Abstract
AIM: To evaluate the risk factors for ampullary adenoma and ampullary cancer.
METHODS: This case-control study included ampullary tumor patients referred to Peking Union Medical College Hospital. Controls were randomly selected from an existing database of healthy individuals at the Health Screening Center of the same hospital. Data on metabolic syndromes, medical conditions, and family history were collected by retrospective review of the patients’ records and health examination reports, or by interview.
RESULTS: A total of 181 patients and 905 age- and sex-matched controls were enrolled. We found that a history of diabetes, cholecystolithiasis, low-density lipoprotein, and apolipoprotein A were significantly related to ampullary adenomas. Diabetes, cholecystolithiasis, chronic pancreatitis, total cholesterol, high-density lipoprotein, and apolipoprotein A were also significantly related to ampullary cancer.
CONCLUSION: Some metabolic syndrome components and medical conditions are potential risk factors for the development of ampullary tumors. Cholelithiasis, diabetes, and apolipoprotein A may contribute to the malignant transformation of benign ampullary adenomas into ampullary cancer.
Keywords: Metabolic syndromes, Ampullary adenoma, Ampullary cancer, Risk factors
Core tip: Although ampullary tumors are relatively rare, the rapid development of, and advances in, endoscopy and imaging techniques have profoundly increased their discovery rate. Despite the increasing numbers of published studies, the etiology for ampullary tumors is incompletely defined. This is the first study to evaluate the impact of metabolic syndromes on ampullary tumors patients.
INTRODUCTION
Although tumors of the ampulla can be benign or malignant, most are malignant[1-3]. Ampullary cancer is less aggressive and has a better prognosis after curative resection than cancer of the distal bile duct or the pancreas[4,5]. The favorable prognosis is thought to be due to its early clinical presentation with obstructive jaundice[6] and its high resectability rate[2]. Despite a relatively favorable outcome following resection, 32%-44% of patients have a relapse, either locally or distantly.
Ampullary adenoma may occur sporadically or in the setting of familial adenomatous polyposis[7]. It is considered a premalignant lesion leading to ampullary cancer because of its capacity for malignant transformation via the adenoma-carcinoma sequence[3,6,8,9], which is generally accepted as valid for colorectal tumors[10].
Numerous published studies investigated the clinicopathological aspects of ampullary tumors, most of which focused on the prognosis associated with this disease. Studies investigating risk factors associated with the proposed adenoma-carcinoma sequence are scarce, especially regarding metabolic syndrome components and ampullary tumors. The aim of this study was to evaluate, in detail, the relations between metabolic syndromes and other risk factors with ampullary cancer and the precursor lesions.
MATERIALS AND METHODS
Study population and design
We conducted a hospital-based case-controlled study that included 1086 subjects (181 patients with a histologically-confirmed ampullary tumor, 905 healthy controls) from Peking Union Medical College Hospital (PUMCH) in Beijing, China. This hospital is a major diagnostic and treatment center for periampullary adenocarcinoma in China.
Using the PUMCH Patient Information Database, we compiled a list of all patients who had been diagnosed with ampullary adenoma or ampullary cancer between 2006 and 2010. Periampullary adenocarcinomas, such as those of the pancreatic head, distal bile duct, and duodenum, as well as neuroendocrine tumors, were excluded. Patients who had undergone a primary attempt at curative resection and whose diagnoses were confirmed by pathology examination were included in this study. We performed a manual retrospective review of the patients’ records to collect demographic, clinical, and risk factor information. This included a detailed assessment of the family history of cancer, personal medical history, hormone and medication intake, and occupational exposure to chemicals. Data collection, including age, sex, demographic data, history of systemic diseases and gastrointestinal surgery, and a complete physical examination were conducted by the doctors before operating. Other related information was collected by interviewing patients or their family members, and was recorded by a physician in a structured data collection sheet. Such recording is routinely performed in our gastrointestinal oncology clinic, and the forms are kept as part of the patients’ medical records. Age, sex, history of hypertension or diabetes mellitus, hepatitis B virus (HBV) infection, metabolic syndromes, and previous cholecystectomy data were abstracted.
Using the Statistical Package for Social Science Program (version 13; SPSS, Chicago, IL, United States), controls were randomly selected from an existing database of healthy individuals at the PUMCH Health Screening Center. They were frequency matched to cases by sex and exact age at a ratio of 5:1. The database consists of healthy individuals who are genetically unrelated family members, spouses, and friends of patients who had cancer other than gastrointestinal cancer. The PUMCH Health Screening Center is one of the major centers providing routine physical examinations in Beijing. Most of those who come for examinations are native residents.
