrate (pH 7.4). The aromatase activity of the SA adipose and liver tissues presented no changes among the INT, INT+EA and OVX, and the activity of the SA adipose and liver tissue in the OVX+EA increased significantly (p < 0.01) compared with that in the INT, INT+EA and OVX (Fig. 3).
Figure 3.
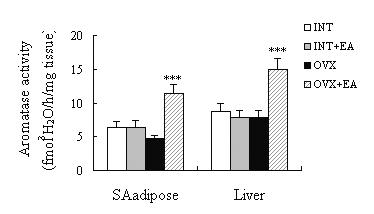
The aromatase activities in SA adipose and liver tissues of the INT (n = 12), INT+EA (n = 12), OVX (n = 12) and OVX+EA (n = 10) rats. ***p < 0.01 vs INT, INT+EA and OVX.
Effects of EA on aromatase expressions by Western blotting
Densitometric analysis of the protein concentration using aromatase/β-actin expressed as the mean with SEM. The ratio of liver aromatase to β-actin in the OVX decreased significantly compared with that in the INT (p < 0.05). And the ratio of the SA adipose produced a higher level in the OVX than in the INT (p < 0.05). The ratio of liver tissues in the OVX+EA increased significantly compared with that in the OVX (p < 0.05). And the ratio of SA adipose tissues in the OVX+EA was as much as that in the OVX, but was higher than in the INT (p < 0.05). No disparity was detected between the INT and INT+EA (Fig. 4). No immunoreactive bands detected in the samples when using antiserum after preabsorption with excessive antigens and omission of the primary antibody.
Figure 4.
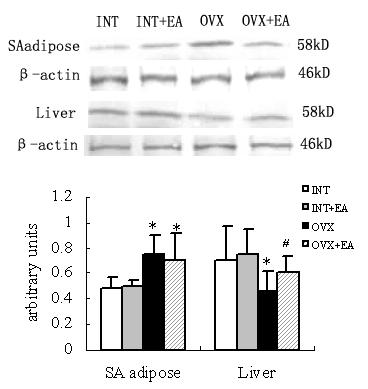
Effects of the electroacupuncture on the aromatase expressions by Western blot analysis. The upper picture shows the Western blot analysis of the aromatase P450. The SA adipose and liver tissue samples (50 mg/lane) were electrophoresed and blotted onto the membrane, and aromatase was then detected using the polyclonal antibody as described in materials and methods. Densitometric analysis of the protein concentration using aromatase/β-actin expressed as the mean with SEM bar (nINT = 12, nINT+EA = 12, nOVX = 12 and nOVX+EA = 10) in each column indicated in the lower panel. * p < 0.05 vs INT, INT+EA and OVX, # p < 0.05 vs OVX
Effects of EA on aromatase expressions by RT-PCR
Comparison of the amplified PCR fragment with rat ovary aromatase sequence revealed 100% homology (data not shown). Densitometric analysis of the mRNA concentration using aromatase/β-actin expressed as the mean with SEM. The ratio of liver aromatase to β-actin in the OVX decreased significantly compared with that in the INT (p < 0.05), and the ratio of liver tissue in the OVX+EA was higher than in the OVX (p < 0.05). No changes occurred on the ratios of SA adipose tissue between the OVX and INT, but a significant increase of the ratio in the OVX+EA (p < 0.05) compared with that in the INT and OVX was detected. However, no disparity was found between the INT and INT+EA (Fig. 5).
Figure 5.
