Abstract
The mechanism through which marijuana produces its psychoactive effects is Δ9- tetrahydrocannabinol (THC)-induced activation of cannabinoid CB1 receptors. These receptors are normally activated by endogenous lipids, including anandamide and 2-arachidonoyl glycerol (2-AG). A logical “first step” in determination of the role of these endocannabinoids in THC’s psychoactive effects is to investigate the degree to which pharmacologically induced increases in anandamide and/or 2-AG concentrations through exogenous administration and/or systemic administration of inhibitors of their metabolism, fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), respectively, share THC’s discriminative stimulus effects. To this end, adult male mice and rats were trained to discriminate THC (5.6 and 3 mg/kg, respectively). In Experiment 1, exogenous administration of anandamide or 2-AG did not substitute for THC in mice nor was substitution enhanced by co-administration of the FAAH or MAGL inhibitors, URB597 and N-arachidonyl maleimide (NAM), respectively. Significant decreases in responding may have prevented assessment of adequate endocannabinoid doses. In mice trained at higher baseline response rates (Experiment 2), the FAAH inhibitor PF3845 (10 mg/kg) enhanced anandamide substitution for THC without producing effects of its own. The MAGL inhibitor JZL184 increased brain levels of 2-AG in vitro and in vivo, increased THC-like responding without co-administration of 2-AG. In rats, neither URB597 nor JZL184 engendered significant THC-appropriate responding, but co-administration of these two enzyme inhibitors approached full substitution. The present results highlight the complex interplay between anandamide and 2-AG and suggest that endogenous increases of both endocannabinoids are most effective in elicitation of THC-like discriminative stimulus effects.
Keywords: anandamide, 2-arachidonoylglycerol, cannabinoids, discriminative stimulus, FAAH, JZL184, MAGL, mice, PF3845, rats, Δ9-tetrahydrocannabinol, URB597
1.0 Introduction
The endocannabinoid system, one of several lipid signaling systems in the brain, is comprised of two G-protein coupled receptors, their signaling pathways, two predominant endogenous ligands, and synthetic and metabolic pathways for these endocannabinoids. Of the two identified receptors, one type (CB1) is found in largest concentrations in the brain (Herkenham et al., 1991) whereas the other type (CB2) is primarily, but not exclusively (Van Sickle et al., 2005; Xi et al., 2011), located in the periphery (Galiegue et al., 1995). Anandamide, the most thoroughly characterized of the endocannabinoids, is produced via the hydrolysis of membrane phospholipid precursors of the N-acyl-phophatidyl-ethanolamine (NAPE) family through a synthesis mechanism that has not been entirely characterized (Leung et al., 2006). Inactivation of anandamide occurs primarily via degradation by fatty acid amide hydrolase (FAAH), an enzyme that also degrades a number of other endogenous fatty acids (Cravatt and Lichtman, 2002). The primary synthetic and metabolic enzymes for the endocannabinoid 2-arachidonoylglycerol (2-AG) have been identified as diacylglycerol (DAGL) and monoacylglycerol lipase (MAGL), respectively (Dinh et al., 2002), albeit other enzymes (e.g., ABHD6 and ABHD12) also contribute to 2-AG metabolism (Marrs et al., 2010). In several brain areas, localization of FAAH is primarily post-synaptic whereas localization of MAGL is pre-synaptic (Gulyas et al., 2004), suggesting some degree of functional segregation of signaling pathways for each endocannabinoid.
