Abstract
AIM: To conduct a systematic review of the published epidemiological studies investigating the association of the interactions between gene variants and dietary intake with gastric cancer risk.
METHODS: A literature search was conducted in PubMed, EMBASE, and MEDLINE for articles published between January 2000 and July 2013, and 38 studies were identified. Previous studies included various dietary factors (e.g., fruits and vegetables, soybean products, salt, meat, and alcohol) and genetic variants that are involved in various metabolic pathways.
RESULTS: Studies suggest that individuals who carry high-risk genetic variants and demonstrate particular dietary habits may have an increased risk of gastric cancer compared with those who do not carry high-risk genetic variants. Distinctive dietary patterns and variations in the frequency of genetic variants may explain the higher incidence of gastric cancer in a particular region. However, most previous studies have limitations, such as a small sample size and a retrospective case-control design. In addition, past studies have been unable to elucidate the specific mechanism in gene-diet interaction associated with gastric carcinogenesis.
CONCLUSION: Additional large prospective epidemiological and experimental studies are required to identify the gene-diet metabolic pathways related to gastric cancer susceptibility.
Keywords: Gastric cancer, Gene, Diet, Interaction
Core tip: Gene-diet interactions related to gastric carcinogenesis may provide a unique environment for cancer growth or suppression in each individual. Gene-diet interactions may explain the large variation in gastric cancer incidence in different populations and the inconsistent findings of previous gene or diet studies. Therefore, this review provides an overview of the published epidemiological studies that have investigated the interactions between gene variants and dietary factors associated with gastric cancer risk.
INTRODUCTION
Although the incidence of gastric cancer has steadily declined in past decades, gastric cancer is the second leading cause of death from cancer worldwide[1,2]. Evidence has indicated that environmental factors such as Helicobacter pylori (H. pylori) colonization, cigarette smoking, and diet may play an important role in gastric carcinogenesis[3]. It has been reported that high intake of fresh fruits and vegetables has a protective effect against gastric cancer, whereas salted, smoked, pickled, and preserved foods rich in salt, nitrites, and preformed N-nitroso compounds are associated with an increased risk of gastric cancer[4,5]. Polymorphisms in genes may also contribute to the etiology of gastric cancer by altering the activity of enzymes that are involved in diverse molecular processes, such as DNA synthesis and repair (e.g., MTHFR), carcinogen-metabolism (e.g., GST and NAT), the inflammatory response (e.g., IL-1B), tumor suppression (e.g., p53), and so on[6].
The incidence of gastric cancer varies geographically; high-risk areas include East Asia (especially China and Japan), Eastern Europe, and parts of Central and South America[7]. Gastric cancer is thought to result from a combination of environmental factors and the accumulation of generalized and specific genetic alterations[8]. Therefore, gene-diet interactions, which provide a unique environment for cancer growth or suppression in each individual, may explain the large amount of variation in gastric cancer in different populations and the inconsistent findings of gene or diet studies[8,9]. In this present study, we reviewed previous studies that have investigated the interactions between dietary intake and gene polymorphisms associated with gastric cancer risk in diverse populations in epidemiological studies.
MATERIALS AND METHODS
The studies were identified in a search of the electronic databases of PubMed, MEDLINE, and EMBASE from January 2000 to July 2013. The search strategy used combinations of the following terms: gastric cancer, stomach cancer, gene, polymorphism, and diet. The following inclusion criteria were used for selecting the studies: (1) measurement of gastric cancer incidence; (2) measurement of dietary factors; (3) epidemiological study with a case-control or cohort study design; and (4) assessment of the interaction effect of dietary factors and genetic variants on gastric cancer risk.
We assessed the relevance of the studies using a hierarchical approach based on the title, the abstract, and the full-text article. The references of the retrieved papers were also examined to identify additional papers; an additional seven articles were included. The identified studies were screened by two authors independently. The study flow chart depicting the literature search and study selection is presented in Figure 1. We identified a total of 38 eligible studies, which were assessed by two reviewers. In terms of gene-diet interactions, many genes and their polymorphisms were examined. Therefore, we summarized previous studies based on the metabolic pathways of genetic variants. The following data were extracted from each eligible study as follow: (1) study (year) and country; (2) study design (number of cases and controls, source of control); (3) exposure [gene (polymorphisms), diets/nutrients]; and (4) outcome (gene, diets/nutrients, interaction).
Figure 1.
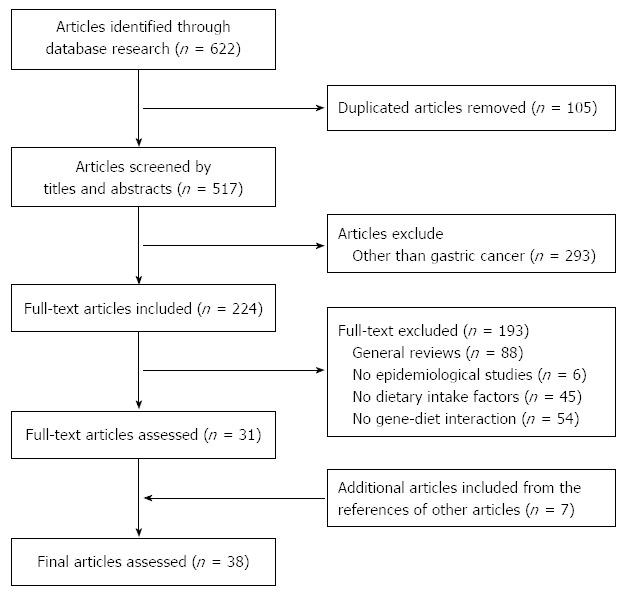
Study flow chart depicting the literature search and study selection.
RESULTS
In this review, we identified 38 studies that investigated the gene-diet interactions. The studies were mostly performed in Asia (n = 23), followed by the Europe (n = 9) and America (n = 6). In Asia, studies performed among Chinese people were most common (n = 11), followed by Korean (n = 8), Japanese (n = 3), and Indian (n = 1) populations. In Europe, two nested case-control studies were conducted within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. An additional seven independent studies were conducted in Poland (n = 3) and Italy (n = 4). In America, Mexico was the most popular study location (n = 3), followed by Brazil (n = 2) and the United States (n = 1). The incidence rates of gastric cancer in the studies were measured in a hospital-setting or in the general population in a specific region of a country.
The studies examined various dietary factors (e.g., fresh fruits and vegetables, meat, salted and preserved foods, and alcohol) and polymorphisms in many genes. We summarized the findings of the previous studies in Tables 1-3, according to the role of the genetic polymorphisms that have been studied in association with gastric cancer. Table 1 presents the interactive effect between DNA synthesis and repair-related genetic polymorphisms (e.g., MTHFR, hOGG1, and XRCC) and various dietary factors on gastric cancer risk. Table 2 shows the effect of diet on gastric cancer risk according to the carcinogen metabolism-related genetic polymorphisms (e.g., CYP2E1, GSTT1, GSTM1, SULT1A1, and ALDH2). Finally, Table 3 presents the effect of diet on gastric cancer risk according to the other selected genetic variants (e.g., IL-1B, IL-10, and p53).
Table 1.
