Key Points
The anti-β2GP1 autoantibody/β2GP1 complex binds to the platelet thrombus, amplifying platelet activation.
Platelets are required for enhanced activation of the endothelium and fibrin generation by the anti-β2GP1 autoantibody/β2GP1 complex.
Abstract
Antiphospholipid syndrome (APS) is defined by thrombosis, fetal loss, and the presence of antiphospholipid antibodies, including anti–β2-glycoprotein-1 autoantibodies (anti-β2GP1) that have a direct role in the pathogenesis of thrombosis in vivo. The cellular targets of the anti-β2GP1 autoantibody/β2GP1 complex in vivo were studied using a laser-induced thrombosis model of APS in a live mouse and human anti-β2GP1 autoantibodies affinity-purified from APS patients. Cell binding of fluorescently labeled β2GP1 and anti-β2GP1 autoantibodies revealed their colocalization on the platelet thrombus but not the endothelium. Anti-β2GP1 autoantibodies enhanced platelet activation, monitored by calcium mobilization, and endothelial activation, monitored by intercellular adhesion molecule-1 expression. When eptifibatide was infused to block platelet thrombus formation, enhanced fibrin generation and endothelial cell activation were eliminated. Thus, the anti-β2GP1 autoantibody/β2GP1 complex binds to the thrombus, enhancing platelet activation, and platelet secretion leads to enhanced endothelium activation and fibrin generation. These results lead to a paradigm shift away from the concept that binding of the anti-β2GP1 autoantibody/β2GP1 complex activates both endothelial cells and platelets toward one in which activation of platelets in response to anti-β2GP1 autoantibody/β2GP1 complex binding leads to subsequent enhanced endothelium activation and fibrin generation.
Introduction
Antiphospholipid syndrome (APS) is characterized by venous or arterial thrombosis and/or pregnancy morbidity and is associated with circulating antiphospholipid (aPL) autoantibodies.1-3 These antibodies, including anti–β2-glycoprotein-1 (anti-β2GP1) autoantibodies, recognize plasma proteins that bind to anionic phospholipids, among which β2GP1 is the major target.4 Antibodies directed against β2GPI5,6 are associated with thrombotic events in APS. Anti-β2GP1 autoantibodies from patients with APS and thrombosis enhance arterial thrombus formation after injury in a mouse model of APS,7 with dramatic increases in platelet thrombus size and fibrin generation.
The mechanisms leading to thrombosis in APS are unresolved. In vitro and in vivo studies using animal models demonstrated that aPL antibodies interact with endothelial cells and monocytes to increase tissue factor expression and complement activation and proinflammatory cytokines.8,9 In vitro, platelet activation occurs after the binding of complexes of anti-β2GP1 antibodies and dimerized β2GP1 to GPIbα and ApoER2.10-12 Furthermore, APS patients exhibit markers of platelet activation.13 The conventional understanding is that the anti-β2GP1/β2GP1 complex binds to receptors on both the endothelial cell and the platelet, leading to their activation. However, which cells are the targets of anti-β2GP1 antibody/β2GP1 complexes in a live animal and which interactions are pathologic in vivo are not known.
To amplify initial thrombus formation, aPL have to (1) bind to target cells; (2) activate those cells; and (3) facilitate intercellular and intermolecular interactions required for thrombus development. To identify the cell against which the anti-β2GP1 autoantibody/β2GP1 complexes in vivo is directed, we examined anti-β2GP1 autoantibody and β2GP1 binding to the vessel wall in a mouse after injury using intravital microscopy. Enhanced platelet activation by anti-β2GP1 autoantibodies was monitored by intracellular calcium mobilization. Enhanced endothelial cell activation was monitored by intercellular adhesion molecule-1 (ICAM-1) expression in the presence or absence of platelets and by calcium mobilization in the absence of platelets. We observe that, in vivo, the anti-β2GP1 autoantibody/β2GP1 complex binds to platelets but not the endothelium; that anti-β2GP1 autoantibodies induce increased activation of thrombus-associated platelets; and that enhanced platelet activation leads to enhanced activation of the endothelium and fibrin generation. In the absence of a platelet thrombus, there is no enhancement of endothelial cell activation or fibrin generation by anti-β2GP1 autoantibodies. These results lead to a paradigm shift from the concept that binding of the anti-β2GP1 autoantibody/β2GP1 complex activates both endothelial cells and platelets toward one in which activation of platelets in response to anti-β2GP1 autoantibody/β2GP1 complex binding leads to subsequent enhanced endothelial cell activation and fibrin generation.
Methods
Patient sera
APS patients were diagnosed14 based on a history of thrombosis and anti-cardiolipin antibodies or anti-β2GP1 (Table 1; see supplemental Figure 1 on the Blood Web site). Anti-β2GP1 autoantibodies were isolated using β2GP1–agarose7 and F(ab′)2 fragments prepared. Immunoglobulin G (IgG) from patients and normal subjects and anti-β2GP1 IgG purified from patients were assayed for anti-cardiolipin and anti-β2GP1 (INOVA). These purified anti-β2GPI antibodies used for these experiments express anti-cardiolipin, anti-β2GPI activity, and lupus anticoagulant activity measured by the dilute Russell's viper venom time. None of the APL serologic properties was lost during purification. This study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Clinical features of APS patients
| P1 | P2 | P3 | P4 | P5 | Control | |
|---|---|---|---|---|---|---|
| Thrombotic events | Arterial and venous | Stroke | Arterial and venous | Stroke | Stroke | None |
| Lupus anticoagulant | + | + | ND | ND | + | — |
| Anti-β2GP1 IgG (SGU) | 199 | 197 | 140 | 23 | 186 | <5 |
Clinical features of patients and healthy individuals (controls).
ND, not determined; P, patient number; SGU, standard IgG units.
Mice
Wild-type C57BL/6J mice were from Jackson Laboratory. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved animal care and procedures.
Reagents
Platelets were imaged in vivo using anti-CD42 antibodies conjugated with Dylight 488 or Dylight 649 (Emfret Analytics). This antibody, directed against GPIβ, whereas von Willebrand factor binds to GPIα, does not interfere with thrombus formation (supplemental Figure 2). Fibrin was detected using a mouse anti-human fibrin monoclonal antibody (clone 59D8) that cross-reacts with mouse fibrin but not fibrinogen. ICAM-1 was detected using a rat anti-mouse ICAM-1 monoclonal antibody (YN1/1.74) (Southern Biotech). Eptifibatide (Bachem); Fluo-4-AM, fura-2-AM, and Alexa Fluors (Invitrogen); Cremophore and human serum albumin (HSA; Sigma-Aldrich); and β2GP1 (Meridian) were available commercially.
Intravital microscopy
Intravital widefield microscopy of the cremaster muscle microcirculation was performed15,16 with modifications. Digital images were captured with a C9300-201 CCD digital camera (Hamamatsu) connected to a VS4-1845 GEN III image intensifier (VideoScope) or an Orca Flash 4.0v2 CMOS camera (Hamamatsu). The Olympus microscope includes a Yokogawa CSU-X1 A1 confocal scanner. The laser source, housed in an acousto-optical tunable filter launch (Intelligent Imaging Innovations), includes 3 solid-state lasers (Cobolt and Coherent). Vessel injury was induced with a Micropoint Laser System (Photonics).15 Image analysis was performed with Slidebook, Version 5.5 (Intelligent Imaging Innovations).16
Platelet thrombus size, fibrin generation, and ICAM-1 expression during thrombus formation
Platelet accumulation, fibrin generation, and ICAM-1 expression were evaluated in vivo using rat IgG against the GPIbβ subunit of the murine GPIb-V-IX complex labeled with Dylight 649 to identify platelets; anti–fibrin-Alexa 488 to visualize fibrin; anti–ICAM-1-Alexa 488 to visualize ICAM-1. Platelet thrombus size, fibrin generation, and ICAM-1 quantitation were determined by calculating median values of the area under the curves for fluorescence at 649 or 488 nm vs time. The increase induced by these antibodies is expressed as a ratio of the median values of the integrated area under the curves after and before infusion of antibodies.
Anti-β2GP1 autoantibody and β2GP1 binding during thrombus formation
The binding of fluorescently labeled anti-β2GP1 autoantibodies to the developing thrombus was examined using anti-β2GP1 F(ab′)2-Alexa 488 and β2GP1-Alexa 647. Control F(ab′)2-Alexa 488 and HSA-Alexa 647 were employed as controls. Platelets were labeled with anti-CD42 antibody conjugated with Dylight 649 or Dylight 488. The kinetics of anti-β2GP1 F(ab′)2, control F(ab′)2, β2GP1, and HSA binding and platelet accumulation at the site of injury were determined from median fluorescence values.
Platelet activation of thrombus-bound platelets in vivo
Platelet activation in mice was imaged using fura-2-AM.17 Infusion of fura-2-AM–loaded platelets in a recipient mouse allowed recording of both platelet accumulation, monitored after excitation at 380 nm, and calcium mobilization, monitored after excitation at 340 nm.
