Abstract
Neuregulin-1 (NRG1) signaling through the tyrosine kinase receptors erbB2 and erbB4 is required for cardiac morphogenesis, and it plays an essential role in maintaining the myocardial architecture during adulthood. The tyrosine kinase receptor erbB2 was first linked to the amplification and overexpression of erbb2 gene in a subtype of breast tumor cells, which is indicative of highly proliferative cells and likely a poor prognosis following conventional chemotherapy. The development of targeted therapies to block the survival of erbB2-positive cancer cells revealed that impaired NRG1 signaling through erbB2/erbB4 heterodimers combined with anthracycline chemotherapy may lead to dilated cardiomyopathy in a subpopulation of treated patients. The ventricular-specific deletion of either erbb2 or erbb4 manifested dilated cardiomyopathy, which is aggravated by the administration of doxorubicin. Based on the exacerbated toxicity displayed by the combined treatment, it is expected that the relevant pathways would be affected in a synergistic manner. This review examines the NRG1 activities that were monitored in different model systems, focusing on the emerging pathways and molecular targets, which may aid in understanding the acquired dilated cardiomyopathy that occurs under the conditions of NRG1-deficient signaling.
Keywords: Ventricular dilation, Cardiotoxicity, Erbb2, Erbb4, Neuregulin, Trastuzumab, Doxorubicin
Core tip: We have reviewed the cardiac requirement of neuregulin-1 (NRG1) signaling through the receptor tyrosine kinase erbB2/erbB4. The evidence indicates that the NRG1/erbB signaling pathway displays a panel of activities implicated in maintaining the myocardial architecture during remodeling, which may explain why the combined treatment with antibodies against erbB2 and anthracycline chemotherapy may evolve into a severe dilated cardiomyopathy. We have further examined the potential molecular targets, which have been either inferred from impaired NRG1 signaling or directly assessed by the administration of NRG1. The current working hypotheses have been delineated towards a prospective molecular understanding of NRG1 signaling in heart.
INTRODUCTION
Dilated cardiomyopathy (DCM) results from the abnormal remodeling of the myocardium with the eccentric growth of cardiomyocytes in response to valve defects, toxic and metabolic causes or gene defects[1,2]. An acquired form of DCM is manifested in a subpopulation of breast cancer patients treated with anthracycline chemotherapy combined with humanized antibodies against erbB2. The amplification and over-expression of erbB2 occurs in 25% of all breast cancer types, inducing a highly invasive tumor that has a poor prognosis when treated using conventional therapies. Targeted therapies with antibodies against erbB2 were shown to be clinically effective for erbB2-positive breast cancer patients through an objective tumor regression analysis, with lower rates of both recurrence and mortality[3]. However, the iatrogenic effect of the combined immunotherapy and chemotherapy results in increased incidence of dilated cardiomyopathy, initially affecting a subpopulation of 27% of treated patients[4].
We reviewed the panel of activities of the NRG1 pathway that affect cardiomyocyte survival, proliferation, differentiation and specification to further focus on the synergistically deregulated molecular pathways under the experimental conditions of impaired NRG1 signaling and doxorubicin therapy.
THERAPEUTIC CONSIDERATIONS
Evidence from long-term retrospective analyses of treatment with the humanized antibodies against erbB2 (trastuzumab) have suggested that deficient NRG1 signaling sensitizes the heart to anthracycline cardiotoxicity[5,6]. These studies prompted the sequential administration of immunotherapy after chemotherapy in patients with no signs of cardiotoxicity to reduce the incidence of cardiomyopathy. The continuous development of immunotherapeutic drugs aimed at improving efficacy in tumor cell death have recently provided a novel humanized monoclonal antibody against erbB called pertuzumab, which prevents erbB receptor dimerization and thus blocks the activity of both erbB2 and erbB4. Currently, there are ongoing clinical trials about the safety and efficacy of these immunotherapeutics at escalating doses, when used to treat a diverse group of patients with epithelial-derived cancers. Thus far, the results from the CLEOPATRA study group indicate the beneficial effect of the combined action of pertuzumab and trastuzumab with docetaxel, which leads to the significantly progression-free and prolonged survival of patients with breast cancer while having a comparable level of cardiotoxicity both to previous formulations and to placebo with trastuzumab and docetaxel[7]. These results prompted the FDA priority review of pertuzumab for its approval and release into the market in June 2012.
In the light of the cardiac adverse effects of the therapeutics used to block the survival of cancer cells, researchers have indicated that drugs, specifically those that target signaling pathways or kinase receptors and have a broad range of effects on cancer cells, should also be studied for cardiac safety[8]. In this regard, the multidisciplinary workshop of the Association of the European Society of Cardiology aimed for a consensus in the management of treatments while prioritizing the awareness of anthracycline cardiotoxicity and the development of new targeted therapeutics[9].
Interestingly, the design of an observational trial on cardiotoxicity in cancer therapies has included an additional evaluation of cardiac risk incidence by analyzing the Single Nucleotide Polymorphism/haplotype variations in the NRG1/ErbB signaling gene components (NCT01173341). In addition to its impact on disease management, the results from this trial may contribute to the knowledge of genetic modifiers by identifying polymorphisms on the genes of the NRG1/erbB pathway that are associated with disease.