Interactive effect between DNA synthesis and repair-related genetic polymorphisms and diet on gastric cancer risk
| Ref. | No. case/control | Gene | Diet/nutrient |
Outcome |
||
| (control) | (polymorphism) | Gene | Diet/nutrient | Interaction | ||
| Takezaki et al[24], 2002, China | 101/198 (Population) | hOGG1 (Ser326Cys) | Meat, soybean, vegetable, garlic, tea, alcohol | No assoc | Pickled vegetable (freq. vs non-freq): OR = 2.53 (1.52-4.21) | The positive assoc between pickled vegetable (P interaction = 0.093) or meat (P interaction = 0.083) and GC risk was stronger in Cys/Cys carriers than in Ser carriers; a frequent alcohol consumption (≥ 2 times/wk) increased GC risk in Cys/Cys carriers (P interaction = 0.086) |
| Stolzenberg-Solomon et al[18], 2003, China | 90/398 (Population) | MTHFR (C677T, A1298C) | Alcohol | No assoc | Unknown | Alcohol drinkers increased GC risk among those with MTHFT 677TT carriers (P interaction = 0.03) |
| MTRR (A66G) | ||||||
| Tsukino et al[23], 2004, Japan | 142/271 (Hospital) | hOGG1 (Ser326Cys) | Salt, fruit, vegetable, Vt C, β-carotene | No assoc | Unknown | The positive assoc between salt and GC risk was observed only in Cys allele carriers (P interaction = 0.01); the protective effect of cruciferous vegetable on GC risk was stronger among Ser/Ser genotype carriers (P interaction = 0.053) |
| Huang et al[25], 2005, Poland | 281/390 (Population) | XRCC (Arg399Gln) XPD (Lys751Gln) MGMT (Ile143Val, Leu84Phe) XRCC3 (Thr241Met) | Fruit/vegetable | No assoc | Fruit (low vs high): OR = 2.2 (1.3-3.6) | The positive assoc between low fruit or vegetable intake was modified by selected polymorphisms in XRCC1, XPD, MGMT (P interaction = 0.1-0.2) |
| Nan et al[26], 2005, South Korea | 110/220 (Hospital) | hMLH1 promoter | Cereal, potato, fruit, vegetable, mushroom, butter/cheese/margarine, protein, Vt C, P, K, Zn, Ca, alcohol | Unknown | Unknown | High vegetables increased GC risk and high potato intake decreased GC risk among cases with hMLH1 promoter hypermethylation; high intake of protein, P, K, Zn, Vt C, and Ca was associated with higher GC risk without hypermethylation of the hMLH1 gene promoter; high alcohol consumption was associated with a higher GC risk among those with hypermethylation of the hMLH1 gene promoter |
| Graziano et al[19], 2006, Italy | 162/164 (Population) | MTHFR (C677T) | Alcohol | 677TT vs CC: OR = 2.95 (1.57-5.55) | Unknown | Alcohol drinking modified the association between MTHFR C677T and GC risk (P interaction = 0.09) |
| Lacasaña-Navarro et al[21], 2006, Mexico | 201/427 (Hospital) | MTHFR (C677T) | Folate, alcohol | 677TT vs CC: OR = 1.62 (1.00-2.59) | No assoc | No interaction |
| Boccia et al[16], 2007, Italy | 102/254 (Hospital) | MTHFR (C677T, A1298C) | Fruit, vegetable, alcohol | 677TT vs CC for diffuse GC: OR = 2.92 (1.19-5.58) | Alcohol (high vs low): OR = 3.74 (1.13-12.45) | Low fruit/vegetable consumers increased GC risk among individuals with 677TT genotype |
| Mu et al[17], 2007, China | 206/415 (Population) | MTHFR (C677T, A1298C) | Fruit, vegetable | 677TT vs CC: OR = 2.80 (1.41-5.56) | No assoc | MTHFR 677TT genotype showed a stronger positive association among low fruit/vegetable intake subjects compared with high intake groups |
| Zhang et al[11], 2007, Poland | 305/427 (Population) | MTHFR (2 SNPs) MTR (1 SNP) MTRR (7 SNPs) | Folate | No assoc | No assoc | No interaction |
| Galván Portillo et al[14], 2009, Mexico | 248/478 (Population) | MTHFR (C677T) | Folate, choline, Vt B6, Vt B12, methionine | 677TT vs C allele: OR = 0.23 (0.06-0.84) | Folate (P = 0.001) | Among individuals with MTHFR 677TT genotype, low folate intake increased, but high folate intake decreased diffuse GC risk (P interaction = 0.055) |
| Duan et al[29], 2012, China | 400/400 for rs751402; 403/403 for rs2296147(Population) | ERCC5 (rs751402, rs2296147) | Alcohol | rs751402(AA vs GG):OR = 1.99 (1.20-3.31); rs2296147(CC vs TT): OR = 2.17 (1.04-4.54) | Unknown | Alcohol drinking substantially increased GC risk for subjects carrying rs2296147 CC homozygous variants, but their interaction was not statistically significant |
| Gao et al[15], 2013, China | 264/535 (Population) | MTHFR (C677T) | Folate | 677TT vs CC: OR = 2.08 (1.28-3.66) | Folate (high vs low): OR = 0.54 (0.34-0.83) | MTHFR 677TT carriers increased GC risk among subjects with low folate intake (P interaction = 0.005) |
ERCC5: Excision repair cross-complementing group 5; hOGG1: Human 8-oxoguanine DNA glycosylase 1; hMLH1: Human mutL homolog 1; MTHFR: Methylenetetrahydrofolate reductase; MGMT: O-6-methylguanine-DNA methyltransferase; MTR: 5-methyltetrahydrofolate-homocysteine methyltransferase; MTRR: 5-methyltetrahydrofolate-homocysteine methyltransferase reductase; XPD: Xeroderma pigmentosum D; XRCC: X-ray repair complementing defective repair in Chinese hamster cells; Freq: Frequent; GC: Gastric cancer; No assoc: No association; OR: Odds ratio; Vt: Vitamin; P: Phosphorous; K: Potassium; Zn: Zinc; Ca: Calcium.
Table 3.