Endothelial cell activation in the absence of platelet thrombus formation
Endothelial cell activation was monitored in vivo after vessel injury using Fluo-4-AM18 following infusion of eptifibatide to block platelet thrombus formation. Fluo-4-AM was infused into the mouse circulation via the femoral artery. Platelet aggregation was inhibited by infusion of eptifibatide every 15 minutes. Changes to endothelial calcium level were observed by excitation at 488 nm. The kinetics of endothelial cell activation at the site of laser-induced injury were determined by calculating median fluorescence values.
Results
Binding of anti-β2GP1 autoantibodies and β2GP1 to vascular cells
In in vitro experiments, anti-β2GP1 autoantibodies, in complex with β2GP1, have been shown to interact with multiple cell types, including monocytes,19,20 endothelial cells,20-22 and platelets.10-12,23 We focused on determining the cell responsible for enhanced thrombus formation in vivo. To identify the vascular cells targeted by β2GP1/anti-β2GP1 complexes in vivo, we examined the binding of fluorescently labeled human anti-β2GP1 autoantibodies isolated from patients with APS and fluorescently labeled β2GP1 in a mouse model of APS using intravital microscopy.
In the absence of vascular injury, we have previously demonstrated that anti-β2GP1 autoantibodies do not lead to thrombus formation in vivo.7 If anti-β2GP1 autoantibodies are infused into a mouse before vascular injury, we observed no evidence of activation of the endothelium, as monitored by ICAM-1 expression (data not shown). Similarly, the addition of human anti-β2GP1 F(ab′)2 and control F(ab′)2 to mouse platelets in vitro did not lead to platelet activation, as monitored by P-selectin expression (data not shown).
Binding of human anti-β2GP1 autoantibodies to the developing platelet thrombus in vivo.
Both human anti-β2GP1 IgG and anti-β2GP1 F(ab′)2 bound to the developing thrombus, and both yielded similar results. To minimize nonspecific interaction, experiments were performed with anti-β2GP1 F(ab′)2. The binding of anti-β2GP1 F(ab′)2-Alexa 488 or control F(ab′)2-Alexa 488 was monitored in the developing thrombus after injury along with platelet accumulation, monitored using anti-CD42. The anti-β2GP1 F(ab′)2 autoantibodies were associated with platelets during thrombus formation (Figure 1A, top lane 1), whereas the control antibodies did not bind (Figure 1A, top lane 2). The kinetics of binding of the anti-β2GP1 F(ab′)2 autoantibodies paralleled platelet accumulation (Figure 1A, bottom).
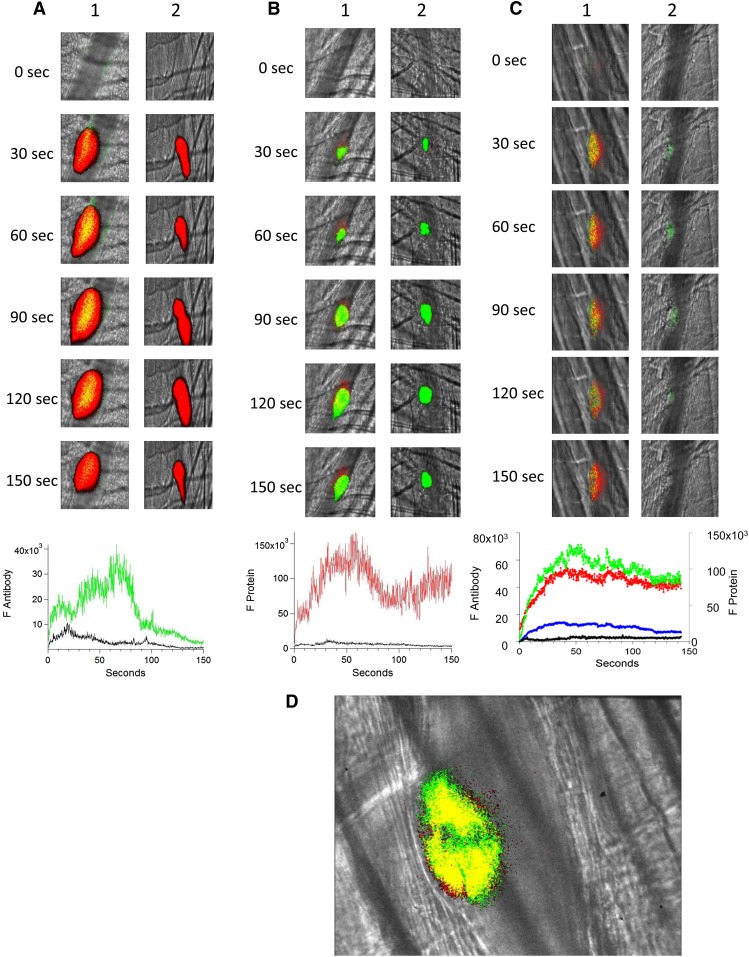
Figure 1.
Binding of fluorescently labeled human anti-β2GP1 F(ab′)2, fluorescently labeled β2GP1, or both to the developing thrombus. (A) Binding of fluorescently labeled human anti-β2GP1 F(ab′)2 to the developing thrombus. Anti-β2GP1 F(ab′)2 (2.5 μg/mouse) or control F(ab′)2 (2.5 μg/mouse), each labeled with Alexa 488, and anti-CD42 antibody labeled with Dylight 649 were infused into the mouse 15 minutes before laser-induced arteriolar wall injury. The results shown are for binding of anti-β2GP1 F(ab′)2 derived from sera from APS patient 1. Similar results were obtained with anti-β2GP1 F(ab′)2 derived from sera from APS patient 2. (Top) Representative images of the fluorescence signal associated with anti-β2GP1 F(ab′)2 (green; lane 1) or control F(ab′)2 (green; lane 2) and platelets (red) over 150 seconds after vessel injury are shown within the context of the brightfield histology. Merge (yellow). (Bottom) The median integrated antibody fluorescence (FANTIBODY) associated with thrombus formation after infusion of anti-β2GP1 F(ab′)2 (51 thrombi, 5 mice) or control F(ab′)2 (51 thrombi, 4 mice) over 150 seconds after vessel wall injury. Anti-β2GP1 F(ab′)2 (green); control F(ab′)2 (black). (B) Binding of fluorescently labeled β2GP1 in the presence of human anti-β2GP1 autoantibodies to the developing thrombus. Mice were infused with β2GP1 (25 μg/mouse) or HSA (25 μg/mouse), each labeled with Alexa 647, anti-β2GP1 autoantibodies (10 μg/mouse), and anti-CD42 antibody labeled with Dylight 488 before laser-induced arteriolar wall injury. (Top) Representative images of the fluorescence signal associated with β2GP1 (lane 1, red) or HSA (lane 2, red) and anti-CD42 antibody labeled with Dylight 488 for platelet detection (green) over 150 seconds after vessel injury are shown within the context of the brightfield histology. Merge (yellow). (Bottom) Median integrated protein fluorescence (Fβ2GP1 or FHSA) associated with thrombus formation in 3 wild-type mice after infusion of β2GP1 conjugated to Alexa 647 (27 thrombi, 3 mice) or HSA control conjugated to Alexa 647 (24 thrombi, 3 mice) over 150 seconds after vessel wall injury. β2GP1 (red); HSA (black). (C) Simultaneous binding of fluorescently labeled anti-β2GP1 autoantibodies and fluorescently labeled β2GP1 during thrombus formation after laser-induced injury. Mice were infused with labeled anti-β2GP1 F(ab′)2 (2.5 μg) derived from patient 2 plus labeled β2GP1 (25 μg) or labeled control F(ab′)2 (2.5 μg) plus labeled HSA (25 μg) 15 minutes before laser-induced arteriolar wall injury. (Top) Representative images of the fluorescence signal associated with Alexa 488-labeled anti-β2GP1 F(ab′)2 (lane 1, green) or Alexa 488–labeled control F(ab′)2 (lane 2, green) and Alexa 647–labeled β2GP1 (lane 1, red) or Alexa 647–labeled HSA (lane 2, red) over 150 seconds after vessel injury are shown within the context of the brightfield histology. Merge (yellow). (Bottom) The median integrated fluorescence, (FANTIBODY) and (FPROTEIN), of antibody and protein, respectively, associated with thrombus formation after infusion of Alexa 488–labeled anti-β2GP1 F(ab′)2 and Alexa 647–labeled β2GP1 (25 thrombi, 3 mice) or Alexa 488–labeled control F(ab′)2 and Alexa 647–labeled HSA (26 thrombi, 2 mice) over 150 seconds after vessel wall injury. Anti-β2GP1 F(ab′)2 (green); control F(ab′)2 (blue); β2GP1 (red); HSA (black). (D) High-resolution confocal intravital imaging of binding of the anti-β2GP1 autoantibody/β2GP1 complex during thrombus formation after laser-induced injury. Confocal image of thrombus formation 60 seconds after vessel wall injury. Alexa 488–labeled anti-β2GP1 F(ab′)2 (green); β2GP1 (red); merge (yellow). A single confocal slice through the center of the thrombus is shown.