We performed a manual retrospective review of the health examination reports to collect demographic, clinical, and risk factor information for the controls. Doctors at the physical examination center collected the data-including age, sex, demographic data, and history of systemic diseases and gastrointestinal surgery, and then performed a complete physical examination. Measurements of height without shoes and weight while clothed were recorded. The body mass index was used as a measurement of obesity. Other related information was collected and recorded in a structured data collection sheet. This information was recorded by examining physicians using the same procedure as was used for the current patients. Controls were interviewed between 2006 and 2010. Two participants were excluded from the study because of incomplete health examination records.
Ampullary tumors were diagnosed by endoscopy. All specimens were classified following an examination at the Department of Pathology, Peking Union Medical College Hospital. Cholelithiasis (including cholecystolithiasis, choledocholithiasis, and hepatolithiasis) and chronic pancreatitis were diagnosed using data from clinical imaging studies - abdominal ultrasonography (US), computed tomography (CT), endoscopic retrograde cholangiopancreatography, and magnetic resonance imaging - and by reviewing medical records. All patients underwent at least one of the aforementioned imaging studies. For control subjects, possible risk factors were determined based on abdominal US data and by reviewing health examination reports. All of the controls had previously undergone US screening for detection of stones.
Laboratory tests
Clinical nurses obtained blood samples via venipuncture from all study participants, who had fasted overnight. The blood was then sent for laboratory examination. Serum lipids, including triglyceride (Tri), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A (ApoA), and apolipoprotein B (ApoB), were measured using the Hitachi modular analytics system (Roche Modular DPP; Hitachi, Tokyo, Japan). Hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc) assays were performed using a second-generation, enzyme-linked immunosorbent assay (Abbott Laboratories, North Chicago, IL, United States). Chronic hepatitis B infection was diagnosed if both HBsAg and anti-HBc assays were positive.
Statistical analysis
Analyses of variables were carried out using SPSS software. Univariate analyses were performed using Fisher’s exact test for categorical variables. Variables with a P value of < 0.05 in the univariate analyses were further adjusted for age and sex in a multiple logistic regression analysis. The model was built using a forward selection process. Variables with a likelihood ratio test P value of < 0.05 were kept in the model and considered statistically significant.
RESULTS
We included 181 patients and 905 controls in the analysis. In the patient group, 57 had ampullary adenomas, and 124 had ampullary cancers. The ampullary tumor patients and the control group had the same mean age (61.8 ± 11.2 years, 61.7 ± 11.2 years, P = 0.934) and there were no differences between the two groups in sex (P = 1.000).
Ampullary adenoma
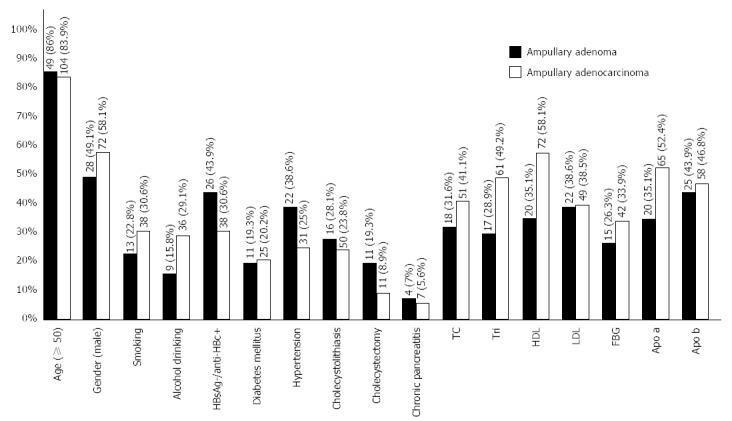
Ampullary adenoma patients had a significantly higher prevalence of hypertension (38.6% vs 23.2%), diabetes (19.3% vs 8.1%), cholelithiasis (28.1% vs 4.2%), cholecystectomy (19.3% vs 2.5%), and chronic pancreatitis (7.0% vs 0.7%). They also had higher levels of TC (31.6% vs 15.5%), LDL (38.6% vs 16.8%), and ApoB (43.9% vs 27.1%). Their HDL (64.9% vs 89.8%) and ApoA (64.9% vs 99.3%) rates were lower than in the controls. During the multivariate logistic regression analysis, hypertension, cholecystectomy, chronic pancreatitis, TC, HDL, and ApoB failed to relate to the development of ampullary adenoma, but the other factors remained significant after adjustment for covariates (Table 1).
Table 1.