THC, the principal psychoactive substituent of the marijuana plant Cannabis sativa (Gaoni and Mechoulam, 1964), acts within the endocannabinoid system to produce characteristic effects in mice [i.e., ‘cannabinoid tetrad’: suppression of activity, antinociception, hypothermia and catalepsy; (Martin et al., 1991)] and distinctive discriminative stimulus effects in rodents and nonhuman primates (Balster and Prescott, 1992; Gold et al., 1992), with the latter being a pharmacologically selective animal model of marijuana’s subjective effects (Balster and Prescott, 1992). While cannabinoid CB1 receptor activation has been shown to be mediate the discriminative stimulus effects of THC (Wiley et al., 1995), the degree to which endogenous cannabinoids contribute to THC’s psychoactive effects has received less research attention. Given that endocannabinoids also activate cannabinoid CB1 receptors, a logical “first step” in determination of the role of endocannabinoids in THC’s psychoactive effects is to investigate whether changes in the levels of one or both of the two best-characterized endocannabinoids, anandamide and 2-AG, mimic the abuse-related effects of THC. In humans, alterations in endocannabinoid concentrations may result from factors such as genetic variation in degradative enzyme levels (Sipe et al., 2002) or through stress-induced changes (Hill and McEwan, 2010). The present study examined the degree to which pharmacologically induced increases in anandamide and/or 2-AG concentrations through exogenous administration and/or systemic administration of FAAH or MAGL inhibitors, respectively, would share THC’s discriminative stimulus effects.
2.0 Materials and Methods
2.1 Subjects
Experimentally naive adult male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were used for both mouse drug discrimination experiments. Adult male ICR mice (Harlan, Dublin, VA) were used for the in vitro experiments. Adult male Long-Evans rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) were used for the rat drug discrimination studies. All rodents were housed individually in clear plastic cages with steel wire fitted tops and wood-chip bedding. They were kept in a light- (12-h light:dark cycle; lights on at 0600) and temperature- (20–22°C) controlled vivarium, except during experimental sessions which occurred during the light component. Mice in the discrimination experiments were maintained at 85–90% of free-feeding body weight. Food was not restricted for mice in the in vitro experiments. Body weights for the rats were determined at approximately 3 months of age and then the rats were gradually reduced to 85% of their free-feeding weights and maintained there by supplemental post-session feedings for the remainder of the study. Water was available ad libitum in the home cage for all rodents. Animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of Virginia Commonwealth University and the ‘Guidelines For The Care And Use Of Mammals In Neuroscience And Behavioral Research’ (National Research Council, 2003).
2.2 Apparatus
Mouse and rat operant chambers (Med-Associates, St. Albans, VT), housed within light- and sound-attenuating cubicles, were used for behavioral training and testing in all of the drug discrimination studies. In the first mouse discrimination experiment (Experiment 1), each inner chamber contained two response levers and a house light. A recessed well centered between the two levers contained a liquid dipper that delivered 0.02 ml of sweetened-condensed milk (by volume: one part condensed milk, one part sugar, and two parts water) as reinforcement. In the mouse chambers used for the second mouse discrimination experiment (Experiment 2), the inner chambers contained two nose poke apertures. A food dispenser delivered 14-mg food pellets (Bioserv Inc., Frenchtown, NJ) to a food cup centered between the two nose poke apertures. The rat chambers contained a food dispenser that delivered 45-mg food pellets (Bioserv) to a food cup located between two response levers. For all discrimination experiments, illumination of lights, delivery of food pellets, and recording of lever presses were controlled by a computer-based system (MED-PC IV, MED Associates Inc., St. Albans, VT).
Identification and quantification of anandamide and 2-AG was performed using an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. A five point calibration curves with concentration ranges of 0.078 to 20 pmol of anandamide and 0.125 to 40 nmols of 2-AG, a blank with and without the internal standards were prepared with each sample batch. A linear regression of the ratio of the peak area counts of anandamide, 2-AG to their corresponding internal standards (anandamide-d8 and 2-AG-d8) versus concentration was used to construct the calibration curves. The lower limit of quantification and lower limit of detection for anandamide were 0.078 pmol and 0.020 pmol, respectively. The lower limit of quantification and the lower limit of detection for 2-AG were 0.125 nmol and 0.0.025 nmol, respectively. All calibration concentrations were calculated to be within 15% of their expected values, except the lower limit of quantification which was within 20 % of its expected concentrations. The lower limit of quantification also had a response at least five times greater than the signal to noise ratio for all sample batch. Lower limit of detection samples were analyzed and verified to have a signal to noise ratio was three times the noise level of the background signal. The linear regression correlation coefficients (r2) for the anandamide and 2-AG calibration curves were 0.990 or better for all calibration curves.