β2GP1, in the presence of anti-β2GP1 autoantibodies, binds to platelets during thrombus formation in vivo.
The binding of human β2GP1-Alexa 647 or HSA-Alexa 647 was monitored by intravital microscopy in the presence of anti-β2GP1 antibodies and endogenous plasma β2GP1 along with platelet accumulation using anti-CD42 antibodies. There was no interaction of β2GP1 with the vessel wall before vessel injury.24 The infusion of labeled β2GP1 showed specific binding of β2GP1 to platelets as well as to the adjacent vessel wall; there was minimal binding of HSA (Figure 1B, top lane 2; bottom). The binding of β2GP1 to the inflamed endothelium at the site of local injury in vivo has been previously described in the absence of anti-β2GP1 autoantibodies.24
Binding of fluorescently labeled anti-β2GP1 autoantibodies and fluorescently labeled β2GP1 during thrombus formation in vivo.
Anti-β2GP1 F(ab′)2-Alexa 488 and β2GP1-Alexa 647 were infused together into mice to monitor the kinetics of binding of the anti-β2GP1 F(ab′)2 and β2GP1 simultaneously. Anti-β2GP1 F(ab′)2 and β2GP1 bound with comparable kinetics to the thrombus (Figure 1C, top lane 1; bottom). A control F(ab′)2-Alexa 488 and HSA-Alexa 647 showed only non-specific interaction (Figure 1C, top lane 2; bottom). These results are consistent with the interaction of the anti-β2GP1/β2GP1 complex with the thrombus.
Confocal imaging of the binding of the anti-β2GP1 autoantibody/β2GP1 complex during thrombus formation following injury.
To demonstrate that most of the anti-β2GP1 F(ab′)2 was in complex with β2GP1 and that this complex bound to the platelet thrombus, we analyzed the distribution of the anti-β2GP1 F(ab′)2/β2GP1 complex via confocal microscopy. Anti-β2GP1 F(ab′)2-Alexa 488 and β2GP1-Alexa 647 were infused, and confocal images obtained through the thrombus center 60 seconds after injury (Figure 1D). All of the anti-β2GP1 F(ab′)2 in the mouse was conjugated to Alexa 488, but only a fraction of the β2GP1 was conjugated to Alexa 647 because most of the β2GP1 is endogenous. The colocalization of anti-β2GP1 F(ab′)2 and β2GP1 is shown as yellow, whereas the interaction of anti–β2GP1 F(ab′)2-Alexa 488 with unlabeled β2GP1 is green. Most of the fluorescence shows likely colocalization of the antibody and antigen, and this colocalization appears solely on the platelet thrombus. The Manders’ coefficient was 0.971 and the Pearson’s coefficient was 0.75. We report both numbers to emphasize that colocalization metrics using an intravital image in a live mouse is at best a loose approximation. No fluorescence is associated with the endothelial surface. The minor appearance of β2GP1 (red) unassociated with antibody is likely from technical issues of registration associated with high-speed intravital imaging of living mice.
Cell activation is amplified by anti-β2GP1 autoantibodies.
To evaluate cell activation in platelets and in endothelial cells during thrombus formation, we monitored calcium mobilization in platelets and endothelial cells, and ICAM-1 expression in endothelial cells after injury.17,18
Anti-β2GP1 autoantibody-enhanced activation of thrombus-bound platelets during thrombus formation.
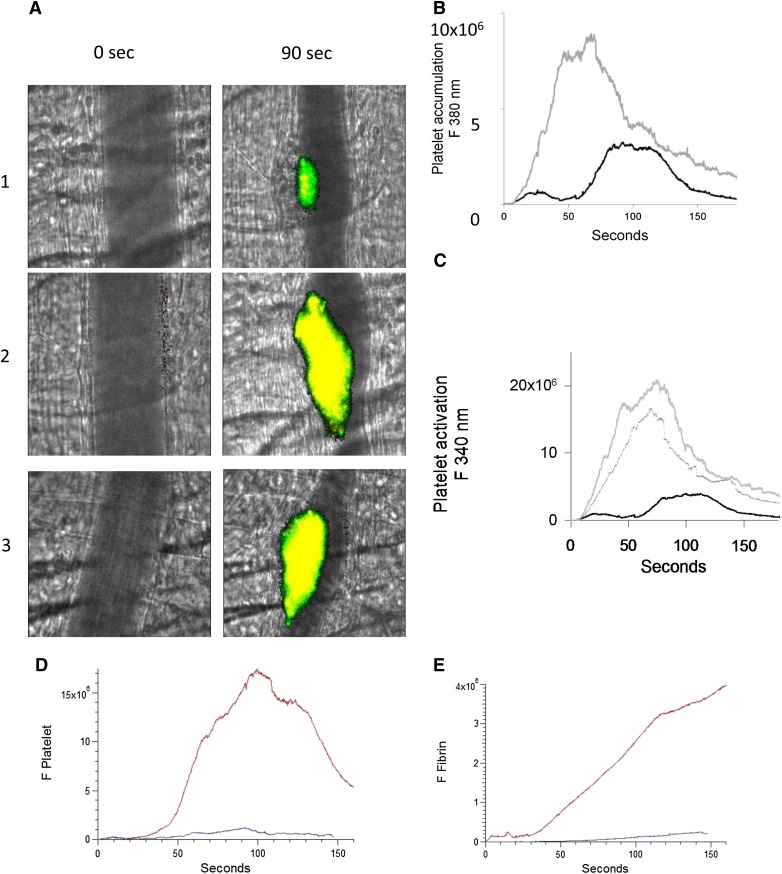
To evaluate whether the number of activated platelets are increased during thrombus formation after infusion of anti-β2GP1 autoantibodies, platelet activation was imaged by calcium mobilization in vivo.17 Platelets isolated from donor mice were loaded with fura-2-AM, and platelets infused into recipient mice. Platelet accumulation and activation were determined after injury. After infusion of anti-β2GP1 autoantibodies, thrombi were generated in recipients, and platelet accumulation and activation again determined. Monitoring fluorescence at 380 nm (unliganded fura-2, green) allowed quantitation of platelet accumulation and monitoring fluorescence at 340 nm (Ca+2 liganded fura-2, red) allowed quantitation of activated platelets (Figure 2A). We found a significant increase in both platelet accumulation (Figure 2B) and platelet activation (Figure 2C) after infusion of the anti-β2GP1 autoantibodies. These results support anti-β2GP1 autoantibody–mediated enhancement of platelet activation and accumulation during thrombus formation. To determine whether intact IgG, which is the form of human anti-β2GP1 autoantibodies that is pathogenic in APS, or F(ab′)2 fragments of anti-β2GP1 both have the ability to amplify platelet aggregation and fibrin generation, we evaluated F(ab′)2 fragments of anti-β2GP1 autoantibodies for their ability to enhance thrombus generation. Amplification of platelet accumulation (Figure 2D) and fibrin generation (Figure 2E) was observed after anti-β2GP1 F(ab′)2 fragment infusion. Similarly, anti-β2GP1 F(ab′)2 fragments amplified platelet activation (Figure 2A, lane 3) as monitored by calcium mobilization.
Figure 2.
Enhanced platelet activation at the site of vascular injury with infusion of anti-β2GP1 autoantibodies. Mice were infused with platelets loaded with fura-2-AM (250 × 106/mouse). Monitoring fluorescence at 380 nm allowed quantitation of platelet accumulation (unliganded fura-2, green) and monitoring fluorescence at 340 nm allowed quantitation of activated platelets (Ca+2 liganded fura-2, red; yellow = merge). (A) Images of the developing thrombus at time 0 and at 90 seconds obtained without (panel 1), with infusion of 10 μg anti-β2GP1 IgG (panel 2) and with infusion of 12 μg anti-β2GP1 F(ab′)2 (panel 3). Resting platelets, green; activated platelets, yellow. (B) Platelet accumulation after laser-induced injury is represented by the median fluorescence intensity of loaded platelets excited at 380 nm over 3 minutes in 20 thrombi in 4 mice before (black) and in 25 thrombi after (gray) infusion of 10 μg of anti-β2GP1 IgG. (C) Platelet activation at the site of laser-induced injury is represented by the median fluorescence intensity of fura-2-AM–loaded platelets excited at 340 nm over 3 minutes in 20 thrombi in 4 mice before (black) and 25 thrombi after (gray) infusion of 10 μg of anti-β2GP1 IgG. (D) F(ab′)2 of anti-β2GP1 IgG-induced enhancement of platelet accumulation. (E) F(ab′)2 of anti-β2GP1 IgG-induced enhancement of fibrin generation. Platelet accumulation and fibrin generation at the site of laser-induced injury were measured before and 20 minutes after infusion of 12 μg of F(ab′)2 of anti-β2GP1 IgG. Platelet and fibrin labeling were performed using anti-CD42 antibody labeled with Dylight 488 (0.1 μg/g) and anti-fibrin antibody labeled with Alexa 647 (0.5 μg/g). F(ab′)2 of anti-β2GP1 IgG-induced changes in platelet thrombus size and fibrin generation were observed in thrombi performed upstream in a single arteriole before (blue, 16 thrombi, 3 mice) and 20 minutes after infusion of 12 μg of F(ab′)2 of anti-β2GP1 IgG (red, 12 thrombi, 2 mice).