Risk factors for ampullary adenoma: univariate and multivariate logistic regression analyses using Fisher’s exact test n (%)
| Risk factor | Controls | Cases |
Univariate analysis |
Multivariate analysis |
||
| P value | OR (95%CI) | P value | OR (95%CI) | |||
| Age | ||||||
| < 50 yr | 45 (15.7) | 8 (14.0) | 1 (reference) | |||
| ≥ 50 yr | 240 (84.3) | 49 (86.0) | 0.843 | 1.148 (0.510-2.588) | ||
| Sex | ||||||
| Female | 145 (50.9) | 29 (50.9) | 1 (reference) | |||
| Male | 140 (49.1) | 28 (49.1) | 1.000 | 1.000 (0.566-1.766) | ||
| Smoking | ||||||
| No | 225 (78.9) | 44 (77.2) | 1 (reference) | |||
| Yes | 60 (21.1) | 13 (22.8) | 0.727 | 1.108 (0.561-2.189) | ||
| Alcohol abuse | ||||||
| No | 209 (73.3) | 48 (84.2) | 1 (reference) | |||
| Yes | 76 (26.7) | 9 (15.8) | 0.094 | 0.516 (0.241-1.101) | ||
| HBV | ||||||
| HBsAg-/anti-HBc- | 147 (51.6) | 32 (56.1) | 1 (reference) | |||
| HBsAg+/anti-HBc+ | 7 (2.5) | _ | _ | _ | ||
| HBsAg-/anti-HBc+ | 131 (45.9) | 25 (43.9) | 0.665 | 0.877 (0.494-1.556) | ||
| Diabetes mellitus | 0.041 | 2.590 (1.055-6.676) | ||||
| No | 262 (91.9) | 46 (80.7) | 1 (reference) | |||
| Yes | 23 (8.1) | 11 (19.3) | 0.015 | 2.274 (1.244-5.965) | ||
| Hypertension | ||||||
| No | 219 (76.8) | 35 (61.4) | 1 (reference) | |||
| Yes | 66 (23.2) | 22 (38.6) | 0.020 | 2.086 (1.145-3.801) | ||
| Cholecystolithiasis | 0.000 | 11.068 (3.395-36.084) | ||||
| No | 273 (95.8) | 41 (71.9) | 1 (reference) | |||
| Yes | 12 (4.2) | 16 (28.1) | 0.000 | 8.878 (3.921-20.103) | ||
| Cholecystectomy | ||||||
| No | 278 (97.5) | 46 (80.7) | 1(reference) | |||
| Yes | 7 (2.5) | 11 (19.3) | 0.000 | 9.497 (3.502-25.755) | ||
| Chronic pancreatitis | ||||||
| No | 283 (99.3) | 53 (93.0) | 1(reference) | |||
| Yes | 2 (0.7) | 4 (7.0) | 0.008 | 10.679 (1.907-59.79) | ||
| TC (mmol/L) | ||||||
| < 5.70 | 240 (84.2) | 39 (68.4) | 1 (reference) | |||
| ≥ 5.70 | 45 (15.8) | 18 (31.6) | 0.008 | 2.462 (1.294-4.682) | ||
| Tri (mmol/L) | ||||||
| < 1.7 | 200 (70.2) | 40 (70.2) | 1 (reference) | |||
| ≥ 1.7 | 85 (29.8) | 17 (29.8) | 1.000 | 1.000 (0.537-1.862) | ||
| HDL (mmol/L) | ||||||
| < 0.93 | 29 (10.2) | 20 (35.1) | 1 (reference) | |||
| ≥ 0.93 | 256 (89.8) | 37 (64.9) | 0.000 | 0.210 (0.108-0.408) | ||
| LDL (mmol/L) | 0.004 | 3.039 (1.412-6.541) | ||||
| < 3.63 | 237 (83.2) | 35 (61.4) | 1 (reference) | |||
| ≥ 3.63 | 48 (16.8) | 22 (38.6) | 0.001 | 3.104 (1.675-5.752) | ||
| FBG (mmol/L) | ||||||
| < 6.1 | 242 (84.9) | 42 (73.7) | 1 (reference) | |||
| ≥ 6.10 | 43 (15.1) | 15 (26.3) | 0.052 | 2.010 (1.026-3.939) | ||
| ApoA (g/L) | ||||||
| < 1 | 2 (0.7) | 20 (35.1) | 1 (reference) | 0.000 | 105.282 (22.229-498.635) | |
| ≥ 1 | 283 (99.3) | 37 (64.9) | 0.000 | 0.013 (0.003-0.058) | ||
| ApoB (g/L) | ||||||
| < 1 | 208 (72.9) | 32 (56.1) | 1 (reference) | |||
| ≥ 1 | 77 (27.1) | 25 (43.9) | 0.017 | 2.110 (1.176-3.788) | ||
| BMI (kg/m2) | ||||||
| < 25.0 | 164 (57.5) | 40 (70.2) | 1 (reference) | |||
| ≥ 25.0 | 121 (42.5) | 17 (29.8) | 0.078 | 0.576 (0.312-1.065) | ||
BMI: Body mass index; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; Tri: Triglyceride; TC: Total cholesterol; FBG: Fasting blood glucose; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen.