Interactive effect between other genetic polymorphisms and diet on gastric cancer risk
| Ref. | No. case/control | Gene | Diet/nutrient |
Outcome |
||
| (control) | (polymorphism) | Gene | Diet/nutrient | Interaction | ||
| Mu et al[47], 2005, China | 206/415 (Population) | GSTM1 (null) | Green tea | Multi-genetic index (≥ 3 vs 0-1): OR = 2.21 (1.02-4.79) | Green tea (high vs low): OR = 0.39 (0.17-0.91) | Among individual carrying p53 codon 72 Pro/Pro genotype, no green tea drinking increased GC risk compared with those with Arg carriers and green tea drinking |
| GSTT1 (null) | ||||||
| GSTP1 (Ile/Val) | ||||||
| p53 codon 72 | ||||||
| (Arg/Pro) | ||||||
| Sul et al[52], 2006, United States | 155/134 (Hospital) | p53 codon 72 (Pro/Arg) | Na, calorie, fiber, fat, Vt C, alcohol | No assoc | Unknown | Possible interactions were observed between Vt C or fat intake and Pro/Pro genotype on GC risk |
| Ko et al[49], 2009, South Korea | 84/336 (Korean Multi-Center Cancer Cohort; nested case-control) | IL-1B (4 SNPs) | Soybean product | No assoc | No assoc | Low intake of soybean product increased GC risk among carriers of IL-10 gene variants (-592 GG/GA, -819 TC/CC, or -1082 AG/GG) |
| IL-2 (2 SNPs) | ||||||
| IL-4 (2 SNPs) | ||||||
| IL-8 (3 SNPs) | ||||||
| IL-10 (3 SNPs) | ||||||
| Wright et al[54], 2009, Poland | 279/414 (Population) | SLC23A1 (4 SNPs) SLC23A2 (9 SNPs) | Vt C | No assoc | Fruit/juice (P < 0.001) | No interaction |
| López Carrillo et al[48], 2012, Mexico | 158/317 (Hospital) | IL-1B (-31C/T) | Capsaicin | No assoc | Unknown | IL-1B-31 C allele carriers infected with H. pylori (CagA+) strains with moderate/high consumption of capsaicin showed an increased GC risk compared to T carriers (P interaction between Cap consumption and IL-1B-31C carrier = 0.04) |
| Oliveira et al[50], 2012, Brazil | 200/246 (Population) | IL-1RN (VNTR) TNF-β (A252G) | Alcohol | IL-1RN (VNTR) L/2+2/2 vs LL; OR = 2.53 (1.66-3.80) | Drinker vs non-drinker: OR = 3.09 (1.91-5.02) | No interaction |
| Agudo et al[53], 2013, Europe | 365/1284 (EPIC study, nested case-control) | HFE (9 SNPs) | Fe | H63D (G allele vs CC) (dominant): OR = 1.73 (1.20-2.51) for non-cardia; OR = 1.93 (1.25-2.98) for intestinal type | No assoc | No interaction |
| Song et al[55], 2013, South Korea | 3245/1700 (Hospital) | PRKAA1 and PTGER4 (rs13361707) ZBTB20 (rs9841504) | Alcohol | rs13361707 (TT vs CC): OR = 1.68 (1.41-2.01) | No assoc | No interaction |
| Zhang et al[56], 2013, China | 401/420 (Hospital) | EGFR (6 SNPs) | Salty food, alcohol | rs2072454 (T allele vs C allele): OR = 0.77 (0.61-0.97) | Salty food (P < 0.001); Alcohol drinking (P < 0.006) | No interaction |
GSTM1: Glutathione S-transferase M1; GSTT1: Glutathione S-transferase T1; GSTP1: Glutathione S-transferase P1; p53: Tumor protein p53; IL: Interleukin; SLC23A1: Solute carrier family 23 (ascorbic acid transporter) member 1; HFE: Hemochromatosis; PRKAA1: 5’-AMP activated protein kinase catalytic subunit alpha-1; PTGER4: Prostaglandin E receptor 4; TNF-β: Tumor necrosis factor beta; ZBTB20: Zinc finger and BTB domain containing protein 20; EGFR: Epidermal growth factor receptor; Fe: Iron; Vt: Vitamin; GC: Gastric cancer; No assoc: No association; Na: Sodium.
Table 2.
Interactive effect between carcinogen metabolism-related genetic polymorphisms and diet on gastric cancer risk
| Ref. | No. case/control | Gene | Diet/Nutrient |
Outcome |
||
| (control) | (polymorphism) | Gene | Diet/nutrient | Interaction | ||
| Nishimoto et al[58], 2000, Brazil | 332/528 (Hospital) | CYP2E1 (RsaI C/A) | Meat | No assoc | The frequency of meat consumption was higher in case only among Japanese, not Brazilians | No interaction |
| Setiawan et al[33], 2000, China | 143/433 (Population) | GSTT1 (null) GSTM1 (null) | Salt, fruit, alcohol | GSTT1 null vs normal: OR = 2.5 (1.01-6.22) | Fruit (P < 0.001) Alcohol (P = 0.051) | High salt intake increased GC risk only among GSTT1 null carriers. |
| Gao et al[42], 2002, China | 98/196 (Population) | CYP2E1 (RsaI) | Meat, soybean, vegetable (pickled or raw), tomato, garlic, tea, alcohol | No assoc | Unknown | No interaction |
| Gao et al[46], 2002, China | 153/223 (Population) | GSTM1 (null) GSTT1 (null) | Tea, alcohol | No assoc | Tea drinking was a protective effect on GC risk: OR = 0.38 (0.24-0.62) | Tea consumption decreased GC risk among those with GSTT1 null genotype; frequent alcohol intake increased GC risk in those with GSTM1 positive genotype. |
| Boccia et al[39], 2005, Italy | 76/260 (Hospital) | SULT1A1 (His/His) | Fruit, vegetable, grilled/barbecued meat, alcohol | His/His vs Arg/Arg: OR = 3.32 (1.17-9.45) | Frequency of GC was higher among those with high fruit intake (P = 0.043) | Among individuals carrying SULT1A1 His/His genotype, high fruit or grilled/barbequed meat intake increased GC risk; alcohol intake increased GC risk among those with His/His genotype. |
| Nan et al[35], 2005, South Korea | 421/632 (Hospital) | CYP1A1 (Ile/Val) CYP2E1 (c1/c2) GSTM1 (null) GSTT1 (null) ALDH2 (*1/*2) | Kimchi, soybean paste, vegetable, allium, seafood, soybean food | CYP1A1 Val carriers vs Ile/Ile: OR = 1.34 (1.04-1.73) | Kimchi (high vs low): OR = 1.57 (1.22-2.01); Soybean pastes (high vs low): OR = 1.62 (1.26-2.09); Non-fermented alliums (high vs low): OR = 0.70 (0.54-0.89); Non-fermented seafood (high vs low): OR = 0.66 (0.51-0.85) | High intake of kimchi or soybean pastes was associated with increased GC risk among carriers with CYP1A1 Ile/Ile, CYP2E1 c1/c1, GSTT1 positive, or ALDH2 *1/*1 genotype; non-fermented alliums were associated with a decreased GC risk among carriers of CYP1A1 Ile/Ile, CYP2E1 c1/c2 or c2/c2, GSTM1 null, GSTT1 positive, or ALDH2 *1/*2 or *2/*2 genotype; non-fermented seafood was associated with a reduced GC risk among carriers with CYP1A1 Ile/Ile, CYP2E1 c1/c1, ALDH2 *1/*1 genotype. |
| Boccia et al[59], 2007, Italy | 107/254 (Hospital) | CYP1A1 (*2A) CYP2E1 (*5A or *6) mEH (rapid, slow, very slow) GSTM1 (null) GSTT1 (null) NAT2 (slow) SULT1A1 (Arg/His, His/His) | Alcohol, fruit/vegetable, grilled meat, meals salt addition | GSTT1 null and NAT2 slow acetylators: OR = 3.00 (1.52-5.93) | Alcohol ( < 7 g/d vs ≥ 7 g/d): OR = 2.10 (1.22-3.60) | Alcohol drinkers carrying the variant allele of CYP2E1 (*5A or *6 alleles) had an increased GC risk compared to those drinking without the variant allele (P for heterogeneity = 0.001) |
| Kobayashi et al[40], 2009, Japan | 149/296 (Hospital) | NAT2 (4 SNPs) CYP1A1 (Ile462Val) CYP1A2 (5’UTR) | Grilled/barbecued meat, HCA | No assoc | No assoc | No interaction |
| Malik et al[60], 2009, India | 108/195 (Population) | GSTM1 (null) GSTT1 (null) GSTP1 (1313Ile/Val) GSTM3 (intron 6 3-bp del) CYP1A1 (6235T/C) CYP2E1 (Rsal-1091C/T) | High salted tea | GSTM1 null vs normal: OR = 1.98 (1.22-3.21); CYP2E1 Rsal c2 vs c1: OR = 2.18 (1.12-4.24); GSTM3 intron 6 3-bp del B vs A: OR = 0.50 (0.27-0.92) | Salted tea (high vs low): OR = 14.78 (8.22-27.23) | No interaction |