Anti-β2GP1 autoantibodies amplify activation of the injured endothelium after laser-induced injury.
Endothelial cell ICAM-1 expression is a marker of endothelial cell activation because ICAM-1 increases significantly after endothelial cell activation.25 Furthermore, ICAM-1 is not expressed on platelets,26 so changes associated with increased ICAM-1 expression can be attributed to the endothelium.27
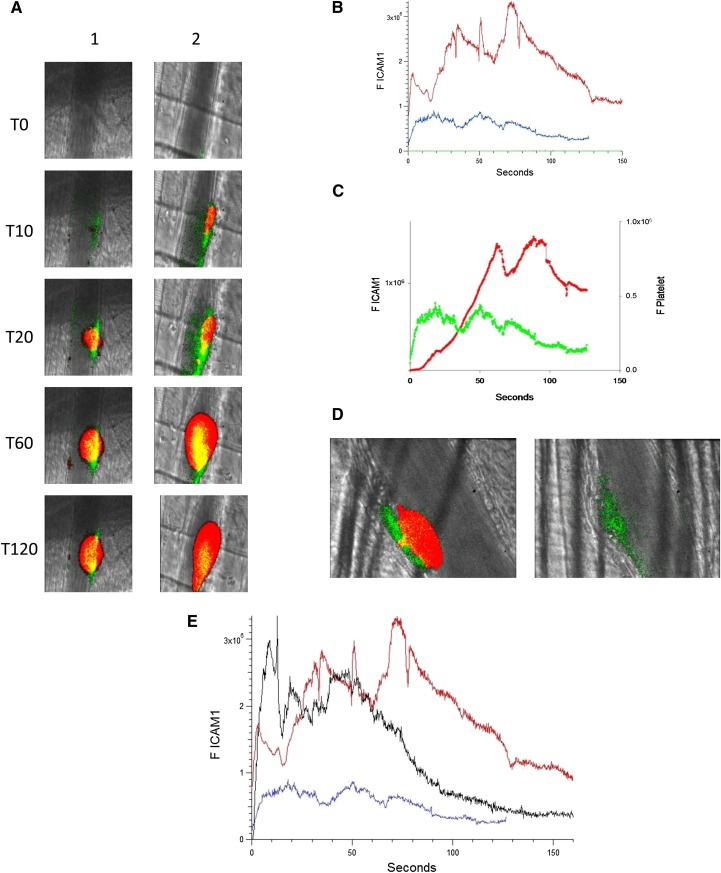
The binding of anti–ICAM-1-Alexa 488 was used to monitor ICAM-1 expression in the developing thrombus after injury; an irrelevant control IgG–Alexa 488 determined nonspecific binding. Platelet accumulation was recorded concomitantly. Upon injury (time 0), an increase in ICAM-1 could be detected along the arteriolar wall at approximately 5 seconds after injury. ICAM-1 peaked at about 50 seconds, then decreased (Figure 3A, left; Figure 3B-C). When anti-β2GP1 autoantibodies were infused into the mouse 15 minutes before injury, ICAM-1 expression was enhanced at the vessel wall (Figure 3A, right). The kinetics of expression of ICAM-1 before and 15 minutes after infusion of anti-β2GP1 autoantibodies indicate that prior infusion of anti-β2GP1 autoantibodies enhances ICAM-1 expression during thrombus formation (Figure 3B). In the absence of anti-β2GP1 autoantibodies, the kinetics of expression of ICAM-1 and platelet accumulation revealed that ICAM-1 expression precedes platelet accumulation (Figure 3C).
Figure 3.
Endothelial cell activation is enhanced at the site of vascular injury in the presence of anti-β2GP1 autoantibodies in vivo. (A) Representative images of ICAM-1 and platelets in the developing thrombus from time 0 to 120 seconds obtained before (left panel, lane 1) and 15 minutes after (right panel, lane 2) infusion of 10 μg of human anti-β2GP1 autoantibodies. ICAM-1, green; platelets, red; merge, yellow. (B) The median integrated ICAM-1 fluorescence (F ICAM-1) associated with thrombus formation before (19 thrombi, 4 mice; blue) and 15 minutes after (27 thrombi, 4 mice; red) infusion of 10 μg of anti-β2GP1 IgG over 150 seconds after vessel wall injury. An irrelevant IgG in place of the anti-ICAM-1 antibody is shown (21 thrombin, 2 mice; green). (C) Comparison of the kinetics of ICAM-1 expression (green) and platelet accumulation (red) during thrombus formation in the absence of anti-β2GP1 IgG. (D) Confocal imaging of ICAM-1 after vascular injury indicates ICAM-1 is localized on the endothelium and not the platelet thrombus. Confocal images of ICAM-1 and platelets were obtained 60 seconds after laser injury during thrombus formation. (Left) ICAM-1 was visualized using anti-ICAM-1 labeled with Alexa 488 (0.4 μg/g mouse) (green) and platelets were visualized using anti-CD42 antibody labeled with Dylight 649 (0.1 μg/g mouse) (red). Merge, yellow. (Right) In the presence of eptifibatide (10 μg/g mouse) and its elimination of platelets, ICAM-1 was visualized on the endothelial surface. Confocal images were obtained through a central section of the thrombus. These confocal images are obtained at high speed in a live mouse where there is minor vessel movement with during each systole. Furthermore, the green and the red images are obtained near simultaneously but not simultaneously. Therefore, the register of the composite image is not perfect. Finally, there is low background noise that we elected not to subtract. We quantitated the fluorescence corresponding to total ICAM-1 fluorescence in the image and quantitated the fluorescence corresponding to the ICAM-1 fluorescence within the thrombus, as defined by platelet fluorescence. (E) F(ab′)2 fragments of anti-β2GP1 autoantibodies enhance activation of endothelial cells similarly to intact anti-β2GP1 autoantibodies. Endothelial cell activation was monitored using anti-ICAM-1 conjugated to Alexa 488 (0.5 μg/g) and platelets were monitored using anti-CD42 antibody conjugated with Dylight 649 (0.1 μg/g) before and 20 minutes after infusion of 12 μg of F(ab′)2 fragments of anti-β2GP1. ICAM-1 expression in endothelial cells was observed in an arteriole before (blue; 19 thrombi, 4 mice) and 20 minutes after infusion of 12 μg of F(ab′)2 fragments of anti-β2GP1 (black; 14 thrombi, 2 mice) or 10 μg of intact anti-β2GP1 (red; 27 thrombi, 4 mice).
To establish whether ICAM-1 is associated with the vessel wall, as would be the case if ICAM-1 is associated specifically with the activated endothelium, or whether ICAM-1 is distributed within the platelet thrombus, as would be the case if leukocytes, leukocyte microparticles, or endothelial cell microparticles contributed to ICAM-1 expression during thrombus formation, confocal images were obtained. ICAM-1 and platelets were imaged simultaneously during thrombus formation aft infusion of anti-β2GP1 autoantibodies. ICAM-1 localized along the vessel wall (Figure 3D, left). There was insignificant ICAM-1–associated fluorescence in the confocal planes through the platelet thrombus. When platelet thrombus formation was blocked with eptifibatide, no platelets were detected but ICAM-1 was visualized along the endothelium (Figure 3D, right). We determined that 17% ± 11% of the total green fluorescence is within the thrombus, and this colocalization is limited to the outer edge of the thrombus. Given the limitations of high-speed intravital microscopy and that no ICAM-1 fluorescence is seen in most of the thrombus, we conclude that there is little or no ICAM-1 in the thrombus. We cannot, however, rule out the possibility of soluble ICAM-1 incorporation into the thrombus.
To determine whether intact IgG, which is the form of human anti-β2GP1 autoantibodies that is pathogenic in APS, or F(ab′)2 fragments of anti-β2GP1 both can amplify endothelial cell activation, we evaluated F(ab′)2 fragments of anti-β2GP1 autoantibodies for their ability to enhance ICAM-1 expression. After laser injury, endothelial cells were activated and expressed ICAM-1. When either F(ab′)2 fragments of anti-β2GP1 autoantibodies or anti-β2GP1 IgG was infused before injury, amplification of ICAM-1 expression was observed (Figure 3E).
Effect of eptifibatide on platelet interaction with the injured endothelium.