Ampullary cancer
In the univariate analyses for ampullary cancer, a history of diabetes (20.2% vs 11.3%), chronic pancreatitis (5.6% vs 0.3%), incidence of cholelithiasis (23.8% vs 4.5%) and cholecystectomy (8.9% vs 3.7%), serum fasting blood glucose (FBG) level (33.9% vs 17.4%), TC (41.1% vs 13.5%), Tri (49.2% vs 30.1%), LDL (39.5% vs 16.1%), and ApoB (46.8% vs 25.3%) were significantly higher in ampullary cancer patients than in controls. The prevalence of HBsAg-/anti-HBc+ (30.6% vs 50.0%) and the levels of HDL (41.9% vs 84.2%) and Apo a (47.6% vs 98.7%) were lower in the cancer patients than in the controls. In the multivariate logistic regression analysis, the incidence of cholecystectomy and HBV infection and the high levels of Tri, LDL, FBG, and ApoB failed to show a relation to the development of ampullary cancer, but the other factors remained significant after adjustment for covariates (Table 2).
Table 2.
Risk factors for ampullary cancer: Univariate and multivariate logistic regression analysis using the fisher exact test n (%)
| Controls | Cases |
Univariate |
Multivariate |
|||
| P value | OR (95%CI) | P value | OR (95%CI) | |||
| Age | ||||||
| < 50 yr | 100 (16.1) | 20 (16.1) | 1 (reference) | |||
| ≥ 50 yr | 520 (83.9) | 104 (83.9) | 1 | 1.000 (0.592–1.689) | ||
| Gender | ||||||
| Female | 259 (41.8) | 52 (41.9) | 1 (reference) | |||
| Male | 361 (58.2) | 72 (58.1) | 1 | 1.000 (0.681–1.488) | ||
| Smoking | ||||||
| No | 448 (72.3) | 86 (69.4) | 1 (reference) | |||
| Yes | 172 (27.7) | 38 (30.6) | 0.513 | 1.151 (0.756-1.752) | ||
| Alcohol drinking | ||||||
| No | 453 (73.1) | 88 (70.9) | 1 (reference) | |||
| Yes | 167 (26.9) | 36 (29.1) | 0.659 | 1.110 (0.724-1.700) | ||
| HBV | ||||||
| HBsAg–/anti-HBc– | 295 (47.6) | 84 (67.7) | 1 (reference) | |||
| HBsAg+/anti-HBc+ | 15 (2.4) | 2 (1.6) | 0.546 | 0.468 (0.105–2.088) | ||
| HBsAg–/anti-HBc+ | 310 (50.0) | 38 (30.6) | 0 | 0.430 (0.284–0.652) | ||
| Diabetes mellitus | 0.021 | 4.75 (2.739-9.853) | ||||
| No | 550 (88.7) | 99 (79.8) | 1 (reference) | |||
| Yes | 70 (11.3) | 25 (20.2) | 0.011 | 1.984 (1.198–3.285) | ||
| Hypertension | ||||||
| No | 452 (72.9) | 93 (75.0) | 1 (reference) | |||
| Yes | 168 (27.1) | 31 (25.0) | 0.659 | 0.897 (0.576–1.397) | ||
| Cholecystolithiasis | 0 | 14.06 (6.82-28.986) | ||||
| No | 592 (95.5) | 160 (76.2) | 1 (reference) | |||
| Yes | 28 (4.5) | 50 (23.8) | 0 | 12.469 (7.37–21.095) | ||
| Cholecystectomy | ||||||
| No | 597 (96.3) | 113 (91.1) | 1 (reference) | |||
| Yes | 23 (3.7) | 11 (8.9) | 0.018 | 2.527 (1.198–5.328) | ||
| Chronic pancreatitis | 0.038 | 8.863 (1.132-69.388) | ||||
| No | 618 (99.7) | 117 (94.4) | 1 (reference) | |||
| Yes | 2 (0.3) | 7 (5.6) | 0 | 18.487 (3.793–90.10) | ||
| TC (mmol/L) | 0 | 5.303 (2.188-12.853) | ||||
| < 5.70 | 536 (86.5) | 73 (58.9) | 1 (reference) | |||
| ≥ 5.70 | 84 (13.5) | 51 (41.1) | 0 | 4.458 (2.914-6.821) | ||
| Tri (mmol/L) | ||||||
| < 1.7 | 415 (66.9) | 63 (50.8) | 1 (reference) | |||
| ≥ 1.7 | 205 (30.1) | 61 (49.2) | 0.001 | 1.960 (1.327-2.894) | ||
| HDL (mmol/L) | 0 | 4.053 (2.122-7.741) | ||||
| < 0.93 | 522 (84.2) | 52 (41.9) | 1 (reference) | |||
| ≥ 0.93 | 98 (15.8) | 72 (58.1) | 0 | 7.375 (4.861-11.19) | ||
| LDL (mmol/L) | ||||||
| < 3.63 | 520 (83.9) | 75 (60.5) | 1 (reference) | |||
| ≥ 3.63 | 100 (16.1) | 49 (39.5) | 0 | 3.397 (2.235-5.165) | ||
| FBG (mmol/L) | ||||||
| < 6.1 | 512 (82.6) | 82 (66.1) | 1 (reference) | |||
| ≥ 6.10 | 108 (17.4) | 42 (33.9) | 0 | 2.428 (1.586-3.717) | ||
| Apo a (g/L) | 0 | 48.58 (19.126-123.393) | ||||
| < 1 | 612 (98.7) | 59 (47.6) | 1 (reference) | |||
| ≥ 1 | 8 (1.3) | 65 (52.4) | 0 | 84.280 (38.578-184.121) | ||
| Apo b (g/L) | ||||||