THE COMPONENTS OF THE NRG1 PATHWAY
Neuregulins are transmembrane proteins of four isotypes (NRG1-4). Neuregulin-1 is classified into at least three subgroups (type I-III) and has approximately 30 isoforms as a result of its synthesis from different promoters and splicing variants[10]. Neuregulins: of types I and II are processed at the membrane by metalloproteinase, ADAM17, 19 and are cleaved by α-secretase activity[11]. The release rate of the amino-terminal active domain is modulated by protein kinase C (PKC)-delta[12]. The active peptide of the NRG is related to the epidermal growth factor (EGF), which contains a cysteine-rich domain that binds to and activates the tyrosine kinase receptors erbB4 and erbB3, which belong to the EGF receptor (erbB1) family. The active forms of erbB2 and erbB3 receptors are considered heterodimers because they lack either an opened ligand binding domain or tyrosine kinase activity, respectively, as opposed to the potential function of erbB4 receptor homodimers[13].
In the heart, the active domain of NRG1 secreted from endothelial cells binds and activates the erbB2/erbB4 heterodimers expressed in cardiomyocytes (Figure 1). The NRG1 pathway, which was initially characterized as inducing cardiomyocyte differentiation and specification, has been identified as inducing a broader panel of activities according to the experimental model system and the induced heart condition (Table 1).
Figure 1.
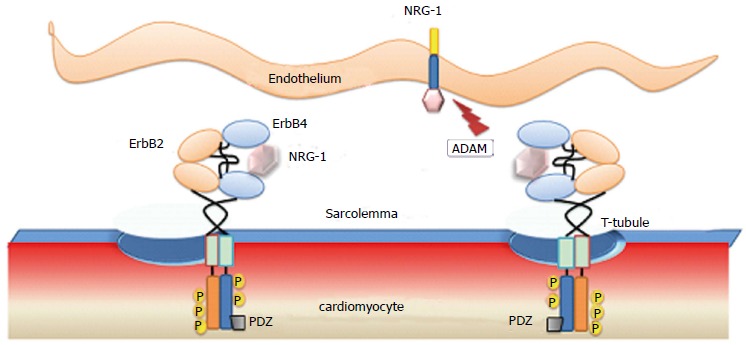
Endothelium-Cardiac muscle interactions through paracrine neuregulin-1 signaling. Secreted neuregulin-1 from endothelial cells binds to erbB4 inducing auto- and trans-phosphorylation of ErbB2/ErbB4 heterodimers, expressed in cardiomyocytes. NRG-1: Neuregulin-1; PDZ: Postsynaptic density 95, disc large and zona occludens-1 homologous protein domain; ADAM: A desintegrin and metallopeptidase; P: Phosphorylated tyrosine.
Table 1.
Biological effects of neuregulin-1/erbB in embryonic and postnatal cardiomyocytes
| Experimental system | Monitored response | Intracellular signal | Ref. |
| Cultured cells | |||
| NRG1 administration in neonatal cardiomyocytes | Sarcomeric F-actin polymerization | PI 3’-kinase | [16] |
| Myofibrillogenesis - Growth | Ras-Mek-erk1/2 | [17] | |
| Proliferation | PI 3’-kinase | [17] | |
| NOS activation | [42] | ||
| Muscarinic activation | [43] | ||
| Karyo- cytokinesis | [44] | ||
| NRG1 administration in human ESC | Specification of cardiomyocytes of the conduction lineage | [45] | |
| Animal models | |||
| NRG1b injection to ex vivo developing E12.5 dpc mouse | Differentiation of trabeculae | [17] | |
| DNA synthesis, Proliferation | PI 3’-kinase | [17] | |
| NRG1 administration in mouse with heart failure | Improved cardiac performance | [40] | |
| Hypomorphic NRG1 deficiency in E8.5dpc mouse | Destabilization of gene regulatory network in the left ventricle | Erk1/2 | [39] |
| Mouse ventricular-erbB2-KO | Overt dilation and reduced survival in erbb2 F/- postnatal mouse | [31] | |
| Mouse ventricular-erbB2-KO | Dilation with cellular apoptosis | [32] | |
| Mouse ventricular-erbB4-KO | Overt ventricular dilation at 3 mo and reduced survival | [33] | |
| Lapatinib inhibition of erbB1/erbB2 in Mouse | erbB2-physiologic hypertrophy in pregnancy | MEK1/erk1/2 | [34] |
| Adult ventricular-erbB4-KO | Cell division in myocardial infarction | [44] | |
Summary of cardiac activities of neuregulin-1 (NRG1)-erbB2/erbB4 signaling inferred from in vitro system and animal model studies. The NRG1 activities, -proliferation, myofibrillogenesis, ventricular remodeling, and repair-, were assessed based on the outcomes of the exogenous administration of NRG1 or by the complete or partial loss of erbB2/erbB4 signaling. NOS: Nitric oxide synthase; MEK1: Mitogen activated kinase erk kinase 1; ESC: Embryonic stem cells.
THE NRG1 INTRACELLULAR SIGNALING CASCADE
The erbB-dependent intracellular cascades have been extensively studied because of their important role in cancer cells, thereby providing a basis for analyses of signaling mechanisms in other cell types. The NRG activation of erbB receptors mediates the auto- and trans-phosphorylation of tyrosine residues at the receptor intracellular domain. A subgroup of phosphotyrosine residues bind specific adaptor molecules (e.g., Grb, Shc, Src, SH3 domain)[14], ultimately inducing intracellular pathways, e.g., MAP kinase and PI 3’-kinase cascades, PLC γ, the regulation of the Ca2+-dependent PKC and Nuclear Factor of Activated T cells activity (Figure 2)[13,15]. A link between NRG1 and focal adhesion kinase (FAK) has been observed in proliferative and migrating cells.
Figure 2.