2.3 Drugs
For the mouse drug discrimination studies, anandamide (Organix, Inc., Woburn, MA), 2-AG (Organix), N-arachidonyl maleimide (NAM; provided by Cayman Chemical, Ann Arbor, Michigan), N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-piperidinecarboxamide (PF3845; provided by Pfizer, Kent, CT), and 4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184; provided by Dr. Benjamin Cravatt, The Scripps Research Institute, La Jolla, CA) were mixed in a vehicle of 5% ethanol, 5% emulphor (Rhone-Poulenc, Inc., Princeton, NJ) and 90% saline. THC [National Institute on Drug Abuse (NIDA), Rockville, MD] was also mixed with this vehicle (Experiment 1 in mice and in rat drug discrimination). Rimonabant (NIDA) and THC (Experiment 2 in mice) were mixed in 0.78% Tween-80 and 99.22% saline. For the mouse study, cyclohexylcarbamicacid 3′-carbamoyl-biphenyl-3-yl ester (URB597; Cayman Chemicals) was mixed in a vehicle of 1% dimethyl sulfoxide (DMSO)/1% Tween 80/98% saline, with addition of 0.2M NaOH buffer for a final ~ pH 8.0. For the rat study, URB597 (provided by Merck, Inc., & Co., Inc., Rahway, NJ) was first dissolved in DMSO under gentle heat at a concentration equivalent to 250 mg/ml and then diluted to test concentrations with a vehicle composed of 37% polyethylene glycol 200 (Sigma-Aldrich, St. Louis, MO), 42% water hydroxypropyl-β-cyclodextrin 40% solution (Sigma-Aldrich), and 21% sterile water. JZL184 was prepared in a vehicle of 40% polyethylene glycol, 10% Tween-80, and 50% sterile water. Routes and times of administration for each procedure are provided in the specific procedure section.
For the in vitro experiments, guanosine 5′ diphosphate (GDP), bovine serum albumin (BSA), and adenosine deaminase were purchased from Sigma-Aldrich. GTPγS was purchased from Roche Diagnostics (Branchburg, NJ). [35S]GTPγS (1150–1300 Ci/mmol) was obtained from Perkin Elmer Life Sciences (Boston, MA). Scintillation fluid (ScinitSafe Econo 1) was purchased from Fisher Scientific (Pittsburgh, PA).
2.4 THC Discrimination in Mice
Training in the two mouse discrimination procedures was similar to that described previously (Vann et al., 2009). Briefly, each mouse was placed in a standard operant conditioning chamber with two response manipulanda (two levers for Experiment 1 and two nose poke apertures for Experiment 2). Mice were trained to respond on one of the two manipulanda following administration of THC and to respond on the other manipulanda following vehicle injection according to a fixed ratio 10 (FR10) reinforcement schedule, under which 10 consecutive responses on the correct (drug-appropriate) manipulandum resulted in delivery of a small dipper of milk (Experiment 1) or a food pellet (Experiment 2). The training dose of THC was 5.6 mg/kg in both experiments. Responses on the incorrect manipulandum reset the ratio requirement on the correct manipulandum. Daily injections were administered on a double alternation sequence of THC and vehicle (e.g., drug, drug, vehicle, vehicle). For both studies, daily 15 min training sessions were held Monday-Friday until the mice consistently met two criteria: (1) the first completed FR10 was on the correct manipulandum and (2) ≥ 80% of the total responding occurred on the correct manipulandum. Due to the probable floor effect engendered by the low response rates in Experiment 1, Experiment 2 included the additional criterion of response rates consistently ≥ 0.16 responses/s. When the criteria were met in each experiment, acquisition of the discrimination was established and substitution testing began.