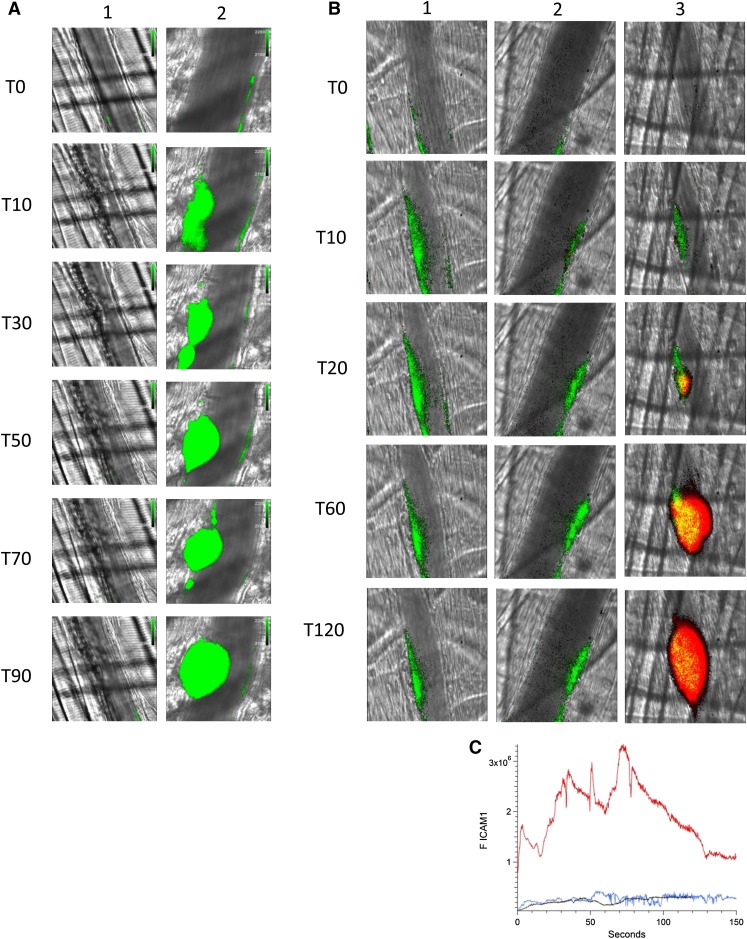
Eptifibatide, an inhibitor of α2bβ3, blocks platelet aggregation. Using a high-resolution camera that permits visualization of individual platelets, we demonstrated that infusion of eptifibatide not only inhibits platelet aggregation in this model but also inhibits platelet–endothelium interaction. Initial infusion of eptifibatide eliminated visualization of platelets on the endothelium after laser injury (Figure 4A, lane 1). However, eptifibatide is cleared rapidly and platelets begin to accumulate in arterioles at 20 minutes and a platelet thrombus is formed at 60 minutes (Figure 4A, lane 2).
Figure 4.
Platelet thrombus is required for enhanced endothelial cell activation in the presence of anti-β2GP1 autoantibodies. (A) Infusion of eptifibatide (10 μg/g mouse) initially inhibits platelet interaction with the injured vessel wall but its effect is gone by 60 minutes. Platelets were labeled using anti-CD42 antibody conjugated to Dylight 488 (0.1 μg/g) and platelet fluorescence (green) imaged at 488 nm. Images were obtained with the high-resolution CMOS camera that resolves individual platelets. (Lane 1) Image at time 0. (Lane 2) Image at time 60 minutes after eptifibatide infusion. (B) Representative images of ICAM-1 and platelets in the developing thrombus from time 0 to 120 seconds obtained in the presence of eptifibatide (10 μg/g mouse) (lane 1); human anti-β2GP1 autoantibodies (10 μg) and eptifibatide (10 μg/g mouse) (lane 2); human anti-β2GP1 autoantibodies (10 μg) in the absence of eptifibatide (lane 3). ICAM-1, green; platelets, red. (C) The median integrated ICAM-1 fluorescence (F ICAM-1) associated with thrombus formation in wild-type mice infused with human anti-β2GP1 autoantibodies (27 thrombi, 4 mice; red); human anti-β2GP1 autoantibodies and eptifibatide (27 thrombi, 3 mice; blue); and eptifibatide (18 thrombi, 3 mice; black).
Platelets are required for anti-β2GP1 autoantibody–induced enhancement of endothelial activation in vivo.
Endothelial cell binding of anti-β2GP1 antibodies and anti-β2GP1 antibody–induced endothelial cell activation in in vitro experiments have been extensively described.28-38 Two prior in vivo studies have confirmed antibody-induced endothelial cell activation in wild-type mice.22,33 We determined whether enhanced endothelial cell activation was dependent upon the presence of platelets.
Monitoring ICAM-1, we explored endothelial cell activation in vivo after laser-induced injury in the absence of platelet thrombus formation. Eptifibatide was infused into the mouse before vessel injury and continued every 15 minutes. ICAM-1, visualized with anti–ICAM-1-Alexa 488, reached maximal fluorescence by 10 to 15 seconds in the absence of anti-β2GP1 autoantibodies (Figure 4B, lane 1). The magnitude of ICAM-1 expression was similar in the presence of anti-β2GP1 autoantibodies (Figure 4B, lane 2). In the absence of eptifibatide and in the presence of anti-β2GP1 autoantibodies, ICAM-1 expression is significantly enhanced in the presence of a platelet thrombus (Figure 4B, lane 3). These results indicate a role for platelets in endothelial cell activation. The kinetics of ICAM-1 expression confirmed that ICAM-1 expression was similar regardless of the presence or absence of anti-β2GP1 autoantibodies in the absence of a platelet thrombus (Figure 4C). When a platelet thrombus is allowed to form, anti-β2GP1 autoantibodies enhance ICAM-1 fluorescence by 15- to 20-fold. These results suggest that a platelet thrombus contributes to enhanced endothelial cell activation after vessel injury.
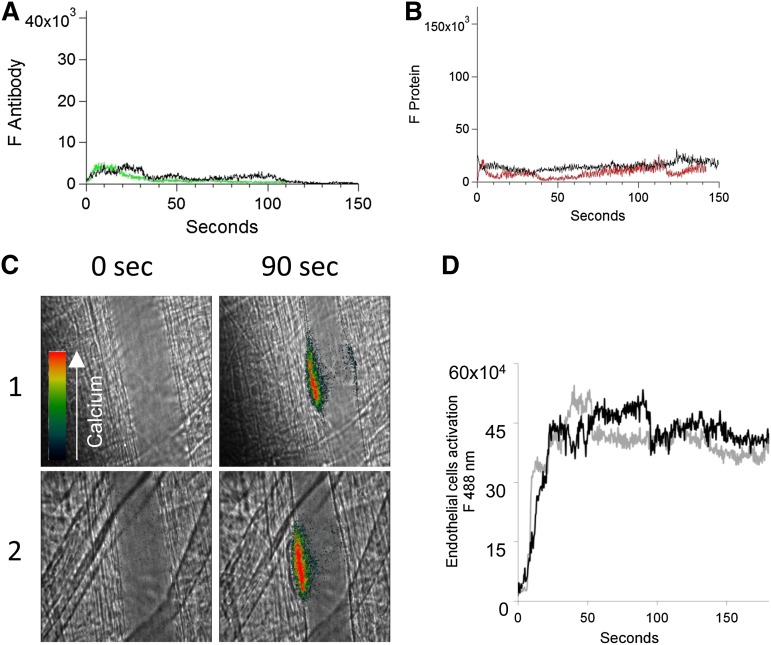
To confirm the importance of platelets in anti-β2GP1 antibody–enhanced endothelial cell activation, we monitored both anti-β2GP1 antibody and β2GP1 binding to the endothelium as well as endothelial cell activation in vivo by calcium mobilization in the absence of a platelet thrombus. After blocking platelet accumulation with eptifibatide, we monitored anti-β2GP1 F(ab′)2-Alexa 488 or control F(ab′)2-Alexa 488 after injury (Figure 5A). No significant binding of anti-β2GP1 F(ab′)2 was observed in the absence of a platelet thrombus. Similarly, after blocking platelet accumulation with eptifibatide, no significant binding of β2GP1 was observed (Figure 5B). These results indicate that, in the absence of a platelet thrombus, neither anti-β2GP1 F(ab′)2 nor β2GP1 bind significantly to the endothelium after vascular injury.
Figure 5.