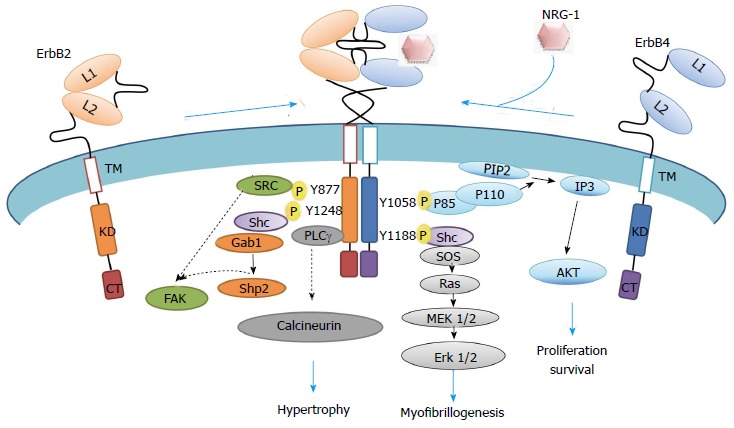
Representation of neuregulin-1-erbB2/erbB4 intracellular signaling cascade. Schematic representation of active ErbB2/ErbB4 heterodimers through phosphorylation, which phosphosites are docking sites for intracellular molecules involved in pathways that modulate myocyte biology. Specific non-phosphorylated residues interact to PDZ domain proteins. CT: Cytoplasmic tail; KD: Kinase domain; L: Ligand binding site; TM: Transmembrane domain; NRG-1: Neuregulin-1; PIP2: Phosphoinositol-2-phosphate; SOS: Son of sevenless; IP3: Inositol triphosphate; AKT: Thymoma viral oncogene homolog 1, a serine/threonine protein kinase; MEK: Mitogen activated kinase erk kinase; Shc: Src homology domain containing transforming protein; Shp: Protein tyrosine phosphatase; FAK: Focal adhesion kinase; Gab: Binding protein of growth factor bound protein Grb2; P: Phosphorylated tyrosine residues.
The Ras/MAPK/erk1/2 pathway was required for the NRG1-driven myofibrillogenesis in cultured cardiomyocytes. This activity was mimicked by a constitutively active form of Ras and inhibited by both its dominant negative form and the MEK1 inhibitor PD98059[16,17]. The NRG1-induced ability of cardiomyocytes to proliferate was manifested by its combined administration with Insulin-like growth factor I (IGF-I). The NRG1/IGF-I induction of cardiomyocyte DNA synthesis in both ex vivo embryonic development and cultured neonatal cardiomyocytes was prevented by wortmannin, an inhibitor of PI3’-Kinase. Cellular transfection with an adenovirus harboring a constitutively active Akt mimicked the proliferative and protective activities, which were inhibited by a dominant negative form of Akt in the presence of NRG1/IGF-I (Figure 2)[17].
Alternative pathways may be activated by the cross-communication of erbB2 and G protein coupled receptors. Pro-hypertrophic GPCR agonists (e.g., angiotensin II, endothelin I and isoproterenol) have been implicated in the transactivation of EGFR and erbB2, thereby inducing hypertrophic and survival stimuli in cardiac cells[18,19].
ErbB non-phosphorylated interactions
Intracellular signaling also depends on the binding ability of specific non-phosphorylated residues of erbB receptors to PDZ (postsynaptic density 95, discs large and Zonula occludens-1) domain-containing proteins. This interaction with PDZ domain proteins is relevant for the specific location of erbB proteins in particular membrane compartments and for the modulation of the receptor stability and activity[20]. Despite the significance of PDZ domain proteins in the heart (e.g., MAGUK, actinin binding proteins), there is not yet evidence for the specific PDZ-erbB-interacting proteins in cardiomyocytes. The erbB4 receptors, which are endocytosis-impaired, are also regulated through proteolysis, which is mediated by the proteasome system and the alternative transcriptional activity of the cleavable juxtamembrane isoform JMa[21,22]. These mechanisms either drive the erbB4 protein degradation or induce the nuclear translocation of the JMa intracellular domain. As occurs for the release of the NRG1 active peptides, the release of the erbB4 JMa C-terminal domain is modulated by the activation of PKC and cleaved by the activity of the tumor necrosis factor-alpha converting enzyme and of γ-secretase at the plasma membrane[23]. In the heart, the identified erbB4 protein in cardiomyocytes is the JMb non-cleavable splice variant[24], which may be proteolytically modulated by the proteasome system. Three PPXY motifs couple erbB4 with WW domain proteins, such as Wwox and ubiquitin ligases, thereby either modulating the transcriptional activity of the c-terminal domain when translocated into the nucleus or promoting the isoform degradation[25]. Of the two cytoplasmic splice variants CYT1 and CYT2, CYT1 mediates specific interactions with SH2 and WW binding domain proteins (e.g., PI 3’-kinase, ubiquitin ligases)[26].
Interestingly, erbb4 polymorphisms and splicing variants of the erbb4 gene and, more recently, of Nrg-1 and erbb2 have been linked to the pathogenesis of non-neoplastic disorders[27-30]. However, further experimentation is still needed to address the regulation and specific functions of isoforms in cardiovascular, psychiatric, and other diseases.