For all mice that successfully acquired the discrimination, stimulus substitution tests were conducted on Tuesdays and Fridays during 15 min test sessions, with maintenance of training continuing on intervening days. During test sessions, responses on either manipulandum delivered reinforcement according to an FR10 schedule. To be tested in either experiment, mice must have completed the first FR10 on the correct manipulandum and ≥ 80% of the total responding must have occurred on the correct manipulandum during the prior day’s training session. In addition, the mouse must have met these same criteria during previous training sessions with the alternate training compound (training drug or vehicle). A dose-effect curve determination with THC was conducted in each group followed by tests with the other compounds. Throughout both mouse drug discrimination studies, doses of all drugs were administered at a volume of 10 ml/kg and in ascending order for each dose-effect curve. THC was administered intraperitoneally (i.p., Experiment 1) or subcutaneously (s.c., Experiment 2) 30 min prior to the test session. In Experiment 1, anandamide (0.1–10 mg/kg) and 2-AG (0.1–30 mg/kg) were administered i.p. 10 min prior to test sessions. In Experiment 2, anandamide (1–10 mg/kg) was administered s.c. 30 min before the start of the session. URB597 (0.3 mg/kg) and NAM (1 mg/kg) were injected i.p. 50 min before administration of anandamide (0.1–1 mg/kg) or 2-AG (0.1–1 mg/kg), respectively. Previous work has shown that these doses of URB597 and NAM enhanced the cannabimimetic effects of anandamide and 2-AG, respectively, but did not exhibit in vivo cannabimimetic activity when administered alone (Burston et al., 2008; Solinas et al., 2007). PF3845 (1–10 mg/kg) and JZL184 (3–30 mg/kg) were injected i.p. 120 min before the start of the session.
2.5 THC Discrimination in Rats
The rats were initially trained in daily (Monday - Friday) 15 min sessions to press one of two levers under a fixed-ratio 1 (FR1) schedule of reinforcement in which each lever press resulted in a food pellet delivery. The response requirement was gradually increased to FR10. During the next few sessions, the rats were reinforced only for pressing the opposite lever until they pressed reliably under FR10 contingencies. Rats were then injected i.p. with 3 mg/kg THC or vehicle 30 min prior to the start of the session. For each rat, the experimenter designated one lever as correct (and pressing it resulted in pellet delivery) following THC administration and the other as correct following vehicle administration. The lever on which the rats were initially trained and on which they acquired the lever-press response was designated as the “vehicle-correct” lever. Alternation of THC and vehicle injections followed a two-monthly cycle (Month #1: TVVTV, VTTVT, VTVTV, TVTVT; Month #2: VTTVV, TVTVT, TVVTT, VTVTV; in which T=THC, V=vehicle). Lever pressing produced pellet delivery only on the injection-appropriate lever during a training session. Incorrect presses reset the response requirement on the correct lever.
Testing commenced when: (1) a rat completed the first fixed-ratio on the correct lever on at least 8 of 10 consecutive sessions; and (2) at least 80% of the total responses were made on the correct lever during those 8 sessions. Tests were conducted on Tuesdays and Fridays provided that the rat completed the first fixed ratio on the correct levers during the most recent THC- and vehicle-training sessions, otherwise, a training session was administered. During test sessions, responding on either lever was reinforced with food pellet delivery. Initially, vehicle and doses of THC (0.1–10 mg/kg) were tested followed by URB597 (3–56 mg/kg) and its vehicle. Subsequently, JZL184 (16 mg/kg) alone and in combination with URB597 (30 mg/kg) were tested. This dose of URB597 was chosen because it was the highest dose of URB597 that did not alter response rates when tested alone. JZL184 dose choice was based upon our prior work with this compound (Long et al., 2009a). After this set of tests was completed, the rats were retrained at 1 mg/kg THC until criteria as described above were met, and retested with THC (0.01–10 mg/kg) and URB597 (3–56 mg/kg). For all rat drug discrimination experiments, THC and its vehicle were given i.p. in a volume equivalent to 1 ml/kg body weight 30 min pre-session; URB597 and its vehicle were injected i.p. in a volume equivalent to 3 ml/kg and were administered 120 min pre-session. JZL184 and its vehicle were injected i.p. in a volume of 1 ml/kg and administered 120 min pre-session.