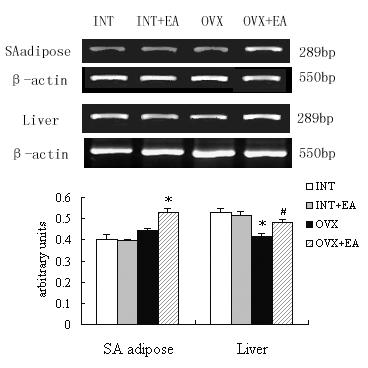
Effects of the electroacupuncture on the aromatase mRNA expressions by RT-PCR analysis. The upper picture shows the gel electrophoresis of the RT-PCR products for the aromatase. Total RNA fractions were isolated from the SA adipose and liver tissues of the INT, INT+EA, OVX and OVX+EA rats, and the cDNAs were prepared using standard techniques, as described in materials and methods. The RT-PCR products for aromatase were fractionated by electrophoresis through 2% agarose gels. Densitometric analysis of the mRNA concentration using aromatase/β-actin expressed as the mean with SEM bar (nINT = 12, nINT + EA = 12, nOVX = 12 and nOVX + EA = 10) in each column indicated in the lower panel. * p < 0.05 vs INT and INT+EA, # p < 0.05 vs OVX.
Discussion
Extragonadal aromatization has been in a general sense recognized, although its significance is only becoming to be appreciated, as will be explained further. It has been reported that aromatization in the adipose tissue is not negligible under normal and pathological conditions [13]. Due to the highest conversion of C19 precursor such as androstenedione to estrogen was seen in adipose tissue obtained from the subcutaneous abdominal wall but not from intraperitoneal cavity [14], our research focused on the aromatase activity of the SA adipose tissues. Hemsell and co-workers [15] first addressed the significance of adipose tissue as a major source of estrogen production, i.e. there is a progressive increase in the conversion efficiency with advancing age, and the increase of estrogen production as a function of obesity [16,17]. In our results, increased aromatase protein expressions were observed in the OVX and OVX+EA rats' SA adipose tissue. It is known that the extent of extragonadal aromatization is influenced by weight [18]. The weight of ovariectomized rats increases significantly [19], and the mesenchymal cells from the SA adipose tissue can be active, which may be closely related to the higher expression of the aromatase. Yet, only in OVX+EA was there an associated increase in aromatase activity of the SA adipose tissue. The factors possibly involved in the regulation of aromatase expression and activity are still poorly understood [20]. Many studies have been performed to assess the possible dependence of the enzyme on androgens. However, the data available are conflicting [20,21]. So further explorations are still needed on the way by which the EA stimulation activates the aromatase enzyme in ovariectomized rats. And there is sometimes inconsistency on the levels between transcription and translation of genes, which was verified by the discrepancy of the increased protein expression and unchanged mRNA expression of the aromatase in the SA adipose tissues that occurred in the present results.
Though it has been reported that the splanchnic tissue is a minor site for extraglandular aromatization of androgens [22], there is a significant conversion of androstenedione to estrone by liver tissues [17]. In adult liver homogenates, C19 norsteroid (19-nortestosterone; NT) is readily aromatised to estrogens [23,24]. The present results showed that the aromatase activity of the liver tissue was higher in the OVX+EA than that in the INT and OVX, which is closely related to the elevated aromatase mRNA and protein expressions. On the other hand, after ovariectomy, the diminution of C19 precursor from ovaries for aromatisation may induce the decreased expressions of aromatase in the OVX rats compared with the gonad-intact rats in our results. However, in the present study, the aromatase expressions in the OVX rats did not show consistent changes between the SA adipose and liver tissues, which may suggest that the role of SA adipose and splanchnic tissues in the extraglandular aromatization might be different. Nonetheless, in the final analysis, our results suggested that both the SA adipose and liver tissues contributed to the effects of EA on extragonadal aromatization to promote the circulation estrogen concentrations. Previous investigation [25,26] has shown that aromatase activity is regulated mainly at the transcriptional level. But our results indicated that the EA stimulated the transcription of the aromatase gene or inhibited the breakdown of its mRNA in the liver tissue but not in the SA adipose tissue. However, determination of the relative mRNA contents did not help us distinguish these two alternatives. Despite that, we concluded that the EA stimulated the activity of aromatase.