| < 1 | 463 (74.7) | 66 (53.2) | 1 (reference) | |||
| ≥ 1 | 157 (25.3) | 58 (46.8) | 0 | 2.592 (1.743-3.853) | ||
| BMI (kg/m2) | ||||||
| < 25.0 | 364 (58.7) | 67 (54.1) | 1 (reference) | |||
| ≥ 25.0 | 256 (41.3) | 57 (45.9) | 0.37 | 1.210 (0.821-1.782) | ||
BMI: Body mass index; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; Tri: Triglyceride; TC: Total cholesterol; FBG: Fasting blood glucose; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen.
DISCUSSION
The relation between risk factors and ampullary tumors has been rarely investigated. We believe this to be the first and the largest hospital-based case-control study to evaluate metabolic syndromes for ampullary tumors. Our results provide evidence that some metabolic syndromes (diabetes, ApoA-related) and cholecystolithiasis are associated with an increased risk of ampullary cancer. There is also evidence that its premalignant lesion, ampullary adenoma, may be involved in an adenoma-carcinoma transformation.
Diabetes is one of the major public health challenges in both industrialized and developing countries. Increasing epidemiological evidence supports the idea that long-standing diabetes is one of the most important risk factors for overall cancer incidence[11-13]. A recent case-control study reported a 4.7-fold increased risk of cancer in persons with diabetes[11]. However, few studies have addressed the association of diabetes with ampullary tumor risk. In this study, not only did we find a positive link between diabetes and ampullary cancer (odds ratio: 4.75), we also found that diabetes increases the risk of ampullary adenoma by 2.59 times. It is the first time that diabetes and the development of ampullary tumors have been linked by published evidence. However, unlike pancreatic adenocarcinoma, as reported by Gapstur et al[14] and Butler et al[15] and in our previous study, we did not find a positive relation between plasma glucose concentration and ampullary tumor development.
The exact role of diabetes in ampullary tumor development is still unknown, but some biological mechanisms have been used to explain this relationship between cancer and diabetes. People with diabetes have been known to generate more reactive oxygen species than healthy controls. Normal cell DNA may be damaged by direct oxidation or by interference with cell DNA repair[16], thus potentially leading to tumor development. Another mechanism may be hyperinsulinemia; in diabetic patients, insulin resistance and hyperinsulinemia is a common phenomenon[17]. Insulin is a growth promoter, it can upregulate the production of insulin-like growth factor-1, which can promote tumor development by inhibiting apoptosis, stimulating cell proliferation, and enhancing angiogenesis[18-20]. It has also been reported that insulin may promote the growth of most human pancreatic cancers[21,22] and increase the replication markers of pancreatic ductal carcinoma.
Hyperlipidemia has been recently reported to be associated with cancer development, which is generally characterized by high levels of TC, Tri, and LDL, and a low level of HDL in serum[23-26]. Some experts suggest that dyslipidemia are risk factors for prostate, colon, and breast cancers. However, to our knowledge, no previous studies have investigated the association between lipid metabolism and risk of ampullary tumor development. We found that dyslipidemia is indeed a risk factor for the development of ampullary tumors. Patients with a high level of LDL and a low level of ApoA were at high risk of developing ampullary adenoma. A high level of TC and low levels of ApoA and HDL were also contributing risk factors for ampullary cancer development.