2.6 Quantification of Brain Concentrations of 2-AG and Anandamide
Adult male ICR mice were injected i.p. with vehicle (1:1:18), 5 mg/kg NAM, or 1 mg/kg URB597 one hour before decapitation or with 16 mg/kg JZL184 two hours prior to decapitation. After decapitation, the cerebellum was harvested and rapidly cooled by immersion in liquid nitrogen. Tissue was stored at −80°C until use. Anandamide and 2-AG were then extracted using a methanol/chloroform extraction (Burston et al., 2008; Hardison et al., 2006). Samples were homogenized on ice in 2 mL chloroform: methanol (2:1, v/v). The internal standards, 1 pmol anandamide-d8 and 2 nmol 2-AG-d8, were added to each sample, calibrator or control. Samples were mixed and centrifuged after the addition of 0.2 mL of a 0.73% sodium chloride solution. The chloroform was collected and evaporated to dryness with nitrogen. The extracts were reconstituted with 100 μL methanol and placed in autosampler vials for analysis. The injection volume was 20 μL and the auto sampler temperature was set at 5°C. The chromatographic separation of anandamide and 2-AG was performed on a Discovery® HS C18 column 15cm × 2.1mm, 3μm (Supelco: Bellefonte, PA) kept at 40°C. The mobile phase was 10 % water with 1g/L ammonium acetate and 0.1% formic acid, and 90% methanol with 1g/L ammonium acetate and 0.1% formic acid. The flow rate was 0.3 mL/min. The following transition ions (m/z) were monitored in positive multiple reaction monitoring (MRM) mode: anandamide, 348>62 & 348>91; anandamide-d8, 356>62; 2-AG, 379>287 & 279>269; and 2-AG, 387>296. Total run time was 10 min.
2.7 Agonist-Stimulated [35S]GTPγS Binding
Tissue was placed in 5 mL cold membrane buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4) and homogenized. URB597 (50 nM) was then incubated with the homogenate for 30 min at 30°C, in order to ensure that there was significant inhibition of FAAH before anandamide was added to the protein homogenate. The samples were then centrifuged at 50,000 g at 5°C for 10 min. The supernatant was removed and samples were re-suspended in 5mL assay buffer A (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4). Protein concentration was determined by Bradford method (Bradford, 1976). Prior to assay, membranes (4–8 μg protein) were pre-incubated for 25 min at 30°C with adenosine deaminase (3 μU/ml) in assay buffer A. Concentration-effect curves were generated by incubating the appropriate amount of membrane protein (4–8 mg) in assay buffer B (assay buffer A plus 1.25 g/L BSA) with 0.1–60 μM of anandamide plus URB597 (10 nM) in the presence of 30 μM GDP and 0.1 nM [35S]GTPγS in 0.5 mL total volume for 2 hours at 30°C. Basal binding was measured in the absence of agonist and non-specific binding was measured in the presence of 20 μM unlabeled GTPγS. The reaction was terminated by vacuum filtration though Whatman GF/B glass fiber filters, followed by three washes with 4°C Tris buffer (50 mM Tris-HCl, pH 7.4). Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency after 10-h extraction in ScintiSafe Econo 1 scintillation fluid.
2.8 Data Analysis
For mouse and rat discrimination studies, the percentage of responses on the drug manipulandum and response rate (responses/s) were calculated. When appropriate, ED50 values were calculated on the linear part of the drug manipulandum selection dose-response curve for each drug using least squares linear regression analysis, followed by calculation of 95% confidence intervals (CI). Since animals that responded less than 10 times during a test session did not respond sufficiently on either manipulandum to earn a reinforcer, their data were excluded from analysis of drug manipulandum selection, but response rate data were included. Response-rate data were analyzed using a repeated-measures analysis of variance (ANOVA). Mean substitution was used in cases where an individual animal was not tested on one of the doses. Repeated measures ANOVAs were also used to analyze percentage drug manipulandum responding for drug combination tests (e.g., URB597 and JZL184, rimonabant and JZL184). Significant ANOVAs were further analyzed with Tukey post hoc tests (α = 0.05) to specify differences between means. All indications of significance in the results section refer to the results of these post hoc tests.