ERBB REQUIREMENT IN POSTNATAL AND ADULT HEART
The clinical implication of the NRG1 signaling in cardiology results from the increased incidence of dilated cardiomyopathies in a subpopulation of breast tumor patients undergoing the combined administration of anthracycline chemotherapy and humanized antibodies against erbB2 protein[4]. The cardiac effect of the antibodies (i.e., trastuzumab, pertuzumab), which are species specific and do not cross-react with the mouse protein, were experimentally assessed through the ventricular cardiomyocyte-specific deletion of either the Erbb2 or the Erbb4 gene in mice. A ventricular-specific mutation in either of these genes caused dilated cardiomyopathy during adulthood (Figure 3)[31-33]. These murine models were useful for demonstratingthe cardiomyocyte-autonomous requirement of erbB2/erbB4 during the postnatal remodeling of the myocardium. In agreement with the role of the NRG1-erbB2/erbB4 pathway to prevent ventricular dilation during remodeling, the lapatinib-mediated inhibition of erbB2 phosphorylation in mice resulted in a pathological pregnancy-related dilation of the ventricular chambers that occurred without apparent apoptotic cell death (Table 1)[34].
Figure 3.
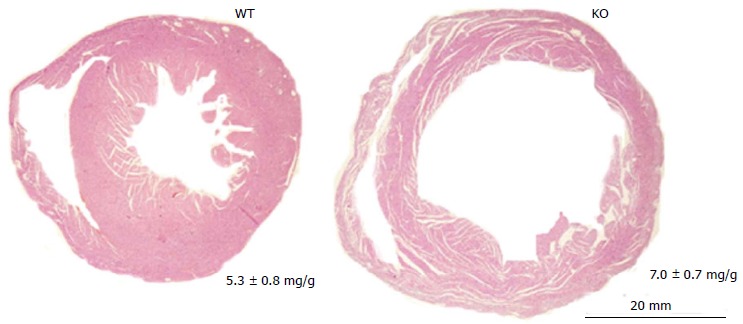
Ventricular specific erbB4-knockout leads to adult dilated cardiomyopathy. Representative image of transverse ventricular sections stained with hematoxilin-eosin. Camber dilation is overt in mouse erbB4-KO hearts in the adulthood. WT: Wild type; KO: Knock-out.
The association of changes in the NRG1/erbB pathway with disease was also suggested by the downregulation of both erbB2 and erbB4 expression during the pathologic remodeling of the myocardium in rodents under pressure overload and in humans with a failing myocardium[35]. However, it is plausible that a different isoform than the normally expressed JMb could be expressed in the failing myocardium. In this regard, the human erbB4 JMa CYT1 isoform manifested a similar activity to the endogenous erbB4 JMb isoform in cardiac morphogenesis in transgenic mice[36]. A different role than the classical activity of transmembrane tyrosine kinase receptor is displayed by the cleavable erbB4 isoform, which acts as a transcriptional co-activator or co-repressor[37]. The nuclear localization of the full-length erbB4 protein has recently been observed in cultured adult rat cardiomyocytes under the stress conditions of cell isolation, and the protein was suggested to participate in DNA damaging processes[38].
Subcellular localization of erbB2/erbB4
Clues about a local cardiomyocyte effect of NRG1 on the maintenance of the myocardial architecture may arise from the subcellular localization of the receptors. The erbB2/erbB4 proteins accumulated within the T-tubule membrane system of cardiomyocytes and the intercalated discs (ID) (Figure 4)[33].
Figure 4.
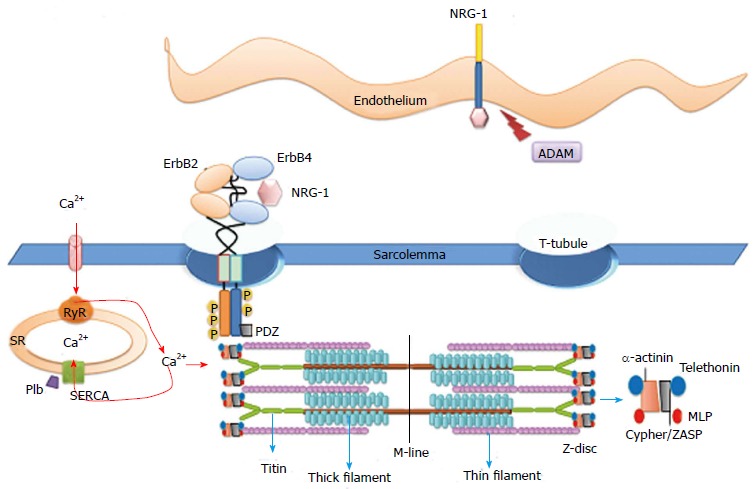
Functional interaction of molecules placed at the T-tubules. The ErbB2 and ErbB4 proteins are localized to the T-tubules. This compartment of the sarcolemma provides specific sites for functional interactions with molecules at the sarcoplasmic reticulum and at the myofibril Z-band. Molecular interactions at the T-tubules and at the intercalated discs provide the electric-contractile coupling of the myocardium. Scheme of the sarcomeric units of thin and thick filaments are anchored by titin and actin at the Z-band via α-actinin scaffold protein complex. Muscle LIM domain protein (MLP), telethonin (T-cap) and PDZ and LIM domain protein (ZASP). PDZ: Postsynaptic density 95, disc large and zonula occludens-1; ADAM: A desintegrin and metallopeptidase; SERCA: Sarcoplasmic reticulum calcium ATPase; Plb: Phospholamban; RyR: Ryanodine receptor; SR: Sarcoplasmic reticulum.
The relevance of the receptor localization is that the T-tubules place membrane molecules in close apposition with the myofibril Z-disc, thereby providing a specific context for the functional interactions among molecules at the plasma membrane, sarcoplasmic reticulum (SR) and the myofibril Z-disc. The ID are highly specialized Z-disc structures at the cell-cell contact that provide the anchorage of the sarcomeres to the membrane and place the connexin-43 gap junctions, assuring electric transmission and rhythmic contraction of cardiomyocytes.