| Piao et al[43], 2009, South Korea | 2213/1699 (Population) | GSTM1 (null) GSTT1 (null) | Alcohol | No assoc | No assoc | No interaction |
| Zhang et al[36], 2009, South Korea | 471/471 (Hospital) | NAT2 acetylator | Meat, Vt B6, Fe, nut, stew, Kimchi, soybean paste, soybean food, allium, seaweeds, alcohol | No assoc | High intake of stews, kimchi, soybean paste, sodium, well-done meat, and alcohol increased GC risk; high intake of nuts, non-fermented soybean foods, non-fermented alliums decreased GC risk. | High intake of kimchi, stews, soybean paste, and alcohol were increased GC risk in slow/intermediate acetylators. |
| Shin et al[44], 2011, South Korea | 445/370 (Hospital) | ALDH2 (*1/*2) | Alcohol | No assoc | Ex-drinker vs never-drinker: OR = 1.68 (1.07-2.64) | There was an interaction between drinking status and ALDH2 genotype (ALDH*1/*1 vs ALDH2 *1/*2, P interaction = 0.048). |
| Duell et al[41], 2012, Europe | 364/1272 (EPIC study; nested case-control) | ADH1A (2 SNPs) ADH1B (5 SNPs) ADH1C (9 SNPs) ADH7 (10 SNPs) ALDH2 (2 SNPs) | Alcohol | Allelic OR ADH1A rs1230025: OR = 1.30 (1.07-1.59); ADH1C rs283411: OR = 0.59 (0.38-0.91); ALDH2 rs16941667: OR = 1.34 (1.00-1.79) | Alcohol (high vs low): OR = 2.37 (1.37-4.10) | Alcohol intake modified the association between ALDH1A rs1230025 and GC risk. |
| Zhang et al[61], 2012, China | 618/1147 (Population) | GSTP1 (Ile105Val) | Alcohol | Ile/Ile vs Val/Val: OR = 3.32 (1.79-6.17) | Alcohol (drinker vs nondrinker): P < 0.002 | Alcohol drinking increased GC risk among Val/Val carriers compared with Ile/Ile carriers |
| Matsuo et al[45], 2013, Japan | 697/1372 (Hospital) | ALDH2 (Glu504Lys) | Alcohol, fruit/vegetable | Alcohol (heavy vs non-drinker): OR = 1.72 (1.17-2.52) | A significant interaction between alcohol drinking and ALDH2 Lys allele (P = 0.0054) | |
| Eom et al[37], 2013, South Korea | 477/477 (Hospital) | CYP1A2 (3 SNPs) CYP2E1 (3 SNPs) EPHX1 (3 SNPs) GSTM1 (null) GSTT1 (null) | Aflatoxin B1 | CYP1A2 (CT vs CC): OR = 0.72 (0.52-0.98) | Aflatoxin B1 (low vs high): OR = 1.94 (1.43-2.63) | No interaction |
CYP2E1: Cytochrome P450, family 2, subfamily E, polypeptide 1; CYP1A1: Cytochrome P450, family 1, subfamily A, polypeptide 1; EPHX1: Microsomal epoxide hydrolase 1; GSTP1: Glutathione S-transferase P1; GSTT1: Glutathione S-transferase T1; GSTM1: Glutathione S-transferase M1; SULT1A1: sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1; ADH: Alcohol dehydrogenase; ALDH2: Aldehyde dehydrogenase 2 family; NAT2: N-acetyltransferase 2; Fe: Iron; Vt: Vitamin; GC: Gastric cancer; No assoc: No association; HCA: Heterocyclic amine.
DISCUSSION
This review summarizes the previous studies that have investigated the gene-diet interactions according to the roles of the genetic polymorphisms that have been studied in association with gastric cancer.
Effect of DNA synthesis and repair-related genetic polymorphisms and dietary factors on gastric cancer risk
Polymorphisms of genes associated with DNA synthesis and repair enzymes may contribute to an increased risk of gastric carcinogenesis by altering the function or efficiency of DNA synthesis and repair[10].
Genetic variation in one-carbon metabolism may affect normal patterns of DNA methylation and synthesis and therefore determine susceptibility to gastric cancer[11]. Methylenetetrahydrofolate reductase (MTHFR) irreversibly converts 5,10-methylene-tetrahydrofolate to 5-methyltetrahydrofolate, which is the main circulating form of folate and a methyl group donator[6]. A meta-analysis by Boccia et al[12] revealed a higher risk of gastric cancer among individuals with the MTHFR 677TT genotype compared with those with wild-type homozygotes, because of reduced enzyme activity[13]. Several studies have investigated the association between dietary factors (e.g., fruits, vegetables, folate, and alcohol) and MTHFR polymorphisms and have reported significant interactions[14-19]. In a population-based case-control study in China, individuals carrying the MTHFR 677TT genotype who had low folate intake had a higher gastric cancer risk compared with those with the CC genotype (P for interaction < 0.05)[15]. Another population-based case-control study in Mexico also reported that MTHFR 677TT carriers with high folate intake had a lower risk of diffuse-type gastric cancer, whereas those with low folate intake had a higher risk of intestinal-type gastric cancer compared with C allele carriers[14]. Stolzenberg-Solomon et al[18] found that alcohol consumption increased gastric cancer risk among MTHFR 677TT carriers in China. The increased gastric risk can be explained by DNA hypomethylation caused by the decrease in MTHFR enzyme activity among the individuals carrying the T allele who consume few fruits and vegetables and large amounts of alcohol[17]. This dietary habit may impair folate metabolism, particularly in those with folate deficiencies[20]. In contrast, some studies have found no association between MTHFR polymorphisms and either folate intake[11,21] or alcohol intake[16,21] and gastric cancer risk. It has been suggested that the differences in folate status between different populations may have caused the inconsistent findings on the role of MTHFR genetic variants in gastric carcinogenesis[17]. In addition to MTHFR polymorphisms, Zhang et al[11] also investigated the modifying effect of polymorphisms of methionine synthesis reductase (MTRR) and methionine synthase (MTR), but found no interactions.
Human oxoguanine glycosylase 1 (hOGG1) is responsible for repairing 8-hydroxy-deoxyguanosine residue, which is the major form of oxidative DNA damage induced by reactive oxygen species (ROS)[22]. hOGG1 Ser-Cys substitution at codon 326 results in decreased DNA repair activity, and thus might increase gastric cancer risk[23]. Tsukino et al[23] reported that high cruciferous vegetable intake had a protective effect against gastric cancer only among individuals carrying the Ser/Ser genotype (P for interaction = 0.053) and that high salt intake was positively associated with gastric cancer risk only among Cys carriers (P for interaction = 0.001). These results may suggest that the protective effect of dietary antioxidant compounds and other natural compounds (e.g., phenols, flavonoids, and isothiocyanates) against gastric cancer is stronger among subjects with the hOGG1 Ser/Ser genotype[23]. Takezaki et al[24] also found that a greater risk of gastric cancer associated with the frequent consumption of pickled vegetables, meat, and alcohol among the subjects carrying the Cys/Cys genotype than among the Ser carriers, but these interactions had only borderline significance. Oxidative damage due to dietary factors may induce carcinogenesis in the stomach, and it is more pronounced in Cys/Cys carriers, who have less ability to repair DNA damage[24].