Of the factors influencing plasma estradiol concentrations, plasma testosterone is the most important determinant. In the present results, the testosterone circulated at concentrations, which may be an order of magnitude greater than those of estradiol in the blood of ovariectomized rats. It is now recognized that much of the physiology of androgens is explicable in terms of concept that testosterone functions as a circulating pro-hormone, which is converted in target tissues, on the one hand to 5α-dihydrotestosterone (DHT) and on the other hand to estradiol [6]. Though we detected the blood concentrations of testosterone of the animals, it is still far from able to elucidate the androgen origin for the extraglandular aromatization. Tissues could possibly form the biologically active steroids E2 from the precursor androstenedione, dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S), C19 steroids of exclusively adrenal origin [27,28]. They form a large reservoir of precursor, which is available for conversion to testosterone and thus to estrogens in numerous peripheral tissue sites, and it is also relatively insensitive, in the short term, to changes in secretion rates [6,28]. It is well known that adrenal is the principle organ to secrete sexual hormones except ovarian in females [29]. In our previous research, we observed the effects of EA on the adrenals of animals, which showed that the mean weight of adrenals in the OVX+EA rats increased significantly than that in the OVX and INT rats [9], and the argyrophilia of NOR proteins (AgNORs) [30] number in reticulate zone of the OVX+EA was considerable more than that of the OVX and INT rats [5], which suggested that the adrenal function might be activated by the EA. In addition, the contents of blood corticosterone in the OVX+EA rats were markedly raised [9], but with no statistical difference of those in the OVX and INT rats, providing further evidence that the adrenal cortex cells were activated in the OVX+EA rats. All these results suggest that EA may activate the adrenal compensatory mechanism in ovariectomized rats.
The results including the changes of blood steroids contents, the tissue aromatase activity and our previous reports [4,5,7,9] suggest that the effects of acupuncture in the regulation of HPOA may be exerted via enhancing the extraglandular aromatization and promoting the function of HPAA, thereby resetting the negative feedback of estrogen to HPOA. Our results, however, did not show the same effects in the INT+EA rats, suggesting that the EA may play a normalizing role. It is unlikely that the changes can be caused by the factors contributed by the enforced binding in the laboratory. While treated with EA, the animals did not show any violent reactions since they were fixed as comfortably as possible under no anaesthesia. It is known that maturation and of vaginal epithelia cells are a reaction dependent on estrogen level, and no mature epithelia was observed in the smears of the OVX+C rats, which verified the specificity of the EA effects.
Disruption of reproductive function in mammals is a well-known consequence of stress. But when considering the potential effects exerted by a stressor, one realizes that, each description of the response of the HPOA to stimuli must be accompanied by the precise characterization of these stimuli. Sometimes electroacupuncture can be considered a stressor for a conscious rat, i.e., strong electrical stimulation. But in our studies, the blood levels of corticosterone, which are considered the most reliable indicator of stress, detected in the same experimental animals do not produce any changes in INT+EA. The present results of increased E2 concentrations and the increased aromatase activity of SA adipose and liver tissues in the OVX+EA group might not be a stress response. It is known that stress increases the release and production of steroids from fat tissue. Glucocorticoids are known to increase aromatase expression in fat [31]. But the use of acupuncture for reducing anxiety and stress possible through its sympathinhibitory property and impact on β-endorphin levels has well been reviewed [32,33]. And acupuncture may provide an excellent alternative for stress reduction in women undergoing infertility treatment [34]. Nevertheless, the present study evaluated the effect of electroacupuncture on extragonadal aromatization in ovariectomized rats, which is valuable in research for the mechanism of acupuncture on reproductive disorders.
Conclusions
Both the subcutaneous abdominal adipose tissues and the liver tissues contributed to the effects of electroacupuncture on the extragonadal aromatization to promote the blood concentrations of estrogen in the ovariectomized rats.
Authors' contributions
Hong Zhao and Zhanzhuang Tian designd the study, performed the molecular genetic studies and the statistical analysis, and drafted the manuscript. Lina Cheng participated in the radiometric assay. Boying Chen conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This project was financed by the National Natural Science Foundation of China (No. 39870914) and Shanghai Medical Health Bureau (the Key Project 20003 on Traditional Chinese Medicine).
Contributor Information
Hong Zhao, Email: zh2000h@hotmail.com.
Zhanzhuang Tian, Email: tianzhanzhuang@hotmail.com.