Concerning the erbB subcellular context, it is speculated that NRG1 may act on cues at the cytoskeletal pathways that are required for myocardial remodeling. A connection to the cytoskeleton may be required for the feedback modulation of NRG1 signaling with wall stress and contractile parameters during cardiac chamber morphogenesis[39].
CARDIOPROTECTIVE AND REGENERATIVE ACTIVITIES
The in vivo administration of NRG1, under different conditions of mouse cardiac pathology, contributed to the amelioration of ventricular contraction (e.g., reduced left ventricular end systolic dimensions, increased ejection fraction). In these experiments, the NRG1-mediated functional performance of the ventricular chambers was correlated with the increased phosphorylation of the myosin regulatory light chain (RLC) (Table 1)[40]. However, the administration of NRG1 in knockout mice for the myosin light chain kinase (Mlck) gene improves cardiac function without increasing the phosphorylation level of RLC[41], thus implicating additional mechanisms for contractile improvement.
In cultured cardiomyocytes, NRG1 exerts a negative inotropic effect through either NOS activity or the activation of the muscarinic response (Table 1)[42,43]. Both nitric oxide and active muscarinic receptors modulate the inotropic response to beta-adrenergic stimulation, which may result in an improved fractional shortening. Indeed, there is a general lack of evidence for either a direct NRG1-mediated inotropic effect or an induced change in calcium handling or in myofilament calcium sensitivity that may provide a molecular explanation for the NRG1-mediated enhancement of cardiac systolic function.
Additional repair activities have been suggested through the induced proliferation of cardiomyocytes in a murine model of cardiac infarction. In this setting, NRG-1/erbB4 was shown to induce mononucleated cardiomyocytes to proliferate, thereby contributing to the cardiac repair mechanisms in one-week-old myocardial infarction without affecting the level of apoptotic cell death (Table 1)[44]. This activity is particularly interesting for the renewed research of the cardiomyocytes’ re-entry into the cell cycle and the use of stem cells to re-populate the injured myocardium[45-47]. In this regard, pluripotent cells may also repair damage myocardial areas through the secretion of relevant growth factors[48].
The evidence for the essential role of NRG1-erbB2/erbB4 prompted an evaluation of the safety and efficacy of NRG1 administration in patients with chronic heart failure and focal ischemia (Phase I NCT01258387, Phase III NCT01439893, NCT01541202 trials)[49,50]. The panel of activities displayed upon NRG1 administration has been well reviewed[51] and indicates a pleiotropic effect on cardiac muscle and vascular cells[51]. The ultimate contribution of each identified NRG1-mediated activity on cardiac performance remains to be defined. Moreover, the employment of NRG1 for the treatment of cardiac dysfunction still requires a mechanistic understanding of how this signal exerts its effect as well as which critical molecular targets of this pathway affect cardiac remodeling.
MECHANISMS OF CARDIOTOXICITY: THE ROLE OF NRG1/ERBB SIGNALING
The evidence of the critical activity of NRG1 towards anthracycline cardiotoxicity led to improvements in the clinical management of administering chemotherapy and antibodies against erbB2. Further investigation is required to understand the molecular link of a NRG1 deficiency and exacerbated cardiotoxicity. The individual therapies with either antibodies against erbB2 or anthracyclines exert a cardiotoxic effect at a lower incidence rate compared to the combined treatment. Chemotherapy with anthracycline derivatives may result in both immediate and delayed cardiomyocyte toxic events. The induction of the oxidative stress response that underlies anthracycline cardiotoxicity has been related to cellular apoptosis and necrosis as the mechanism of toxicity. However, employing antioxidants in radiation- or chemotherapy-treated patients resulted in an unclear improvement in cardiac function. These results led to the conclusion that oxidative stress may be viewed as a two-way process by which radical oxygen species (ROS) mediate tumor cell death and promote cell survival through the degradation of anthracyclines[52]. In addition, clinical studies aimed to reduce the oxidative and inflammatory process during ischemic dilated cardiomyopathy and chronic heart failure have not yet provided a therapeutic strategy because of dissimilar explanations among the trial results[53]. It is therefore likely that ROS-independent mechanisms may play a more important role in the doxorubicin-induced myocardial damage than previously evaluated[54].
Direct evidence for the role of NRG1 against anthracycline cardiotoxicity was provided by the protective activity of NRG1 against doxorubicin-mediated myofibril disarray and in preventing the toxic degradation of troponins in cultured cardiomyocytes (Table 2)[55,56]. The cardioprotective activity of NRG1 was also inferred in vivo by the doxorubicin-aggravated contractile dysfunction in heterozygous NRG1 mutant mice and by the exacerbated cardiac chamber dilation in the ventricular-specific erbB4-knockout mice (Table 2)[57,58].
Table 2.
Biological effects of combined neuregulin-1/erbB signaling and anthracyclines
| Experimental system | Monitored response | Ref. |
| Cultured cardiomyocytes | ||
| NRG-1 administration | Prevented myofibril disarray | [55] |
| Attenuated troponin degradation | [56] | |
| Animal models | ||
| NRG1-deficiency/doxorubicin (NRG1 heterozygous mouse) | Induced heart failure | [57] |
| NRG1-deficiency/doxorubicin (Ventricular specific-erbB4-KO) | Deregulated Protein Homeostasis Autophagic vacuolization | [58] |
Summary of effects mediated by anthracycline-induced toxicity and by neuregulin-1 (NRG1)-erbB2/erbB4 signaling in in vitro system and animal models. The NRG-1 activities on cardiomyocyte survival, myofibril organization and ventricular homeostasis were assessed based on the exogenous administration of NRG1 or by the outcome of an impaired signaling as discussed throughout the article.