Several other genetic variants related to DNA repair pathways were also examined. In a population-based case-control study in Poland, Huang et al[25] investigated four DNA repair genes (e.g., XRCC1, XPD, MGMT, and XRCC3), which represent four repair pathways. The risk associated with low fruit or vegetable intake tended to be modified by selected polymorphisms in XRCC1, XPD, and MGMT (P for interaction = 0.1-0.2)[25]. Additionally, Nan et al[26] found that high vegetable intake and low potato intake were associated with an increased risk of gastric cancer caused by hypermethylation of the hMLH1 promoter, which is correlated with the loss of gene expression in South Korea. It is assumed that pickled vegetables are a major source of vegetables in Korean diets; thus, high salt consumption may affect gastric carcinogenesis[27]. In this study, high intake of butter, cheese, and margarine was associated with a lower risk of gastric cancer regardless of hypermethylation of the hMLH1 promoter, which is in agreement with the relatively low incidence of gastric cancer in countries that consume large amounts of these foods[28]. Duan et al[29] investigated two polymorphisms in excision repair cross-complementing group 5 (ERCC5) promoter region in China and found their genetic effect on increased gastric cancer risk, especially for the diffuse subtype. They also found that alcohol drinking appeared to elevate the risk of gastric cancer among variant carriers, but their interaction effects were not statistically significant.
Effect of carcinogen metabolism-related genetic polymorphisms and dietary factors on gastric cancer risk
Potential carcinogens are activated by phase I enzymes [e.g., cytochrome P450 (CYP)] or detoxified by phase II enzymes [e.g., glutathione S-transferases superfamily (GST), N-acetyltransferase (NAT) and sulfotransferase (SULT)]. Genetic polymorphisms of these enzymes may alter enzyme activity and thus affect susceptibility to gastric cancer[6,30].
Salt and salted food consumption is known to elevate gastric cancer risk directly by damaging the gastric mucus or indirectly through correlating with H. pylori infection[31,32]. Setiawan et al[33] reported a positive association between high salt intake and gastric cancer among GSTT1 null carriers in China. GSTM1 and GSTT1 genes exhibit homozygous deletion (null) genotype polymorphisms. Individuals carrying one of these variants have no enzyme activity, and thus are more susceptible to carcinogens[34]. In South Korea, Nan et al[35] found that high intake of kimchi and soybean pastes fermented with salt and other chemicals is a risk factor for gastric cancer among individuals carrying CYP1A1 Ile/Ile and CYP2E1 c1/c1, as well as the GSTM1-positive, GSTT1-positive, or ALDH2 *1/*1 genotypes. N-nitroso compounds from kimchi and soybean pastes would not be rapidly metabolized in individuals with the CYP1A1 Ile/Ile or CYP2E1 c1/c1 genotypes, and the increased exposure of the gastric mucosa to N-nitroso compounds may elevate the risk of gastric cancer[35]. High plasma concentrations of glutathione-conjugated carcinogens in individuals with the GSTM1-positive genotype or the GSTT1-positive genotype may increase the risk of gastric cancer[35]. In addition, Zhang et al[36] found that high consumption of salted foods was associated with a higher risk of gastric cancer in slow/intermediate acetylators than in rapid NAT2 acetylators in Koreans. It has been suggested that the slow/intermediate NAT2 acetylator genotype may have less capacity to detoxify the carcinogens in the diet and thus may increase susceptibility to gastric cancer in individuals who consume large amounts of foods containing those carcinogens[36]. Eom et al[37] investigated the interactions between dietary intake of aflatoxin B1 and the polymorphisms of aflatoxin B1 metabolic enzymes (CYPA2, CYP2E1, EPHX1, GSTM1, and GSTT1), but found no interactions.
Prolonged cooking of meat at a high temperature produces several potent carcinogens including heterocyclic amines (HCAs)[38]. Boccia et al[39] reported that the positive association between gastric cancer risk and high consumption of grilled/barbecued meat in Italy was more pronounced among SULT1A1 His/His carriers compared with Arg/Arg carriers. It is possible that low enzyme activity in the individuals with SULT1A1 His/His genotype may result in less detoxification of HCAs from high consumption of grilled/barbequed meat[39]. However, Kobayashi et al[40] did not find any interaction between the consumption of grilled or barbequed meat and polymorphisms in NAT2, CYP1A1, and CYP1A2. The authors assumed that the relatively low intake of HCAs among the Japanese might have affected the findings.
The increased acetaldehyde levels induced by heavy alcohol drinking may lead to DNA damage and subsequently increase gastric cancer risk[41]. Several studies have investigated the differential role of alcohol on gastric cancer risk according to polymorphisms in various genes (e.g., GSTT1, GSTM1, CYP2E1, SULT1A1, and ALDH2)[33,36,39,41-45]. In the EPIC study, Duell et al[41] investigated 29 polymorphisms in alcohol metabolism-related genes, alcohol intake, and gastric cancer risk. They found that genetic variants at the ADH1 and ALDH2 loci may influence gastric cancer risk and that alcohol consumption may modify the effect of ADH1 rs1230025. Similarly, Matsuo et al[45] reported that heavy drinking was associated with an increased risk of gastric cancer among ALDH2 Lys allele carriers in a Japanese population. Boccia et al[39] found that alcohol intake increased gastric cancer risk among individuals with the SULT1A1 His/His genotype who had a low SULT1A1 enzyme activity. They hypothesized that high alcohol consumption may elevate gastric cancer risk more among individuals with low enzyme efficiency in their detoxification reactions.
Tea consumption has a protective effect against gastric carcinogenesis[32]. Gao et al[46] found that regular tea consumption among those with the GSTM1 or the GSTT1 null genotype was associated with decreased gastric cancer risk in China. This result suggests that the protective effect of tea consumption against gastric cancer is independent of the detoxification mechanisms involving GSTM1 and GSTT1. However, Mu et al[47] found no interactions between tea consumption and these polymorphisms. Unexpectedly, Boccia et al[39] reported that the positive association between the SULT1A1 His/His genotype (compared with the Arg/Arg genotype) and gastric cancer risk was more pronounced among individuals with high fruit intake in Italy. The authors assumed that the His/His genotype was possibly associated with unknown risk factors, such as heavy contamination with herbicides or pesticides related to gastric cancer risk.
Effect of other selected genetic polymorphisms and dietary factors on gastric cancer risk
Individual differences in the inflammatory response may contribute to the variation in the malignant transformation of the gastric mucosa, which may be modulated by genetic variants of inflammation-related cytokines[6]. López-Carrillo et al[48] reported that moderate to high capsaicin consumption synergistically increased the risk of gastric cancer in genetically susceptible individuals (IL-1B-31C allele carriers) infected with the more virulent H. pylori (CagA-positive) strains in Mexico. They suggested that capsaicin consumption, H. pylori infection, and IL-1B-31C genotypes may affect gastric carcinogenesis via the same metabolic pathway (e.g., an increased inflammatory response and an altered gastric acidic environment). In South Korea, Ko et al[49] investigated several inflammation-related polymorphisms and found that IL-10 genetic variants and low intake of soybean products increased gastric cancer risk compared with the same variants combined with high consumption of soybean products. Soybeans are a major source of isoflavones (e.g., genisten and daidzein), which have anti-inflammatory and anti-oxidative effects. In those who consume fewer soybeans, IL-10 genetic variants may allow infection with more virulent H. pylori strains and increase gastric inflammation[49]. Oliveira et al[50] investigated the modifying effects of alcohol on the association between genetic polymorphisms anti- or pro-inflammatory cytokines and gastric cancer risk in Southeast Brazilians but found no significant interactions.