While the exact molecular mechanism by which hyperlipidemia is involved is unclear, inflammation is a potential risk factor for cancer[27,28]. Increased levels of triglycerides, LDL, and total cholesterol, and decreased levels of ApoA and HDL in serum have been reported to have a relationship with increased proinflammatory cytokines, such as interleukin-6, tumor necrosis factor-α, and interleukin-1[29,30]. Additionally, increased levels of LDL are associated with oxidized LDL, which is linked with an increase in reactive oxygen species[31,32]. It is well known that reactive oxygen species can cause DNA damage by activating oncogenes and inactivating tumor suppressor genes[33], all of which have been found to play a role in carcinogenesis. Together, these data suggest that dyslipidemias may play a role in ampulla tumor development. Our retrospective cohort study did not confirm the exact roles of the various lipids in ampullary adenoma and ampullary cancer. Although further studies are needed to clarify the potential roles, we believe that dyslipidemia plays a key role in the development of ampullary tumors.
Observational studies in humans have shown relations between several medical conditions and an increased risk of pancreaticobiliary adenocarcinoma, including cholecystitis[34,35], cholecystectomy[36], chronic pancreatitis[12,37-39], obesity[40], and HBV infection[41,42]. However, findings to support these associations have been somewhat contradictory. In the present study, cholecystolithiasis was an independent factor for an increased risk of developing ampulla adenoma (odds ratio: 11.068) and ampulla cancer (odds ratio: 14.06). It may even be involved in the transformation from adenoma to carcinoma. It is reported that there might be some relationship between diabetes and cholelithiasis[43,44]. Given the possibility that the relationship between ampullary tumors and cholelithiasis might be confounded by diabetes, we excluded the diabetes patients and found that the results did not change the association (data not shown).
We believe we are the first to observe a significant positive linear trend that chronic pancreatitis might be a risk factor for ampullary tumors, which is also a strong risk factor for both pancreatic and ampullary cancers (OR = 8.863). The χ2 test indicated that chronic pancreatitis is associated with the risk of developing ampullary adenoma, but a multivariate logistic regression test indicated that chronic pancreatitis is not correlated with ampullary adenoma formation.
Hepatitis B virus infection is a major public health burden in China[45-47]. The association between HBV and pancreaticobiliary adenocarcinoma has been proven, however, our results do not show a relationship between HBV infection and ampullary tumors development, whereas cigarette smoking, alcohol abuse, cholecystectomy, and obesity are risk factors.
This study revealed that some metabolic syndromes and medical conditions independently increased the risk of ampullary tumor development. Among them, a history of diabetes, cholelithiasis, and a high ApoA level were associated with progression to malignancy and may be involved in the adenoma-carcinoma transformation (Figure 1). Our study is the first one to evaluate risk factors involved in the adenoma-carcinoma sequence, so our results should be of value to the surgical and oncological communities.
Figure 1.
Risk factors for ampullary adenomas and ampullary cancer.
COMMENTS
Background
Tumors of the ampulla can be benign or malignant, although in most series the majority of ampullary neoplasms are malignant. Although ampullary tumors are relatively rare, the rapid development of, and advances in, endoscopy and imaging techniques have profoundly increased their discovery rate. Despite the increasing numbers of published studies, the etiology for ampullary tumors is incompletely defined. This is the first study to evaluate the impact of metabolic syndromes on ampullary tumor patients.
Research frontiers
Numerous published studies investigated the clinicopathological aspects of ampullary tumors, most of which focused on the prognosis associated with the disease. Studies investigating risk factors associated with the proposed adenoma-carcinoma sequence are scarce, especially regarding metabolic syndrome components and ampullary tumors.
Innovations and breakthroughs
In recent years, numerous published studies investigated the clinicopathological aspects of ampullary tumors, most of which focused on the prognosis associated with this disease. This study found that some metabolic syndrome components and medical conditions are potential risk factors for the development of ampullary tumors. Cholelithiasis, diabetes, and apolipoprotein A may contribute to the malignant transformation of benign ampullary adenomas into ampullary cancer.
Applications
This study revealed that some metabolic syndromes and medical conditions independently increased the risk of ampullary tumor development. Better understanding of the underlying pathophysiology of the association between the risk factors and ampullary tumors may provide new insights into the potential additive or synergistic effects of the components of the metabolic syndromes and the development of ampullary tumor treatment modalities. This will strengthen the clinical relevance of treating patients who meet the criteria for this syndrome.