Lina Cheng, Email: chen_bo_ying@hotmail.com.
Boying Chen, Email: chen_bo_ying@hotmail.com.
References
- Clement JV, Mcloughlin L, Lowry PJ, Besser GM, Rees LH, Wen HL. Acupuncture in heroin addicts: changes in Met-enkephalin and beta-endorphin in blood and cerebrospinal liquid. Lancet. 1979;2:380–383. doi: 10.1016/S0140-6736(79)90401-X. [DOI] [PubMed] [Google Scholar]
- Kim EH, Kim YJ, Lee HJ, Huh Y, Chung JH, Seo JC, Kang JE, Lee HJ, Yim SV, Kim CJ. Acupuncture increases cell proliferation in dentate gyrus after transient global ischemia in gerbils. Neurosci Lett. 2001;297:21–24. doi: 10.1016/S0304-3940(00)01656-6. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Waldenstrom U, Tagnfors U, Lundeberg T, Lindsted TG, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2000;79:180–188. doi: 10.1034/j.1600-0412.2000.079003180.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Tian ZZ, Chen BY. An important role of corticotropin-releasing hormone in electroacupuncture normalizing the subnormal function of hypothalamus-pituitary-ovary axis in ovariectomized rats. Neurosci Lett. 2003;349:25–28. doi: 10.1016/S0304-3940(03)00676-1. [DOI] [PubMed] [Google Scholar]
- Chen BY. Acupuncture normalizes dysfunction of hypothalamic-pituitary-ovarian axis. Acupunct Electrother Res. 1997;22:97–108. doi: 10.3727/036012997816356734. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Sources of estrogen and their importance. J Ster Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Zhao H, Tian ZZ, Chen BY. Increased corticortropin-releasing hormone release in ovariectomized rats' paraventricular nucleus: effects of electroacupuncture. Neurosci Lett. 2003;253:37–40. doi: 10.1016/j.neulet.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Shi XM. Acupuncture therapeutics. 1. People health publisher, Beijing; 2001. pp. 367–435. [Google Scholar]
- Gao H, Ji S, Chen B. [Electroacupuncture promotes enlargement of adrenals and enhances concentration of blood corticosterone in ovariectomized rats] Zhen Ci Yan Jiu. 1995;20:55–58. Chinese. [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M. In situ estrogen synthesized by aromatase p450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141:3852–3861. doi: 10.1210/en.141.10.3852. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol Reprod. 1998;58:919–926. doi: 10.1095/biolreprod58.4.919. [DOI] [PubMed] [Google Scholar]
- Vague J, Sardo J. Aromatization of androgens (author's transl) Sem Hop. 1981;57:1467–1476. [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham_Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrin Rev. 1994;15:342–355. doi: 10.1210/er.15.3.342. [DOI] [PubMed] [Google Scholar]
- MacDonald PC, Edman CD, Hemsell DL, Porter JC, Siiteri PK. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1978;130:448–455. doi: 10.1016/0002-9378(78)90287-9. [DOI] [PubMed] [Google Scholar]
- Bray GA. The underlying basis for obesity: relationship to cancer. J Nutr. 2002;132:3451S–3455S. doi: 10.1093/jn/132.11.3451S. [DOI] [PubMed] [Google Scholar]
- Frost PG, Reed MJ, James VH. The aromatization of androstenedione by human adipose and liver tissue. J Steroid Biochem. 1980;13:1427–1431. doi: 10.1016/0022-4731(80)90055-2. [DOI] [PubMed] [Google Scholar]
- Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in post-menopausal women. J Clin Endocrinol Metab. 1973;36:207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Zhang Y, Stewart KG. A comparison of ovariectomy models for estrogen studies. Am J Physiol Regul Integr Comp Physiol. 2001;280:R904–R907. doi: 10.1152/ajpregu.2001.280.3.R904. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Motta M, Martini L, Celotti F. Aromatase expression and activity in male and female cultured rat hypothalamic neurons: effect of androgens. Mol Cel Endocrin. 2001;178:1–10. doi: 10.1016/S0303-7207(01)00442-7. [DOI] [PubMed] [Google Scholar]
- Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Brain Res Dev Brain Res. 1997;98:74–81. doi: 10.1016/S0165-3806(96)00170-8. [DOI] [PubMed] [Google Scholar]
- Longcope C, Billiar RB, Takaoka Y, Reddy PS, Richardson D, Little B. Tissue sites of aromatization in the female rhesus monkey. Endocrinology. 1983;113:1679–1682. doi: 10.1210/endo-113-5-1679. [DOI] [PubMed] [Google Scholar]
- Harada N, Ota H, Yoshimura N, Katsuyama T, Takagi Y. Localized aberrant expression of cytochrome P450 aromatase in primary and metastatic malignant tumors of human liver. J Clin Endocrinol Metab. 1998;83:697–702. doi: 10.1210/jc.83.2.697. [DOI] [PubMed] [Google Scholar]
- Yoshiji S, Yamamoto T, Okada H. Aromatization of androstenedione and 19-nortestosterone in human placenta, liver and adipose tissues. Nippon Naibunpi Gakkai Zasshi. 1986;62:18–25. doi: 10.1507/endocrine1927.62.1_18. (in Japanese) [DOI] [PubMed] [Google Scholar]
- Schmidt M, Renner C, Loffler G. Progesterone inhibits glucocorticoid-dependent aromatase induction in human adipose fibroblasts. J Endocrinol. 1998;158:401–407. doi: 10.1677/joe.0.1580401. [DOI] [PubMed] [Google Scholar]
- Evans CT, Corbin CJ, Saunders CT, Merrill JC, Simpson ER, Mendelson CR. Regulation of estrogen biosynthesis in human adipose stromal cells. Effects of dibutyryl cyclic AMP, epidermal growth factor, and phorbol esters on the synthesis of aromatase cytochrome P-450. J Biol Chem. 1987;262:6914–6920. [PubMed] [Google Scholar]
- Martel C, Melner MH, Gagné D, Simard J, Labrie F. Widespread tissue distribution of steroid sulfatase, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD), 17 beta-HSD 5 alpha-reductase and aromatase activities in the rhesus monkey. Mol Cell Endocrinol. 1994;104:103–111. doi: 10.1016/0303-7207(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Role of aromatase in sex steroid action. J Mol Endocrinol. 2000;25:149–156. doi: 10.1677/jme.0.0250149. [DOI] [PubMed] [Google Scholar]
- Meikle AW, Daynes RA, Araneo BA. Adrenal androgen secretion and biologic effects. Endocrinol Metab Clin North Am. 1991;20:381–400. [PubMed] [Google Scholar]
- De Capoa A, Baldini A, Marlekaj P, Natoli C, Rocchi M, Archidiacono N, Cianfarani S, Spadoni GL, Boscherini B. Hormone-modulated rRNA activity is visualized by selective staining of the NORs. Cell Biol Int Rep. 1985;9:791–796. doi: 10.1016/0309-1651(85)90097-9. [DOI] [PubMed] [Google Scholar]
- McTernan PG, Anderson LA, Anwar AJ, Eggo MC, Crocker J, Barnett AH, Stewart PM, Kumar S. Glucocorticoid regulation of P450 aromatase activity in human adipose tissue: gender and site differences. J Clin Endocrinol Metab. 2002;87:1327–1336. doi: 10.1210/jc.87.3.1327. [DOI] [PubMed] [Google Scholar]
- Chen A. An introduction to sequential electric acupuncture (SEA) in the treatment of stress related physical and mental disorders. Acupunct Electrother Res. 1992;17:273–283. doi: 10.3727/036012992816357675. [DOI] [PubMed] [Google Scholar]
- Dong JT. Research on the reduction of anxiety and depression with acupuncture. Am J Acupunct. 1993;21:327–330. [Google Scholar]
- Chang R, Chung PH, Rosenwaks Z. Role of acupuncture in the treatment of female infertility. Fertil Steril. 2002;78:1149–1153. doi: 10.1016/S0015-0282(02)04348-0. [DOI] [PubMed] [Google Scholar]