The tumor protein 53 gene (p53) is the most frequently studied tumor suppressor gene[51]. It has been reported that the p53 codon 72 Arg/Arg genotype may induce apoptosis with faster kinetics and suppress transformation more efficiently than the Pro/Pro variant[47]. Sul et al[52] reported that the p53 codon 72 Pro/Pro genotype combined with low vitamin C intake showed a strong positive relationship with distal gastric cancer. Vitamin C, an important anti-oxidant, inhibits carcinogenesis by neutralizing ROS that can damage DNA. Additionally, Mu et al[47] also reported that the p53 codon 72 Pro/Pro genotype was more strongly associated with an increased risk of gastric cancer among non-green-tea drinkers compared with green-tea drinkers who were Arg carriers. They also found that individuals with a high multi-genetic index (e.g., GSTM1, GSTT1, GSTP1, and p53 codon 72 genotype) who consumed green tea were at higher risk of developing gastric cancer in China, suggesting that the combination of multiple genes from different pathways may contribute to the development of gastric cancer[47].
Some studies have investigated the modifying effect of some dietary factors on the roles of other genetic variants in gastric carcinogenesis, but did not find any significant interactions[53-56]. Because iron overload can increase oxidative stress and DNA damage, Agudo et al[53] investigated the interaction between the Hemochromatosis (HFE) gene mutation and iron overload in gastric carcinogenesis. In the EPIC study, individuals carrying the mutation of HFE polymorphism (H63D) had an increased risk of non-cardia and intestinal-type gastric cancer due to iron overload, but they found no interaction with iron intake[53]. In addition, it has been suggested that the common genetic variation in SLC23A1 and SLC23A2 could impact gastric cancer risk based on the role of ascorbic acid in the stomach, but Wright et al[54] found no association between SLC23A2 and ascorbic acid intake.
Limitations of studies investigating gene-diet interactions and gastric cancer risk
Previous studies investigating the gene-diet interaction effects on gastric cancer have several limitations. First, a majority of the studies were case-control studies, which are susceptible to both recall and selection bias. In particular, hospital-based controls may not adequately represent the prevalence of genetic variants or dietary habits in the general population because some of them may be related to their diseases. Second, many studies are underpowered to detect the interactions between genetic polymorphisms and dietary factors because they have a relatively small sample size or effect size. The lack of statistical power may explain why the results of the analysis of the interactions between polymorphisms and dietary status did not reach statistical significance. Third, the dietary data may not be entirely accurate because information about dietary intake was based on a questionnaire that assessed the average intake frequencies and portion size. In addition, exposure to dietary carcinogens may not develop into gastric cancer for 20 years or more, and recent dietary habits might not reflect the past eating habits accurately. Fourth, the etiology of gastric cancer varies according to the histological type (diffuse vs intestinal) and the anatomic location (cardia vs non-cardia)[57]. Although some studies have reported differential gene-diet interactions according to the subtype of gastric cancer[14], most studies did not conduct stratified analyses. Given these limitations, the findings from these studies should be interpreted with caution.
Different dietary habits and frequencies of genetic polymorphisms are observed in diverse populations. In addition, previous studies also provide some evidence of possible gene-diet interaction effects on gastric cancer risk, indicating differential effects of dietary factors on gastric carcinogenesis in genetically susceptible individuals. It may explain the geographical variations in gastric cancer and the inconsistent findings from previous studies investigating the role of either diet or gene in gastric carcinogenesis. However, additional large prospective studies are required to confirm these findings. We speculate that findings from gene-diet interaction studies may provide a basis for identifying at-risk subpopulations and promoting primary prevention of gastric cancer.
COMMENTS
Background
The incidence of gastric cancer varies geographically, which implies that environmental and genetic factors play a role in gastric carcinogenesis. Overall, this review summarized 38 research studies conducted on gene-diet interaction associated with gastric cancer risk.
Research frontiers
This study is related with genetics, nutrition, and cancer field. It may be helpful to understand the general mechanism of cellular processes (e.g., DNA synthesis) and genetic transitions (e.g., p53 gene), which significantly affects the development of cancer. Also, it is important to understand the role of nutrition related with cancer risk.
Innovations and breakthroughs
The previous research studies have shown that the alteration in DNA synthesis, the effects of carcinogen metabolism-related genetic polymorphisms and other selected polymorphisms with dietary intake may increase the risk of gastric cancer in diverse populations. This evidence suggests the modifications in genetic variants, particularly from dietary intake, are associated with gastric carcinogenesis.
Applications
Future studies may include a larger study population to determine consistent findings compared with the previous studies. This review may be expanded to further investigation on a specific role of genetic variants with dietary intake in additional regions associated with gastric cancer risk.
Terminology
Genetic variation is the phenotypic and genotypic differences among individuals in a population. Polymorphism is one of two or more variants of a particular DNA sequence. Genotype is the genetic constitution of an organism or cell.
Peer review
Indeed, genetic variants interacting with dietary intake are possibly associated with gastric cancer risk among individuals in different regions. This review may be useful to understand the increase risk of gastric cancer from the alterations in genetic variants and their interaction with nutrition.
Footnotes
Supported by A grant from the National Cancer Center, South Korea, No. 1110300 and No. 1410260
P- Reviewer: Gu QL, Pimanov SI S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton JP, Meltzer SJ. A review of the genomics of gastric cancer. Clin Gastroenterol Hepatol. 2006;4:416–425. doi: 10.1016/j.cgh.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, Tanizaki Y, Matsumoto T, Iida M, Kiyohara Y. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008;168:1409–1415. doi: 10.1093/aje/kwn276. [DOI] [PubMed] [Google Scholar]
- 4.Palli D. Epidemiology of gastric cancer: an evaluation of available evidence. J Gastroenterol. 2000;35 Suppl 12:84–89. [PubMed] [Google Scholar]
- 5.Pickled vegetables. IARC Monogr Eval Carcinog Risks Hum. 1993;56:83–113. [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Z, Ajani JA, Wei Q. Molecular epidemiology of gastric cancer: current status and future prospects. Gastrointest Cancer Res. 2007;1:12–19. [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114–33; discussion 133. doi: 10.1002/jso.20214. [DOI] [PubMed] [Google Scholar]