Terminology
Metabolic syndrome, also known as insulin resistance syndrome, has become a major public health problem worldwide. It consists of obesity, hyperglycemia, hyperinsulinemia, dyslipidemia, and hypertension.
Peer review
This paper is a well-written, original, and the first study to evaluate the impact of metabolic syndromes on ampullary tumors patients. The findings in this study will strengthen the clinical relevance of treating patients who meet the criteria for this insidious syndrome.
Footnotes
Supported by A grant (in part) from the Municipal Key Discipline of Beijing, China, No. HK100230446; the National Natural Science Foundation of China, No. 81372578; International Science and Technology Cooperation Projects, No. 2010DFB33720; Program for New Century Excellent Talents in University, No. NCET-11-0288
P- Reviewer: Labori KJ S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Heinrich S, Clavien PA. Ampullary cancer. Curr Opin Gastroenterol. 2010;26:280–285. doi: 10.1097/MOG.0b013e3283378eb0. [DOI] [PubMed] [Google Scholar]
- 2.Qiao QL, Zhao YG, Ye ML, Yang YM, Zhao JX, Huang YT, Wan YL. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg. 2007;31:137–43; discussion 144-6. doi: 10.1007/s00268-006-0213-3. [DOI] [PubMed] [Google Scholar]
- 3.Ruemmele P, Dietmaier W, Terracciano L, Tornillo L, Bataille F, Kaiser A, Wuensch PH, Heinmoeller E, Homayounfar K, Luettges J, et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am J Surg Pathol. 2009;33:691–704. doi: 10.1097/PAS.0b013e3181983ef7. [DOI] [PubMed] [Google Scholar]
- 4.Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598–605. doi: 10.1002/jso.21374. [DOI] [PubMed] [Google Scholar]
- 5.Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000;12:75–79. doi: 10.1097/00042737-200012010-00014. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Iida M, Nakamura S, Hizawa K, Yao T, Tsuneyoshi M, Fujishima M. Natural history of ampullary adenoma in familial adenomatous polyposis: reconfirmation of benign nature during extended surveillance. Am J Gastroenterol. 2000;95:1557–1562. doi: 10.1111/j.1572-0241.2000.02094.x. [DOI] [PubMed] [Google Scholar]
- 7.Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, Hochwald SN. Contemporary results with ampullectomy for 29 “benign” neoplasms of the ampulla. J Am Coll Surg. 2008;206:466–471. doi: 10.1016/j.jamcollsurg.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006;38:521–525. doi: 10.1055/s-2006-925263. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser A, Jurowich C, Schönekäs H, Gebhardt C, Wünsch PH. The adenoma-carcinoma sequence applies to epithelial tumours of the papilla of Vater. Z Gastroenterol. 2002;40:913–920. doi: 10.1055/s-2002-35414. [DOI] [PubMed] [Google Scholar]
- 10.Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70:1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Lee MY, Lin KD, Hsiao PJ, Shin SJ. The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism. 2012;61:242–249. doi: 10.1016/j.metabol.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Lipworth L, Zucchetto A, Bosetti C, Franceschi S, Talamini R, Serraino D, McLaughlin JK, La Vecchia C, Negri E. Diabetes mellitus, other medical conditions and pancreatic cancer: a case-control study. Diabetes Metab Res Rev. 2011;27:255–261. doi: 10.1002/dmrr.1162. [DOI] [PubMed] [Google Scholar]
- 13.Bao B, Wang Z, Li Y, Kong D, Ali S, Banerjee S, Ahmad A, Sarkar FH. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta. 2011;1815:135–146. doi: 10.1016/j.bbcan.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 15.Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia. 2010;53:21–26. doi: 10.1007/s00125-009-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gago-Dominguez M, Jiang X, Castelao JE. Lipid peroxidation, oxidative stress genes and dietary factors in breast cancer protection: a hypothesis. Breast Cancer Res. 2007;9:201. doi: 10.1186/bcr1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Li Y, Dong X, Hassan MM, Abbruzzese JL, Li D. Effect of insulin-like growth factor gene polymorphisms alone or in interaction with diabetes on the risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3467–3473. doi: 10.1158/1055-9965.EPI-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 19.Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Found Symp. 2004;262:247–60; discussion 260-68. [PubMed] [Google Scholar]
- 20.Verheus M, Peeters PH, Rinaldi S, Dossus L, Biessy C, Olsen A, Tjønneland A, Overvad K, Jeppesen M, Clavel-Chapelon F, et al. Serum C-peptide levels and breast cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2006;119:659–667. doi: 10.1002/ijc.21861. [DOI] [PubMed] [Google Scholar]