Data for [35S]GTPγS binding assays are reported as mean and standard error of at least four experiments, which were each performed in triplicate. Non-specific binding was subtracted from each sample. Net stimulated [35S]GTPγS binding is defined as agonist-stimulated minus basal [35S]GTPγS binding, and percent stimulation is defined as (net-stimulated/basal [35S]GTPγS binding) × 100%. Nonlinear iterative regression analyses of agonist concentration-effect curves were performed with Prism 4.0 (GraphPad Software, Inc., San Diego, CA). For mass spectrometry data in mice, mean and standard error were determined for anandamide (pmol) and 2-AG (nmol) per g of cerebellum for each condition. ANOVA, followed by Dunnett’s post hoc tests, was used to determine significant differences between basal and drug-induced concentrations of each endocannabinoid. Statistical analysis was performed using Sigma Stat, version 3.1 (Systat Software, Inc., San Jose, CA). Significance was defined as P < 0.05.
3.0 Results
3.1 Mouse Drug Discrimination: Experiment 1
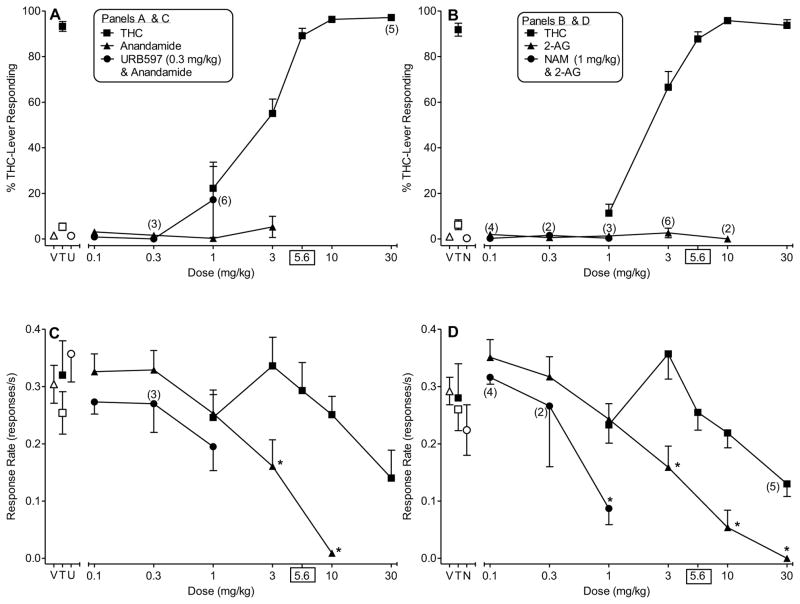
Figure 1 shows the results of tests with THC, anandamide, 2-AG, and the combinations of 0.3 mg/kg URB597/anandamide and 1 mg/kg NAM/2-AG in two groups of mice trained to discriminate 5.6 mg/kg THC from vehicle. As expected, dose-dependent substitution was observed with THC in both groups, with ED50 values of 2.27 (95% CI: 1.88 – 2.74) mg/kg and 2.40 (95% CI: 2.10 – 2.74) mg/kg for mice later tested with anandamide (Fig. 1, panel A) and 2-AG (Fig. 1, panel B), respectively. In contrast, neither endocannabinoid substituted for THC when evaluated alone or in combination with an inhibitor of its primary metabolic enzyme, the FAAH inhibitor URB597 (Fig. 1, panel A) or the nonselective MAGL inhibitor NAM (Fig. 1, panel B) for anandamide and 2-AG, respectively. Further, neither URB597 (0.3 mg/kg) nor NAM (1 mg/kg) substituted for THC when administered alone, as shown at the left side of panels A and B, respectively. Response rates following administration of any of the doses of THC did not significantly differ from rates obtained after vehicle injection for either discrimination group (Fig. 1, panels C and D). In contrast, significant decreases in response rates (compared to vehicle) were observed with each of the endocannabinoids [anandamide: F(6,42)=25.23, P<0.05; 2-AG: F(7,63)=23.38, P<0.05]. Significant attenuation of responding was noted at the 3 and 10 mg/kg doses of anandamide and the 3–30 mg/kg doses of 2-AG (Fig. 1, panels C and D, respectively). Compared to response rates after vehicle injection, the combination of 0.3 mg/kg URB597 and lower doses of anandamide did not significantly affect response rates (Fig. 1, panel C) whereas the combination of 1 mg/kg NAM and the 1 mg/kg dose of 2-AG significantly suppressed responding [F(4,24)=8.84, P<0.05] (Fig. 1, panel D).