- 8.Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–628. doi: 10.1007/s00439-009-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S. A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics. 2008;9:361–374. doi: 10.2174/138920208785699544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte MC, Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, Silva AE. Polymorphisms of DNA repair genes XRCC1 and XRCC3, interaction with environmental exposure and risk of chronic gastritis and gastric cancer. World J Gastroenterol. 2005;11:6593–6600. doi: 10.3748/wjg.v11.i42.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang FF, Terry MB, Hou L, Chen J, Lissowska J, Yeager M, Zatonski W, Chanock S, Morabia A, Chow WH. Genetic polymorphisms in folate metabolism and the risk of stomach cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:115–121. doi: 10.1158/1055-9965.EPI-06-0513. [DOI] [PubMed] [Google Scholar]
- 12.Boccia S, Hung R, Ricciardi G, Gianfagna F, Ebert MP, Fang JY, Gao CM, Götze T, Graziano F, Lacasaña-Navarro M, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol. 2008;167:505–516. doi: 10.1093/aje/kwm344. [DOI] [PubMed] [Google Scholar]
- 13.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 14.Galván-Portillo MV, Cantoral A, Oñate-Ocaña LF, Chen J, Herrera-Goepfert R, Torres-Sanchez L, Hernandez-Ramirez RU, Palma-Coca O, López-Carrillo L. Gastric cancer in relation to the intake of nutrients involved in one-carbon metabolism among MTHFR 677 TT carriers. Eur J Nutr. 2009;48:269–276. doi: 10.1007/s00394-009-0010-5. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Ding LH, Wang JW, Li CB, Wang ZY. Diet folate, DNA methylation and polymorphisms in methylenetetrahydrofolate reductase in association with the susceptibility to gastric cancer. Asian Pac J Cancer Prev. 2013;14:299–302. doi: 10.7314/apjcp.2013.14.1.299. [DOI] [PubMed] [Google Scholar]
- 16.Boccia S, Gianfagna F, Persiani R, La Greca A, Arzani D, Rausei S, D’ugo D, Magistrelli P, Villari P, Van Duijn CM, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and susceptibility to gastric adenocarcinoma in an Italian population. Biomarkers. 2007;12:635–644. doi: 10.1080/13547500701546766. [DOI] [PubMed] [Google Scholar]
- 17.Mu LN, Cao W, Zhang ZF, Yu SZ, Jiang QW, You NC, Lu QY, Zhou XF, Ding BG, Chang J, et al. Polymorphisms of 5,10-methylenetetralydrofolate reductase (MTHFR), fruit and vegetable intake, and the risk of stomach cancer. Biomarkers. 2007;12:61–75. doi: 10.1080/13547500600945101. [DOI] [PubMed] [Google Scholar]
- 18.Stolzenberg-Solomon RZ, Qiao YL, Abnet CC, Ratnasinghe DL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Esophageal and gastric cardia cancer risk and folate- and vitamin B(12)-related polymorphisms in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1222–1226. [PubMed] [Google Scholar]
- 19.Graziano F, Kawakami K, Ruzzo A, Watanabe G, Santini D, Pizzagalli F, Bisonni R, Mari D, Floriani I, Catalano V, et al. Methylenetetrahydrofolate reductase 677C/T gene polymorphism, gastric cancer susceptibility and genomic DNA hypomethylation in an at-risk Italian population. Int J Cancer. 2006;118:628–632. doi: 10.1002/ijc.21397. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E. Alcohol, one-carbon metabolism, and colorectal cancer: recent insights from molecular studies. J Nutr. 2004;134:2475S–2481S. doi: 10.1093/jn/134.9.2475S. [DOI] [PubMed] [Google Scholar]
- 21.Lacasaña-Navarro M, Galván-Portillo M, Chen J, López-Cervantes M, López-Carrillo L. Methylenetetrahydrofolate reductase 677C>T polymorphism and gastric cancer susceptibility in Mexico. Eur J Cancer. 2006;42:528–533. doi: 10.1016/j.ejca.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 23.Tsukino H, Hanaoka T, Otani T, Iwasaki M, Kobayashi M, Hara M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, et al. hOGG1 Ser326Cys polymorphism, interaction with environmental exposures, and gastric cancer risk in Japanese populations. Cancer Sci. 2004;95:977–983. doi: 10.1111/j.1349-7006.2004.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takezaki T, Gao CM, Wu JZ, Li ZY, Wang JD, Ding JH, Liu YT, Hu X, Xu TL, Tajima K, et al. hOGG1 Ser(326)Cys polymorphism and modification by environmental factors of stomach cancer risk in Chinese. Int J Cancer. 2002;99:624–627. doi: 10.1002/ijc.10400. [DOI] [PubMed] [Google Scholar]
- 25.Huang WY, Chow WH, Rothman N, Lissowska J, Llaca V, Yeager M, Zatonski W, Hayes RB. Selected DNA repair polymorphisms and gastric cancer in Poland. Carcinogenesis. 2005;26:1354–1359. doi: 10.1093/carcin/bgi084. [DOI] [PubMed] [Google Scholar]
- 26.Nan HM, Song YJ, Yun HY, Park JS, Kim H. Effects of dietary intake and genetic factors on hypermethylation of the hMLH1 gene promoter in gastric cancer. World J Gastroenterol. 2005;11:3834–3841. doi: 10.3748/wjg.v11.i25.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Park S, Nam BH. Gastric cancer and salt preference: a population-based cohort study in Korea. Am J Clin Nutr. 2010;91:1289–1293. doi: 10.3945/ajcn.2009.28732. [DOI] [PubMed] [Google Scholar]
- 28.Stadtländer CT, Waterbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195–2208. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
- 29.Duan Z, He C, Gong Y, Li P, Xu Q, Sun LP, Wang Z, Xing C, Yuan Y. Promoter polymorphisms in DNA repair gene ERCC5 and susceptibility to gastric cancer in Chinese. Gene. 2012;511:274–279. doi: 10.1016/j.gene.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 31.Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–2213. doi: 10.3748/wjg.15.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasazuki S, Inoue M, Hanaoka T, Yamamoto S, Sobue T, Tsugane S. Green tea consumption and subsequent risk of gastric cancer by subsite: the JPHC Study. Cancer Causes Control. 2004;15:483–491. doi: 10.1023/B:CACO.0000036449.68454.42. [DOI] [PubMed] [Google Scholar]
- 33.Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, Cordova D, Wang MR, Guo CH, Yu SZ, et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2000;9:73–80. [PubMed] [Google Scholar]
- 34.Egan KM, Cai Q, Shu XO, Jin F, Zhu TL, Dai Q, Gao YT, Zheng W. Genetic polymorphisms in GSTM1, GSTP1, and GSTT1 and the risk for breast cancer: results from the Shanghai Breast Cancer Study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2004;13:197–204. doi: 10.1158/1055-9965.epi-03-0294. [DOI] [PubMed] [Google Scholar]
- 35.Nan HM, Park JW, Song YJ, Yun HY, Park JS, Hyun T, Youn SJ, Kim YD, Kang JW, Kim H. Kimchi and soybean pastes are risk factors of gastric cancer. World J Gastroenterol. 2005;11:3175–3181. doi: 10.3748/wjg.v11.i21.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YW, Eom SY, Kim YD, Song YJ, Yun HY, Park JS, Youn SJ, Kim BS, Kim H, Hein DW. Effects of dietary factors and the NAT2 acetylator status on gastric cancer in Koreans. Int J Cancer. 2009;125:139–145. doi: 10.1002/ijc.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eom SY, Yim DH, Zhang Y, Yun JK, Moon SI, Yun HY, Song YJ, Youn SJ, Hyun T, Park JS, et al. Dietary aflatoxin B1 intake, genetic polymorphisms of CYP1A2, CYP2E1, EPHX1, GSTM1, and GSTT1, and gastric cancer risk in Korean. Cancer Causes Control. 2013;24:1963–1972. doi: 10.1007/s10552-013-0272-3. [DOI] [PubMed] [Google Scholar]