The serum level of both the cardiac troponins (e.g., cTnI) and brain natriuretic peptide (BNP) are relevant markers for the follow-up of acquired cardiac pathology in trastuzumab- and anthracycline-treated patients[59,60]. Indeed, the detection of the cTnI and BNP serum levels was useful for validating the exacerbated cardiotoxicity displayed by an injection of doxorubicin in the ventricular-specific erbB4-knockout mouse. The hypertrophic hallmark, i.e., the natriuretic peptide of the atrial type, was expressed at a relatively low level in doxorubicin-treated wildtype (WTD) animals and at intermediate levels in the doxorubicin-treated erbB4-KO (KOD), compared to the robust expression in erbB4-KO[33,58]. The expression of BNP was at a similar level in the erbB4-KO and in both WTD and KOD mice compared to the wildtype. A contrasting finding between WTD and treated KOD mice was the differential expression of both a group of genes related to oxidative stress and molecules of the apoptotic-death pathway that underlined the WTD (Table 3)[58]. The administration of doxorubicin to erbB4-KO mice led to the synergistic deregulation of the ubiquitin-proteasome system. The observed downregulation of the IGF-I/PI3’-Kinase axis, which may respond to lower levels of PPAR[61] or to a deficient activity of CREB under the control of NRG1[62], may represent a potential mechanism acting on the deregulation of the ubiquitin-proteasome system in erbB4-KOD hearts.
Table 3.
Cardiac phenotypic modifications
| Morphology | WT | KO | WTD | KOD |
| Young adult (1 mo) | ||||
| Heart/body weight (mg/g) | 5.2 ± 0.4 | 5.3 ± 0.6 | 5.2 ± 0.5 | 6.2 ± 0.8a |
| Body weight (g) | 17.4 ± 2.0 | 17.3 ± 2.2 | 17.3 ± 2.0 | 17.2 ± 1.5 |
| Adult (3 mo) | ||||
| Heart/body weight (mg/g) | 5.6 ± 0.6 | 7.0 ± 0.8d | 5.4 ± 0.7 | 6.7 ± 0.8d |
| Body weight (g) | 30.1 ± 2.5 | 30.3 ± 2.2 | 29.4 ± 2.2 | 28.4 ± 2.2 |
| Cardiotoxic gene groups | ||||
| Dilation (ratio) | - | 3 | 1.8 | 2.8 |
| Hypertrophy (ratio) | - | 9.9 | 3.3 | 6.5 |
| Damage (ratio) | - | 3.7 | 3.7 | 3.6 |
| Cell death/ necrosis (Ratio) | - | 3.0 | 4.3 | 3.1 |
| Measured activity | ||||
| Caspase 3 (arbitrary units) | 0.25 ± 0.17 | 0.44 ± 0.17 | 0.84 ± 0.09a | 0.35 ± 0.07 |
| Autophagic vacuolization (n°) | 0.07 ± 0.1 | 0.35 ± 0.3 | 0.2 ± 0.2 | 2.4 ± 2.1a |
| Serum cTnI (mg/mL) | 0.15 ± 0.1 | 3.6 ± 1.5 | 0.7 ± 0.2 | 14.5 ± 4.2a |
| LVDP (mmHg) | 109.2 ± 7.1 | 42 ± 8.2a | 94.5 ± 5.5 | 31.5 ± 5.4a |
| Tau ½ | 37.4 ± 1.9 | 38.1 ± 4.9 | 36.1 ± 3.1 | 34.6 ± 2.9 |
Morphological and biochemical modifications studied in the aggravated cardiotoxic condition of the doxorubicin-treated-ventricular specific erbB4-KO mouse model. Summary of hypertrophic-associated morphological changes determined in mouse at the age of 1 and 3 mo, group of differentially expressed genes clustered into a wide-range of cardiotoxic conditions, biochemical activities associated to cell death and of physiological systolic and diastolic parameters are represented as discussed in the text.
P < 0.05 vs WT,
P < 0.01 vs WT and WTD. WTD: Doxorubicin-treated wildtype; KOD: Doxorubicin-treated erbB4-KO; WT: Treated wildtype.
The deregulated group of genes of the ubiquitin-proteasome system was characterized by the upregulation of ubiquitin-ligase, which induced large protein aggregates in cardiomyocytes within the doxorubicin-treated erbB4-KO. Autophagic vacuolization, which is the recommended term for the cellular appearance of large ubiquitin-positive protein aggregates[63], resulted in a 7-fold increase of affected cardiomyocytes relative to the non-treated erbB4-KO (Table 3). The perturbation of the ubiquitin-proteasome system induced by a genetic modification in mice led to cardiomyocyte necrosis and cardiac chamber dilation[64]. Moreover, the level of protein ubiquitination was documented as a useful predictor of myocardial deterioration in patients to follow cardiac transplantation[65]. The monitoring of necrotic cardiomyocytes, by the determination of the cTnI serum level, is a highly sensitive cardiotoxic marker that is employed in the follow-up of breast tumor patients undergoing trastuzumab and chemotherapy[59,60].
A search for biomarkers in the early detection of doxorubicin cardiotoxicity in both heart and peripheral blood mononuclear cells through the determination of differential gene expression indicated that the most significant group of genes was represented by changes in the canonical NRF2-oxidative stress response pathway, protein ubiquitination and the PI3’K/AKT signaling pathway[66]. Collectively, these results extend our current knowledge by demonstrating that the impaired NRG1 response in erbB4-KO hearts to doxorubicin toxicity has a net result of the induced autophagic vacuolization of cardiomyocytes, which is consistent with the association of abnormal protein homeostasis with a severe cardiac disorder.