In the absence of a platelet thrombus, the anti-β2GP1 autoantibody/β2GP1 complex neither bound the endothelium nor enhanced endothelial calcium mobilization. (A) After blocking platelet accumulation by infusion of eptifibatide (10 μg/g mouse), the median integrated antibody fluorescence (FAntibody) after infusion of anti-β2GP1 F(ab′)2 (24 thrombi, 2 mice) or control F(ab′)2 (26 thrombi, 2 mice) over 150 seconds after vessel wall injury was measured. Anti-β2GP1 F(ab′)2 (green); control F(ab′)2 (black). No significant binding of anti-β2GP1 F(ab′)2 was observed in the absence of a platelet thrombus. (B) After blocking platelet accumulation by infusion of eptifibatide (10 μg/g mouse), the median integrated protein fluorescence (Fβ2GP1 or FHSA) after infusion of Alexa 647 conjugated to β2GP1 (24 thrombi, 2 mice) or Alexa 647 conjugated to HSA (26 thrombi, 2 mice) over 150 seconds after vessel wall injury was measured. β2GP1 (red); control (black). No significant binding of β2GP1 was observed. (C) Fluo-4-AM was delivered into the mouse circulation via the femoral artery, and concurrent platelet aggregation was inhibited by infusion of eptifibatide (10 μg/g mouse) every 15 minutes. After laser-induced vessel wall injury, changes to endothelial Ca2+ levels were observed by excitation at 488 nm and images were recorded over time. Antibody-induced change in endothelial cell activation was examined in the injured vessel performed upstream in 1 arteriole before and 15 minutes after infusion of 10 μg of anti-β2GP1 autoantibodies. Images show calcium elevation in the endothelium in the absence of platelet accumulation obtained at time 0 and at 90 seconds after vessel wall injury before (panel 1) and after (panel 2) injection of 10 μg of anti-β2GP1 antibodies. The fluorescence signal is represented as a pseudocolor intensity map where black represents the least intense and red represents the most intense fluorescence signal. (D) The kinetics of endothelial cell activation at the site of laser-induced injury was determined by calculating median fluorescence values at 488 nm as a function of time. Calcium mobilization is represented by the median fluorescence intensity of Fluo-4-AM–loaded endothelial cells over 3 minutes before (black) and after (gray) infusion of 10 μg of anti-β2GP1 IgG for 15 to 20 thrombi in 2 mice.
Using Fluo-4-AM as a reporter of calcium mobilization in endothelial cells, we examined the consequences of infusion of anti-β2GP1 antibodies into a mouse treated with eptifibatide. After injury and in the absence of platelet accumulation due to eptifibatide, we compared endothelial cell activation, monitored by calcium mobilization, before and 15 minutes after infusion of anti-β2GP1 autoantibodies (Figure 5C). No differences were observed (Figure 5D). This result indicates that the infusion of anti-β2GP1 autoantibodies does not enhance endothelial cell activation at the site of injury in the absence of a platelet thrombus.
Endothelial cells do not support anti-β2GP1 antibody–induced enhancement of fibrin generation in the absence of platelet accumulation.
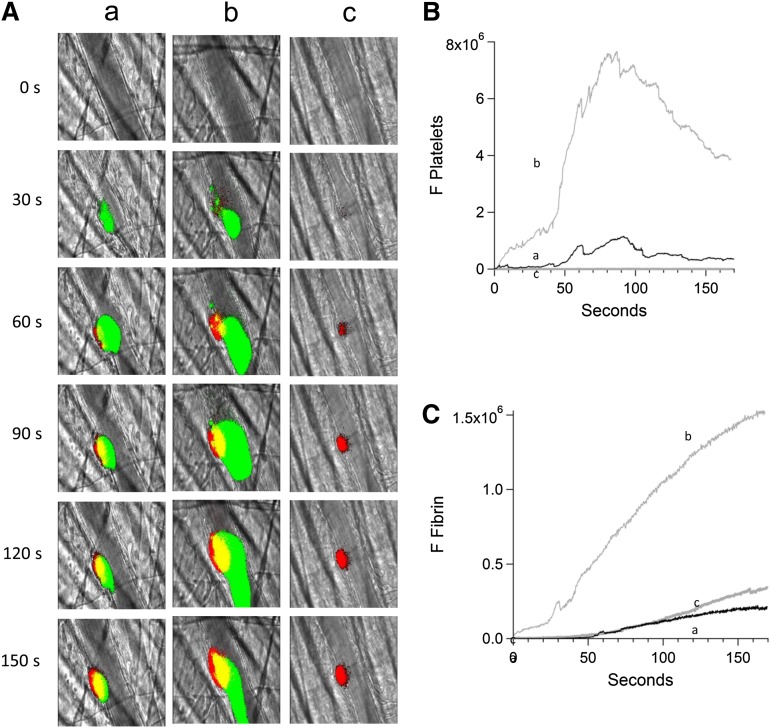
We studied fibrin generation before and after infusion of anti-β2GP1 autoantibodies in the presence and absence of eptifibatide to inhibit platelet accumulation. Anti-β2GP1 autoantibodies enhanced fibrin generation in parallel to platelet accumulation.7 However, in the absence of platelet accumulation at the site of injury, we did not observe an increase in fibrin generation after infusion of anti-β2GP1 autoantibodies (Figure 6). These results indicate that enhancement of fibrin generation by anti-β2GP1 autoantibodies requires the presence of a platelet thrombus.
Figure 6.
Inhibition of platelet thrombus formation with eptifibatide prevents anti-β2GP1 autoantibody–mediated enhancement of fibrin generation. Platelet and fibrin imaging were performed using anti-CD42 antibody labeled with Dylight 488 (0.1 μg/g mouse) and anti-fibrin antibody labeled with Alexa 647 (0.5 μg/g mouse). Platelet thrombus size and fibrin generation at the site of laser-induced injury were determined by calculating median fluorescence values at 488 nm and 647 nm over 3 minutes, respectively. Anti-β2GP1 autoantibody–induced changes in platelet thrombus size and fibrin generation were observed in thrombi performed upstream in a single arteriole before (16 thrombi, 3 mice) and 15 minutes after (20 thrombi, 3 mice) infusion of 10 μg of anti-β2GP1 antibodies. Subsequently, platelet accumulation at the site of injury was prevented by infusion of eptifibatide (10 μg/g mouse) every 15 minutes, and platelet thrombus size and fibrin generation were observed in thrombi (19 thrombi, 3 mice) performed upstream in a single arteriole in the presence of anti-β2GP1 autoantibodies. (A) Representative images of the developing thrombus obtained (a) without antibody, (b) with 10 μg anti-β2GP1 IgG, and (c) with 10 μg anti-β2GP1 IgG and eptifibatide. Platelets, green; fibrin, red; merge, yellow. (B) Platelet accumulation. (C) Fibrin generation. No antibody (a), black; anti-β2GP1 autoantibody (b), light gray; anti-β2GP1 autoantibody and eptifibatide (c), dark gray.
In this model of anti-phospholipid syndrome, platelets are the target of anti-β2GP1/β2GP1 complexes during thrombus formation. In vivo, binding of the anti-β2GP1 antibody/β2GP1 complex to platelets during thrombus formation amplifies platelet activation and accumulation, and these platelets trigger both enhanced activation of the endothelium and increased fibrin generation. In the absence of the platelet thrombus, this complex neither amplifies endothelium activation nor increases fibrin generation.
Anti-β2GP1 autoantibodies amplify platelet accumulation and fibrin generation in venules.
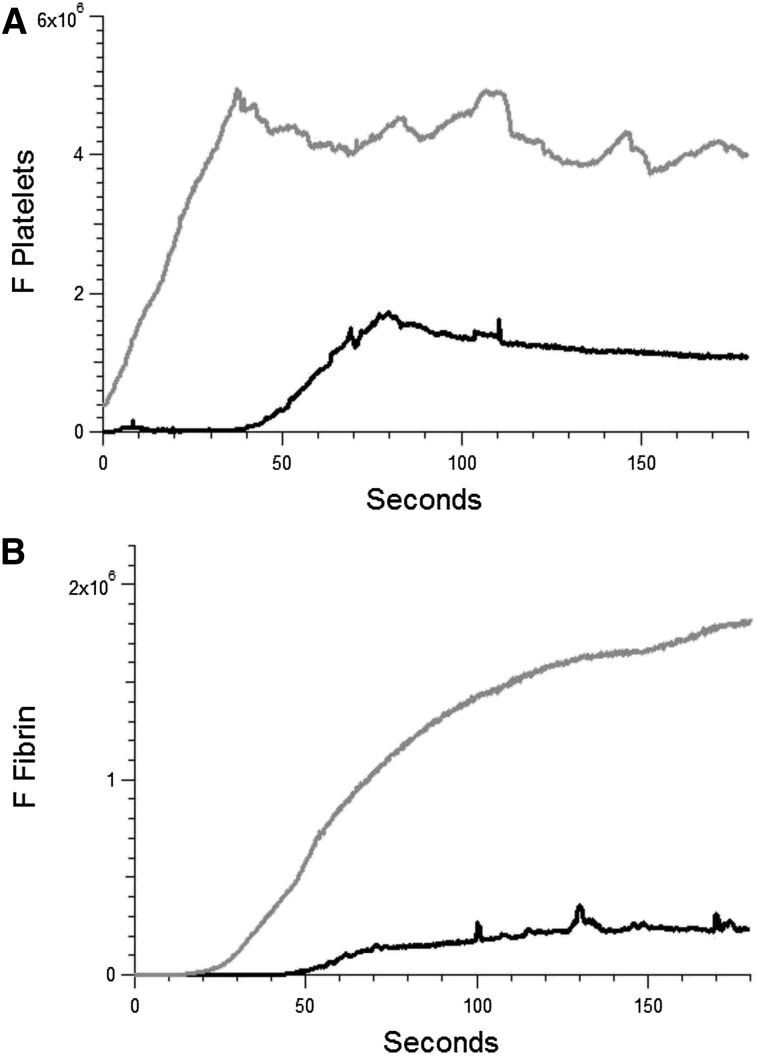
APS is characterized by both arterial and venous thrombosis. Our studies have focused on arterial thrombosis amplified by anti-β2GP1 autoantibodies. To determine whether these autoantibodies have a similar effect in venules in our model, the effect of anti-β2GP1 autoantibodies on laser-induced injury to the venule was studied. Thrombus formation was initiated by laser injury to the venule wall and platelet thrombus size determined based upon fluorescence associated with anti-CD42. Subsequently, anti-β2GP1 autoantibodies were infused and platelet accumulation and fibrin generation monitored (Figure 7). These results demonstrate that anti-β2GP1 autoantibodies are capable of greatly enhancing thrombus size in venules.