- 38.Sugimura T. Carcinogenicity of mutagenic heterocyclic amines formed during the cooking process. Mutat Res. 1985;150:33–41. doi: 10.1016/0027-5107(85)90098-3. [DOI] [PubMed] [Google Scholar]
- 39.Boccia S, Persiani R, La Torre G, Rausei S, Arzani D, Gianfagna F, Romano-Spica V, D’Ugo D, Ricciardi G. Sulfotransferase 1A1 polymorphism and gastric cancer risk: a pilot case-control study. Cancer Lett. 2005;229:235–243. doi: 10.1016/j.canlet.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Sakamoto H, Yoshida T, Tsugane S. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of stomach cancer: a hospital-based case-control study in Japan. Gastric Cancer. 2009;12:198–205. doi: 10.1007/s10120-009-0523-x. [DOI] [PubMed] [Google Scholar]
- 41.Duell EJ, Sala N, Travier N, Muñoz X, Boutron-Ruault MC, Clavel-Chapelon F, Barricarte A, Arriola L, Navarro C, Sánchez-Cantalejo E, et al. Genetic variation in alcohol dehydrogenase (ADH1A, ADH1B, ADH1C, ADH7) and aldehyde dehydrogenase (ALDH2), alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Carcinogenesis. 2012;33:361–367. doi: 10.1093/carcin/bgr285. [DOI] [PubMed] [Google Scholar]
- 42.Gao C, Takezaki T, Wu J, Li Z, Wang J, Ding J, Liu Y, Hu X, Xu T, Tajima K, et al. Interaction between cytochrome P-450 2E1 polymorphisms and environmental factors with risk of esophageal and stomach cancers in Chinese. Cancer Epidemiol Biomarkers Prev. 2002;11:29–34. [PubMed] [Google Scholar]
- 43.Piao JM, Shin MH, Kweon SS, Kim HN, Choi JS, Bae WK, Shim HJ, Kim HR, Park YK, Choi YD, et al. Glutathione-S-transferase (GSTM1, GSTT1) and the risk of gastrointestinal cancer in a Korean population. World J Gastroenterol. 2009;15:5716–5721. doi: 10.3748/wjg.15.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin CM, Kim N, Cho SI, Kim JS, Jung HC, Song IS. Association between alcohol intake and risk for gastric cancer with regard to ALDH2 genotype in the Korean population. Int J Epidemiol. 2011;40:1047–1055. doi: 10.1093/ije/dyr067. [DOI] [PubMed] [Google Scholar]
- 45.Matsuo K, Oze I, Hosono S, Ito H, Watanabe M, Ishioka K, Ito S, Tajika M, Yatabe Y, Niwa Y, et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis. 2013;34:1510–1515. doi: 10.1093/carcin/bgt080. [DOI] [PubMed] [Google Scholar]
- 46.Gao CM, Takezaki T, Wu JZ, Li ZY, Liu YT, Li SP, Ding JH, Su P, Hu X, Xu TL, et al. Glutathione-S-transferases M1 (GSTM1) and GSTT1 genotype, smoking, consumption of alcohol and tea and risk of esophageal and stomach cancers: a case-control study of a high-incidence area in Jiangsu Province, China. Cancer Lett. 2002;188:95–102. doi: 10.1016/s0304-3835(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 47.Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You NC, Setiawan VW, Zhou XF, Ding BG, Wang RH, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer. 2005;116:972–983. doi: 10.1002/ijc.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Carrillo L, Camargo MC, Schneider BG, Sicinschi LA, Hernández-Ramírez RU, Correa P, Cebrian ME. Capsaicin consumption, Helicobacter pylori CagA status and IL1B-31C>T genotypes: a host and environment interaction in gastric cancer. Food Chem Toxicol. 2012;50:2118–2122. doi: 10.1016/j.fct.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko KP, Park SK, Cho LY, Gwack J, Yang JJ, Shin A, Kim CS, Kim Y, Kang D, Chang SH, et al. Soybean product intake modifies the association between interleukin-10 genetic polymorphisms and gastric cancer risk. J Nutr. 2009;139:1008–1012. doi: 10.3945/jn.108.101865. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira JG, Duarte MC, Silva AE. IL-1ra anti-inflammatory cytokine polymorphism is associated with risk of gastric cancer and chronic gastritis in a Brazilian population, but the TNF-β pro-inflammatory cytokine is not. Mol Biol Rep. 2012;39:7617–7625. doi: 10.1007/s11033-012-1596-x. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, Du L, Wei ML, Wu XT. P53 codon 72 polymorphism and gastric cancer: a meta-analysis of the literature. Int J Cancer. 2007;121:1481–1486. doi: 10.1002/ijc.22833. [DOI] [PubMed] [Google Scholar]
- 52.Sul J, Yu GP, Lu QY, Lu ML, Setiawan VW, Wang MR, Guo CH, Yu SZ, Mu L, Cai L, et al. P53 Codon 72 polymorphisms: a case-control study of gastric cancer and potential interactions. Cancer Lett. 2006;238:210–223. doi: 10.1016/j.canlet.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agudo A, Bonet C, Sala N, Muñoz X, Aranda N, Fonseca-Nunes A, Clavel-Chapelon F, Boutron-Ruault MC, Vineis P, Panico S, et al. Hemochromatosis (HFE) gene mutations and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Carcinogenesis. 2013;34:1244–1250. doi: 10.1093/carcin/bgt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright ME, Andreotti G, Lissowska J, Yeager M, Zatonski W, Chanock SJ, Chow WH, Hou L. Genetic variation in sodium-dependent ascorbic acid transporters and risk of gastric cancer in Poland. Eur J Cancer. 2009;45:1824–1830. doi: 10.1016/j.ejca.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song HR, Kim HN, Kweon SS, Choi JS, Shim HJ, Cho SH, Chung IJ, Park YK, Kim SH, Choi YD, et al. Genetic variations in the PRKAA1 and ZBTB20 genes and gastric cancer susceptibility in a Korean population. Mol Carcinog. 2013;52 Suppl 1:E155–E160. doi: 10.1002/mc.22063. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Zhan Z, Wu J, Zhang C, Yang Y, Tong S, Sun Z, Qin L, Yang X, Dong W. Association among polymorphisms in EGFR gene exons, lifestyle and risk of gastric cancer with gender differences in Chinese Han subjects. PLoS One. 2013;8:e59254. doi: 10.1371/journal.pone.0059254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 58.Nishimoto IN, Hanaoka T, Sugimura H, Nagura K, Ihara M, Li XJ, Arai T, Hamada GS, Kowalski LP, Tsugane S. Cytochrome P450 2E1 polymorphism in gastric cancer in Brazil: case-control studies of Japanese Brazilians and non-Japanese Brazilians. Cancer Epidemiol Biomarkers Prev. 2000;9:675–680. [PubMed] [Google Scholar]
- 59.Boccia S, Sayed-Tabatabaei FA, Persiani R, Gianfagna F, Rausei S, Arzani D, La Greca A, D’Ugo D, La Torre G, van Duijn CM, et al. Polymorphisms in metabolic genes, their combination and interaction with tobacco smoke and alcohol consumption and risk of gastric cancer: a case-control study in an Italian population. BMC Cancer. 2007;7:206. doi: 10.1186/1471-2407-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malik MA, Upadhyay R, Mittal RD, Zargar SA, Modi DR, Mittal B. Role of xenobiotic-metabolizing enzyme gene polymorphisms and interactions with environmental factors in susceptibility to gastric cancer in Kashmir Valley. J Gastrointest Cancer. 2009;40:26–32. doi: 10.1007/s12029-009-9072-0. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Sun LP, Xing CZ, Xu Q, He CY, Li P, Gong YH, Liu YP, Yuan Y. Interaction between GSTP1 Val allele and H. pylori infection, smoking and alcohol consumption and risk of gastric cancer among the Chinese population. PLoS One. 2012;7:e47178. doi: 10.1371/journal.pone.0047178. [DOI] [PMC free article] [PubMed] [Google Scholar]