CONCLUSION
The NRG1/erbB pathway is critical for the maintenance of the myocardial structure in the adult heart, and moreover, impaired NRG1 signaling exacerbates anthracycline-mediated cardiotoxicity. The accumulated evidence indicates that NRG1 displays a panel of protective and repair activities in the heart during the lifespan of an individual. In this context, an impaired NRG1 signaling sensitizes the heart to the toxicity of anthracycline. There is an ongoing search for drugs and immunotherapies that can inhibit the erbB receptors implicated in tumorigenesis, which may also display an iatrogenic effect in the heart. The individual treatment with either anthracycline derivatives or the induced deficiency in the NRG1 pathway displayed different gene expression profiles in experimental murine models. The doxorubicin-treated hearts were characterized by an oxidative stress response, which may induce cardiomyocyte apoptosis. The sensitization of the NRG1-deficient heart to the anthracycline toxicity resulted in a potentiated deregulation of the ubiquitin-proteasome system, with a net result of the autophagic vacuolization of cardiomyocytes.
Altogether, the NRG1 activities that affect the myocardial architecture and homeostasis await a mechanistic understanding of how NRG1 modulates remodeling and thereby prevents ventricular dilation. Continuous research in this area will provide critical molecules and targets that may help in the design of diagnostic tools and therapeutics.
Footnotes
P- Reviewer: Anan R, Carbucicchio C S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
Supported by National Research Council of Argentina, CONICET PIP N° 0722
References
- 1.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013;123:19–26. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baselga J. New horizons: gene therapy for cancer. Anticancer Drugs. 1999;10 Suppl 1:S39–S42. [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Popat S, Smith IE. Therapy Insight: anthracyclines and trastuzumab--the optimal management of cardiotoxic side effects. Nat Clin Pract Oncol. 2008;5:324–335. doi: 10.1038/ncponc1090. [DOI] [PubMed] [Google Scholar]
- 6.Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: importance of heterogeneity [corrected] Nat Rev Clin Oncol. 2010;7:139–147. doi: 10.1038/nrclinonc.2009.234. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 9.Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 10.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 11.Qi B, Newcomer RG, Sang QX. ADAM19/adamalysin 19 structure, function, and role as a putative target in tumors and inflammatory diseases. Curr Pharm Des. 2009;15:2336–2348. doi: 10.2174/138161209788682352. [DOI] [PubMed] [Google Scholar]
- 12.Esper RM, Loeb JA. Neurotrophins induce neuregulin release through protein kinase Cdelta activation. J Biol Chem. 2009;284:26251–26260. doi: 10.1074/jbc.M109.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 14.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005.0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–H2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 17.Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J Biol Chem. 1999;274:37362–37369. doi: 10.1074/jbc.274.52.37362. [DOI] [PubMed] [Google Scholar]
- 18.Shah BH, Catt KJ. A central role of EGF receptor transactivation in angiotensin II -induced cardiac hypertrophy. Trends Pharmacol Sci. 2003;24:239–244. doi: 10.1016/S0165-6147(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 19.Chung KY, Walker JW. Interaction and inhibitory cross-talk between endothelin and ErbB receptors in the adult heart. Mol Pharmacol. 2007;71:1494–1502. doi: 10.1124/mol.106.027599. [DOI] [PubMed] [Google Scholar]
- 20.Carraway KL, Sweeney C. Localization and modulation of ErbB receptor tyrosine kinases. Curr Opin Cell Biol. 2001;13:125–130. doi: 10.1016/s0955-0674(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 21.Carraway KL. E3 ubiquitin ligases in ErbB receptor quantity control. Semin Cell Dev Biol. 2010;21:936–943. doi: 10.1016/j.semcdb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veikkolainen V, Vaparanta K, Halkilahti K, Iljin K, Sundvall M, Elenius K. Function of ERBB4 is determined by alternative splicing. Cell Cycle. 2011;10:2647–2657. doi: 10.4161/cc.10.16.17194. [DOI] [PubMed] [Google Scholar]
- 23.Ju CR, Xia XZ, Chen RC. Expressions of tumor necrosis factor-converting enzyme and ErbB3 in rats with chronic obstructive pulmonary disease. Chin Med J (Engl) 2007;120:1505–1510. [PubMed] [Google Scholar]
- 24.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 25.Sundvall M, Korhonen A, Paatero I, Gaudio E, Melino G, Croce CM, Aqeilan RI, Elenius K. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc Natl Acad Sci USA. 2008;105:4162–7. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muraoka-Cook RS, Sandahl MA, Strunk KE, Miraglia LC, Husted C, Hunter DM, Elenius K, Chodosh LA, Earp HS. ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Mol Cell Biol. 2009;29:4935–4948. doi: 10.1128/MCB.01705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 28.McBride KL, Zender GA, Fitzgerald-Butt SM, Seagraves NJ, Fernbach SD, Zapata G, Lewin M, Towbin JA, Belmont JW. Association of common variants in ERBB4 with congenital left ventricular outflow tract obstruction defects. Birth Defects Res A Clin Mol Teratol. 2011;91:162–168. doi: 10.1002/bdra.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huertas-Vazquez A, Teodorescu C, Reinier K, Uy-Evanado A, Chugh H, Jerger K, Ayala J, Gunson K, Jui J, Newton-Cheh C, et al. A common missense variant in the neuregulin 1 gene is associated with both schizophrenia and sudden cardiac death. Heart Rhythm. 2013;10:994–998. doi: 10.1016/j.hrthm.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemieux J, Diorio C, Côté MA, Provencher L, Barabé F, Jacob S, St-Pierre C, Demers E, Tremblay-Lemay R, Nadeau-Larochelle C, et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013;33:2569–2576. [PubMed] [Google Scholar]