Figure 7.
Anti-β2GP1 autoantibodies amplify thrombus formation in venules. Effect of purified anti-β2GP1 autoantibodies on thrombus size and fibrin generation in venules. Anti-β2GP1 autoantibodies were infused into wild-type mice 5 minutes before laser-induced venule wall injury. Platelet and fibrin imaging was performed using anti-CD42 antibody labeled with Dylight 649 (0.1 μg/g mouse) and anti-fibrin antibody labeled with Alexa 488 (0.5 μg/g mouse). Platelet thrombus size and fibrin generation at the site of laser-induced injury were determined by calculating median fluorescence values at 649 nm and 488 nm over 3 minutes, respectively. After initial laser injury of the venule wall, a thrombus composed of platelets (A) and fibrin (B) was generated; 15 minutes after infusion of 10 μg of anti-β2GP1 autoantibodies, anti-β2GP1 autoantibody–induced changes in platelet thrombus size and fibrin generation were observed. The kinetics of the fluorescence signals associated with platelets and fibrin over 180 seconds after vessel injury are shown before (black) and after (gray) infusion of anti-β2GP1 autoantibodies.
Discussion
Many competing hypotheses have been proposed to explain the pathologic mechanism of thrombus induction by the anti-β2GP1 autoantibody/β2GP1 complex in APS. Our animal model allows measurement of thrombus amplification by the anti-β2GP1 autoantibody/β2GP1 complex and the identification of involved components. Human anti-β2GP1 autoantibodies amplify thrombus size.7 In the current study, we evaluated 3 events associated with APS: (1) anti-β2GP1 autoantibody, β2GP1, and anti-β2GP1 autoantibody/β2GP1 complex binding to the platelet thrombus but not the endothelium; (2) anti-β2GP1 autoantibody/β2GP1 complex enhancement of platelet and endothelium activation; and (3) the platelet thrombus requirement for endothelium activation and increased fibrin generation by anti-β2GP1 autoantibodies. The binding of the anti-β2GP1 antibody/β2GP1 complex to cell receptors19,36,39-41 has been studied, and receptors have been proposed to be involved in thrombus enhancement. The inhibition of enhancement of thrombus formation in the absence of specific receptors20-22,42 or in the presence of inhibitors of receptors43 suggest that some receptors may not be directly involved in the pathway initiated by the anti-β2GP1 autoantibody/β2GP1 complex. Therefore, we visualized both cell binding and cell activation induced by the anti-β2GP1 autoantibody/β2GP1 complex in vivo.
Previous animal studies demonstrated amplification of thrombus formation by either IgG derived from whole APS serum44-47 or human anti-β2GP1 autoantibodies.7 In others, endothelium activation was observed in vivo by observation of ICAM-1 expression,48 leukocyte rolling,22,33,49,50 and the expression of endothelial proteins.21,22,48,51,52 Our in vivo results are consistent with and extend these studies demonstrating endothelial cell activation or platelet activation or both by aPL autoantibodies. Furthermore, they are consistent with studies of human subjects showing evidence of activation of the endothelium in APS: endothelial cell microparticles, soluble VCAM1, soluble ICAM-1, von Willebrand factor, soluble thrombomodulin, and soluble P-selectin.53-56 Our results confirm that endothelium activation is enhanced in the presence of aPL antibodies but demonstrate that enhancement is platelet thrombus–dependent. By visualization of the targets of the anti-β2GP1 autoantibody/β2GP1 complex and the functional implications of anti-β2GP1 autoantibody/β2GP1 complex binding to that target, we establish important features of the pathogenesis of aPL autoantibodies. First, we demonstrate with in vivo confocal images that the anti-β2GP1 autoantibody/β2GP1 complex binds exclusively, within limits of detection, to the platelet thrombus and not to the endothelium. Second, the binding of the anti-β2GP1 autoantibody/β2GP1 complex to the developing thrombus leads to amplification of platelet activation. Third, the binding of the anti-β2GP1 autoantibody/β2GP1 complex to the platelet thrombus is followed by endothelium activation. Fourth, in the absence of a platelet thrombus, the anti-β2GP1 autoantibody/β2GP1 complex does not enhance endothelium activation or fibrin generation.
ICAM-1 and other endothelial markers implicated the endothelium in aPL antibody–induced endothelial activation in vivo. Furthermore, in vitro experiments established the ability of aPL antibodies to bind and activate endothelial cells in culture.28-38 Our finding that the anti-β2GP1 autoantibody/β2GP1 complex does not bind to the endothelium but that the endothelium does become activated is consistent with prior experiments. This raises 2 questions: (1) Why does the anti-β2GP1 autoantibody/β2GP1 complex activate endothelial cells in culture in vitro? This may be due to the presence of high arteriolar shear forces in the animal model that are not present in culture or a feature that is unique to cultured endothelial cells. (2) Given the absence of binding of the anti-β2GP1 autoantibody/β2GP1 complex to the endothelium in our mouse model, what is the mechanism by which the endothelium becomes activated? We suspect that enhanced activation of the endothelium is initiated by the releasate of the activated platelets. When eptifibatide was infused to prevent platelet thrombus formation, we observed neither enhanced endothelium activation nor increased fibrin generation associated with the anti-β2GP1 autoantibody/β2GP1 complex. Thus, enhanced activation of the endothelium and increased fibrin generation by the anti-β2GP1 autoantibody/β2GP1 complex is platelet-dependent.
We established that anti-β2GP1 autoantibodies not only enhanced platelet thrombus formation, but also enhanced fibrin generation.7 After vessel injury, fibrin generation occurs even in the absence of a platelet thrombus, as occurs in Par4−/− mice,57 mice treated with eptifibatide,58 or transplanted mice lacking αIIbβ3.59 We now observe that in the absence of a platelet thrombus after administration of eptifibatide, the magnitude of fibrin generation is similar in the presence or absence of anti-β2GP1 autoantibodies. Therefore, antibody-induced fibrin enhancement is platelet thrombus-dependent, although initial fibrin generation is independent of platelets. We have shown that, in vivo, the anti-β2GP1 antibody/β2GP1 complex is targeted at the platelet thrombus. Increased platelet thrombus size and activation in response to injury is responsible for amplifying endothelial cell activation and increased fibrin generation.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung and Blood Institute (P01 HL087203 and R01 HL095084 [B.F.]) and by the Lupus Research Institute.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.P. designed and performed the experiments, analyzed the results, and edited the manuscript; R.A.F. provided patient material, analyzed the results, and edited the manuscript; G.M. developed and improved the intravital microscopy system and edited the manuscript; B.C.F. designed the experiments, analyzed the results, and edited the manuscript; and B.F. designed the experiments, analyzed the results and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Furie, Center for Life Science, Room 903, 3 Blackfan Circle, Boston, MA 02215; e-mail: bfurie@bidmc.harvard.edu.