- 21.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 22.Ding XZ, Fehsenfeld DM, Murphy LO, Permert J, Adrian TE. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas. 2000;21:310–320. doi: 10.1097/00006676-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 24.Furberg AS, Veierød MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–1160. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 25.Michalaki V, Koutroulis G, Syrigos K, Piperi C, Kalofoutis A. Evaluation of serum lipids and high-density lipoprotein subfractions (HDL2, HDL3) in postmenopausal patients with breast cancer. Mol Cell Biochem. 2005;268:19–24. doi: 10.1007/s11010-005-2993-4. [DOI] [PubMed] [Google Scholar]
- 26.Hammarsten J, Högstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- 27.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fogarty AW, Glancy C, Jones S, Lewis SA, McKeever TM, Britton JR. A prospective study of weight change and systemic inflammation over 9 y. Am J Clin Nutr. 2008;87:30–35. doi: 10.1093/ajcn/87.1.30. [DOI] [PubMed] [Google Scholar]
- 29.Feingold KR, Soued M, Adi S, Staprans I, Shigenaga J, Doerrler W, Moser A, Grunfeld C. Tumor necrosis factor-increased hepatic very-low-density lipoprotein production and increased serum triglyceride levels in diabetic rats. Diabetes. 1990;39:1569–1574. doi: 10.2337/diab.39.12.1569. [DOI] [PubMed] [Google Scholar]
- 30.Hardardóttir I, Grünfeld C, Feingold KR. Effects of endotoxin and cytokines on lipid metabolism. Curr Opin Lipidol. 1994;5:207–215. doi: 10.1097/00041433-199405030-00008. [DOI] [PubMed] [Google Scholar]
- 31.Benítez S, Camacho M, Bancells C, Vila L, Sánchez-Quesada JL, Ordóñez-Llanos J. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim Biophys Acta. 2006;1761:1014–1021. doi: 10.1016/j.bbalip.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Wells BJ, Mainous AG, Everett CJ, Gill JM. Iron, cholesterol, and the risk of cancer in an 18-year cohort. Asian Pac J Cancer Prev. 2005;6:505–509. [PubMed] [Google Scholar]
- 33.Feig DI, Sowers LC, Loeb LA. Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc Natl Acad Sci USA. 1994;91:6609–6613. doi: 10.1073/pnas.91.14.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–641. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 35.Thomsen RW, Thomsen HF, Nørgaard M, Cetin K, McLaughlin JK, Tarone RE, Fryzek JP, Sørensen HT. Risk of cholecystitis in patients with cancer: a population-based cohort study in Denmark. Cancer. 2008;113:3410–3419. doi: 10.1002/cncr.23961. [DOI] [PubMed] [Google Scholar]
- 36.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, Schwartz AG, Brown LM, Greenberg RS, Schoenberg JB, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 38.Maisonneuve P, Lowenfels AB, Bueno-de-Mesquita HB, Ghadirian P, Baghurst PA, Zatonski WA, Miller AB, Duell EJ, Boffetta P, Boyle P. Past medical history and pancreatic cancer risk: Results from a multicenter case-control study. Ann Epidemiol. 2010;20:92–98. doi: 10.1016/j.annepidem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Bracci PM, Wang F, Hassan MM, Gupta S, Li D, Holly EA. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control. 2009;20:1723–1731. doi: 10.1007/s10552-009-9424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 41.Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, Zhang DS, Wang FH, Li YH, Xu RH. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012;131:461–468. doi: 10.1002/ijc.26376. [DOI] [PubMed] [Google Scholar]
- 42.Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37:1211–1216. doi: 10.1016/j.humpath.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Shebl FM, Andreotti G, Meyer TE, Gao YT, Rashid A, Yu K, Shen MC, Wang BS, Han TQ, Zhang BH, et al. Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer. 2011;105:1424–1429. doi: 10.1038/bjc.2011.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ata N, Kucukazman M, Yavuz B, Bulus H, Dal K, Ertugrul DT, Yalcin AA, Polat M, Varol N, Akin KO, et al. The metabolic syndrome is associated with complicated gallstone disease. Can J Gastroenterol. 2011;25:274–276. doi: 10.1155/2011/356761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, Zhang SM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30:215–221. doi: 10.1111/j.1478-3231.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 46.Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Tang W. Perspectives on using des-γ-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr. 2013;2:227–231. doi: 10.3978/j.issn.2304-3881.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong S. Some issues regarding the development of general surgery during the last half century. Hepatobiliary Surg Nutr. 2012;1:2–4. doi: 10.3978/j.issn.2304-3881.2012.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]