- 31.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hübner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 33.García-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300:H931–H942. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 35.Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium -- depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 36.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundvall M, Korhonen A, Vaparanta K, Anckar J, Halkilahti K, Salah Z, Aqeilan RI, Palvimo JJ, Sistonen L, Elenius K. Protein inhibitor of activated STAT3 (PIAS3) protein promotes SUMOylation and nuclear sequestration of the intracellular domain of ErbB4 protein. J Biol Chem. 2012;287:23216–23226. doi: 10.1074/jbc.M111.335927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Icli B, Bharti A, Pentassuglia L, Peng X, Sawyer DB. ErbB4 localization to cardiac myocyte nuclei, and its role in myocyte DNA damage response. Biochem Biophys Res Commun. 2012;418:116–121. doi: 10.1016/j.bbrc.2011.12.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai D, Liu X, Forrai A, Wolstein O, Michalicek J, Ahmed I, Garratt AN, Birchmeier C, Zhou M, Hartley L, et al. Neuregulin 1 sustains the gene regulatory network in both trabecular and nontrabecular myocardium. Circ Res. 2010;107:715–727. doi: 10.1161/CIRCRESAHA.110.218693. [DOI] [PubMed] [Google Scholar]
- 40.Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, Yan W, Wang W, Pan J, Xu Y, et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res. 2010;88:334–343. doi: 10.1093/cvr/cvq223. [DOI] [PubMed] [Google Scholar]
- 41.Chang AN, Huang J, Battiprolu PK, Hill JA, Kamm KE, Stull JT. The effects of neuregulin on cardiac Myosin light chain kinase gene-ablated hearts. PLoS One. 2013;8:e66720. doi: 10.1371/journal.pone.0066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 43.Okoshi K, Nakayama M, Yan X, Okoshi MP, Schuldt AJ, Marchionni MA, Lorell BH. Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation. 2004;110:713–717. doi: 10.1161/01.CIR.0000138109.32748.80. [DOI] [PubMed] [Google Scholar]
- 44.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 45.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933–942. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- 47.Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang H, Kloner RA. The combined administration of multiple soluble factors in the repair of chronically infarcted rat myocardium. J Cardiovasc Pharmacol. 2011;57:282–286. doi: 10.1097/FJC.0b013e3182058717. [DOI] [PubMed] [Google Scholar]
- 49.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Iaci JF, Ganguly A, Finklestein SP, Parry TJ, Ren J, Saha S, Sietsma DK, Srinivas M, Vecchione AM, Caggiano AO. Glial growth factor 2 promotes functional recovery with treatment initiated up to 7 days after permanent focal ischemic stroke. Neuropharmacology. 2010;59:640–649. doi: 10.1016/j.neuropharm.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 53.Sawyer DB. Oxidative stress in heart failure: what are we missing? Am J Med Sci. 2011;342:120–124. doi: 10.1097/MAJ.0b013e3182249fcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res. 2013;97:77–87. doi: 10.1093/cvr/cvs309. [DOI] [PubMed] [Google Scholar]
- 55.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 56.Bian Y, Sun M, Silver M, Ho KK, Marchionni MA, Caggiano AO, Stone JR, Amende I, Hampton TG, Morgan JP, et al. Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins. Am J Physiol Heart Circ Physiol. 2009;297:H1974–H1983. doi: 10.1152/ajpheart.01010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, Ho KK, Manning WJ, Marchionni MA, Lorell BH, et al. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H660–H666. doi: 10.1152/ajpheart.00268.2005. [DOI] [PubMed] [Google Scholar]
- 58.Vasti C, Witt H, Said M, Sorroche P, García-Rivello H, Ruiz-Noppinger P, Hertig CM. Doxorubicin and NRG-1/erbB4-Deficiency Affect Gene Expression Profile: Involving Protein Homeostasis in Mouse. ISRN Cardiol. 2012;2012:745185. doi: 10.5402/2012/745185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 60.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.el Azzouzi H, Leptidis S, Bourajjaj M, Armand AS, van der Nagel R, van Bilsen M, Da Costa Martins PA, De Windt LJ. Peroxisome proliferator-activated receptor (PPAR) gene profiling uncovers insulin-like growth factor-1 as a PPARalpha target gene in cardioprotection. J Biol Chem. 2011;286:14598–14607. doi: 10.1074/jbc.M111.220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herndon CA, Ankenbruck N, Lester B, Bailey J, Fromm L. Neuregulin1 signaling targets SRF and CREB and activates the muscle spindle-specific gene Egr3 through a composite SRF-CREB-binding site. Exp Cell Res. 2013;319:718–730. doi: 10.1016/j.yexcr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res. 2011;108:40–50. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vigliano CA, Cabeza Meckert PM, Diez M, Favaloro LE, Cortés C, Fazzi L, Favaloro RR, Laguens RP. Cardiomyocyte hypertrophy, oncosis, and autophagic vacuolization predict mortality in idiopathic dilated cardiomyopathy with advanced heart failure. J Am Coll Cardiol. 2011;57:1523–1531. doi: 10.1016/j.jacc.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 66.Todorova VK, Beggs ML, Delongchamp RR, Dhakal I, Makhoul I, Wei JY, Klimberg VS. Transcriptome profiling of peripheral blood cells identifies potential biomarkers for doxorubicin cardiotoxicity in a rat model. PLoS One. 2012;7:e48398. doi: 10.1371/journal.pone.0048398. [DOI] [PMC free article] [PubMed] [Google Scholar]