References
- 1.de Groot PG, Urbanus RT. The significance of autoantibodies against β2-glycoprotein I. Blood. 2012;120(2):266–274. doi: 10.1182/blood-2012-03-378646. [DOI] [PubMed] [Google Scholar]
- 2.Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Semin Thromb Hemost. 2012;38(4):305–321. doi: 10.1055/s-0032-1311827. [DOI] [PubMed] [Google Scholar]
- 3.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7(6):330–339. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 4.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990;87(11):4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iverson GM, Victoria EJ, Marquis DM. Anti-beta2 glycoprotein I (beta2GPI) autoantibodies recognize an epitope on the first domain of beta2GPI. Proc Natl Acad Sci U S A. 1998;95(26):15542–15546. doi: 10.1073/pnas.95.26.15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Laat B, de Groot PG. Autoantibodies directed against domain I of beta2-glycoprotein I. Curr Rheumatol Rep. 2011;13(1):70–76. doi: 10.1007/s11926-010-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arad A, Proulle V, Furie RA, Furie BC, Furie B. β₂-Glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2011;117(12):3453–3459. doi: 10.1182/blood-2010-08-300715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girardi G, Mackman N. Tissue factor in antiphospholipid antibody-induced pregnancy loss: a pro-inflammatory molecule. Lupus. 2008;17(10):931–936. doi: 10.1177/0961203308094994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110(7):2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi T, Giannakopoulos B, Yan X, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54(8):2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 11.Pennings MT, Derksen RH, van Lummel M, et al. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2007;5(2):369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 12.Lutters BC, Derksen RH, Tekelenburg WL, Lenting PJ, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2′. J Biol Chem. 2003;278(36):33831–33838. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 13.Lellouche F, Martinuzzo M, Said P, Maclouf J, Carreras LO. Imbalance of thromboxane/prostacyclin biosynthesis in patients with lupus anticoagulant. Blood. 1991;78(11):2894–2899. [PubMed] [Google Scholar]
- 14.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 15.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 16.Jasuja R, Passam FH, Kennedy DR, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012;122(6):2104–2113. doi: 10.1172/JCI61228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117(4):953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson BT, Jasuja R, Chen VM, Nandivada P, Furie B, Furie BC. Laser-induced endothelial cell activation supports fibrin formation. Blood. 2010;116(22):4675–4683. doi: 10.1182/blood-2010-05-283986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Döring Y, Hurst J, Lorenz M, et al. Human antiphospholipid antibodies induce TNFalpha in monocytes via Toll-like receptor 8. Immunobiology. 2010;215(3):230–241. doi: 10.1016/j.imbio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus RT, et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117(4):1408–1414. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114(14):3074–3083. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramesh S, Morrell CN, Tarango C, et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J Clin Invest. 2011;121(1):120–131. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2008;6(8):1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 24.Agostinis C, Biffi S, Garrovo C, et al. In vivo distribution of β2 glycoprotein I under various pathophysiologic conditions. Blood. 2011;118(15):4231–4238. doi: 10.1182/blood-2011-01-333617. [DOI] [PubMed] [Google Scholar]
- 25.Pober JS, Gimbrone MA, Jr, Lapierre LA, et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137(6):1893–1896. [PubMed] [Google Scholar]
- 26.Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94(3):1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross PL, Furie BC, Merrill-Skoloff G, Chou J, Furie B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J Leukoc Biol. 2005;78(6):1318–1326. doi: 10.1189/jlb.0405193. [DOI] [PubMed] [Google Scholar]
- 28.Simantov R, LaSala JM, Lo SK, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96(5):2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Papa N, Guidali L, Sala A, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40(3):551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 30.Del Papa N, Raschi E, Catelli L, et al. Endothelial cells as a target for antiphospholipid antibodies: role of anti-beta 2 glycoprotein I antibodies. Am J Reprod Immunol. 1997;38(3):212–217. doi: 10.1111/j.1600-0897.1997.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 31.McCrae KR, DeMichele A, Samuels P, et al. Detection of endothelial cell-reactive immunoglobulin in patients with anti-phospholipid antibodies. Br J Haematol. 1991;79(4):595–605. doi: 10.1111/j.1365-2141.1991.tb08087.x. [DOI] [PubMed] [Google Scholar]
- 32.George J, Blank M, Levy Y, et al. Differential effects of anti-beta2-glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation. 1998;97(9):900–906. doi: 10.1161/01.cir.97.9.900. [DOI] [PubMed] [Google Scholar]
- 33.Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99(15):1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 34.Colasanti T, Alessandri C, Capozzi A, et al. Autoantibodies specific to a peptide of β2-glycoprotein I cross-react with TLR4, inducing a proinflammatory phenotype in endothelial cells and monocytes. Blood. 2012;120(16):3360–3370. doi: 10.1182/blood-2011-09-378851. [DOI] [PubMed] [Google Scholar]
- 35.Allen KL, Hamik A, Jain MK, McCrae KR. Endothelial cell activation by antiphospholipid antibodies is modulated by Kruppel-like transcription factors. Blood. 2011;117(23):6383–6391. doi: 10.1182/blood-2010-10-313072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen KL, Fonseca FV, Betapudi V, Willard B, Zhang J, McCrae KR. A novel pathway for human endothelial cell activation by antiphospholipid/anti-β2 glycoprotein I antibodies. Blood. 2012;119(3):884–893. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunoyer-Geindre S, de Moerloose P, Galve-de Rochemonteix B, Reber G, Kruithof EK. NFkappaB is an essential intermediate in the activation of endothelial cells by anti-beta(2)-glycoprotein 1 antibodies. Thromb Haemost. 2002;88(5):851–857. [PubMed] [Google Scholar]
- 38.Meroni PL, Raschi E, Testoni C, Borghi MO. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol. 2004;112(2):169–174. doi: 10.1016/j.clim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Satta N, Dunoyer-Geindre S, Reber G, et al. The role of TLR2 in the inflammatory activation of mouse fibroblasts by human antiphospholipid antibodies. Blood. 2007;109(4):1507–1514. doi: 10.1182/blood-2005-03-024463. [DOI] [PubMed] [Google Scholar]
- 40.Raschi E, Borghi MO, Grossi C, Broggini V, Pierangeli S, Meroni PL. Toll-like receptors: another player in the pathogenesis of the anti-phospholipid syndrome. Lupus. 2008;17(10):937–942. doi: 10.1177/0961203308095140. [DOI] [PubMed] [Google Scholar]
- 41.Ma K, Simantov R, Zhang JC, Silverstein R, Hajjar KA, McCrae KR. High affinity binding of beta 2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275(20):15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 42.Pierangeli SS, Vega-Ostertag ME, Raschi E, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66(10):1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolyada A, Porter A, Beglova N. Inhibition of thrombotic properties of persistent autoimmune anti-B2GPI antibodies in the mouse model of antiphospholipid syndrome. Blood. 2014;123(7):1090–1097. doi: 10.1182/blood-2013-08-520882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankowski M, Vreys I, Wittevrongel C, et al. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101(1):157–162. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 45.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106(7):2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 46.Pierangeli SS, Liu SW, Anderson G, Barker JH, Harris EN. Thrombogenic properties of murine anti-cardiolipin antibodies induced by beta 2 glycoprotein 1 and human immunoglobulin G antiphospholipid antibodies. Circulation. 1996;94(7):1746–1751. doi: 10.1161/01.cir.94.7.1746. [DOI] [PubMed] [Google Scholar]
- 47.Pierangeli SS, Harris EN. Antiphospholipid antibodies in an in vivo thrombosis model in mice. Lupus. 1994;3(4):247–251. doi: 10.1177/096120339400300408. [DOI] [PubMed] [Google Scholar]
- 48.Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88(2):245–250. doi: 10.1161/01.res.88.2.245. [DOI] [PubMed] [Google Scholar]
- 49.Gharavi AE, Pierangeli SS, Colden-Stanfield M, Liu XW, Espinola RG, Harris EN. GDKV-induced antiphospholipid antibodies enhance thrombosis and activate endothelial cells in vivo and in vitro. J Immunol. 1999;163(5):2922–2927. [PubMed] [Google Scholar]
- 50.Ferrara DE, Liu X, Espinola RG, et al. Inhibition of the thrombogenic and inflammatory properties of antiphospholipid antibodies by fluvastatin in an in vivo animal model. Arthritis Rheum. 2003;48(11):3272–3279. doi: 10.1002/art.11449. [DOI] [PubMed] [Google Scholar]
- 51.Pierangeli SS, Liu X, Espinola R, et al. Functional analyses of patient-derived IgG monoclonal anticardiolipin antibodies using in vivo thrombosis and in vivo microcirculation models. Thromb Haemost. 2000;84(3):388–395. [PubMed] [Google Scholar]
- 52.Espinola RG, Liu X, Colden-Stanfield M, Hall J, Harris EN, Pierangeli SS. E-Selectin mediates pathogenic effects of antiphospholipid antibodies. J Thromb Haemost. 2003;1(4):843–848. doi: 10.1046/j.1538-7836.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 53.Williams FM, Parmar K, Hughes GR, Hunt BJ. Systemic endothelial cell markers in primary antiphospholipid syndrome. Thromb Haemost. 2000;84(5):742–746. [PubMed] [Google Scholar]
- 54.Frijns R, Fijnheer R, Schiel A, Donders R, Sixma J, Derksen R. Persistent increase in plasma thrombomodulin in patients with a history of lupus nephritis: endothelial cell activation markers. J Rheumatol. 2001;28(3):514–519. [PubMed] [Google Scholar]
- 55.Kaplanski G, Cacoub P, Farnarier C, et al. Increased soluble vascular cell adhesion molecule 1 concentrations in patients with primary or systemic lupus erythematosus-related antiphospholipid syndrome: correlations with the severity of thrombosis. Arthritis Rheum. 2000;43(1):55–64. doi: 10.1002/1529-0131(200001)43:1<55::AID-ANR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 56.Dignat-George F, Camoin-Jau L, Sabatier F, et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91(4):667–673. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 57.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci U S A. 2007;104(1):288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116(22):4665–4674. doi: 10.1182/blood-2010-04-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho J, Kennedy DR, Lin L, et al. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood. 2012;120(3):647–655. doi: 10.1182/blood-2011-08-372532. [DOI] [PMC free article] [PubMed] [Google Scholar]