Figure 1.
Effects of THC (filled squares) on % THC-lever responding (upper panels) and response rate (lower panels) in two groups of mice trained to discriminate 5.6 mg/kg THC vs. vehicle. Left panels (A and C) show the effects of anandamide in combination with vehicle (unfilled circles) and in combination with 0.3 mg/kg URB-597 (filled circles) on % THC-lever responding and response rate, respectively. Right panels (B and D) show the effects of 2-AG in combination with vehicle (unfilled circles) and in combination with 1 mg/kg NAM (filled circles) on % THC-lever responding and response rate, respectively. Points above V and T represent the results of control tests with vehicle conducted prior to the dose-effect curve determination for the endocannabinoid (unfilled triangles) and for THC (unfilled squares), respectively. Filled squares above T represent the results of control tests with 5.6 mg/kg THC. Unfilled circles above U (left panels) and N (right panels) represent results of tests with 0.3 mg/kg URB597 and 1 mg/kg NAM, respectively (each in combination with vehicle). All compounds were administered i.p. Values represent the mean (±S.E.M.) of data from 7–10 male mice in each group of discriminators unless indicated otherwise on the figure (numbers in parentheses). Asterisks (*) designate a significant (P < 0.05) decrease in response rate compared to mean rate of responding during the control test with vehicle.
3.2 Mouse Drug Discrimination: Experiment 2
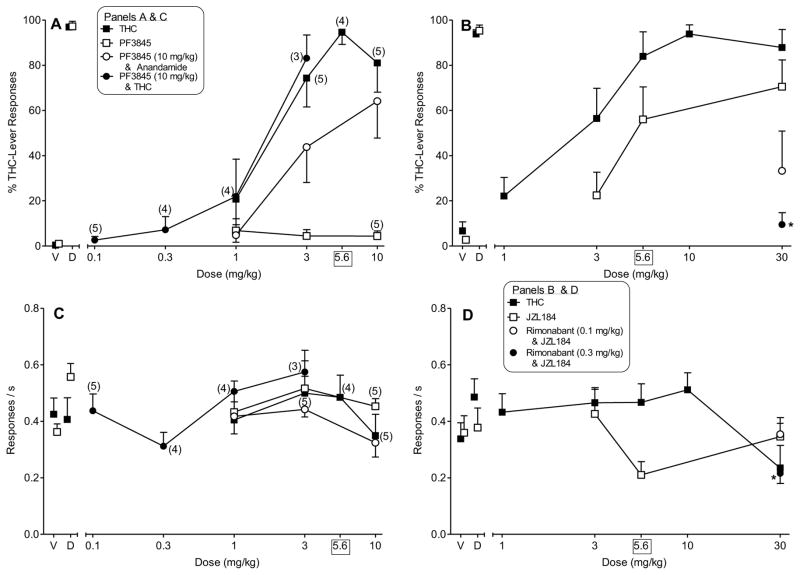
Figure 2 shows the results of tests with THC, anandamide, and FAAH and MAGL inhibitors (PF3845 and JZL184, respectively) in mice trained to discriminate 5.6 mg/kg THC from vehicle. THC was tested twice, once at the beginning of the study and again at the end of the study. In both instances, mice dose-dependently selected the THC lever, with ED50 values of 2.22 mg/kg (95% CI: 1.39 – 3.55 mg/kg) and 1.72 mg/kg (95% CI: 1.08 – 2.74 mg/kg) at the start (Fig. 2, panel A) and the end (Fig. 2, panel B) of the study, respectively. In contrast, PF3845 did not substitute for THC when evaluated alone (Fig. 2, panel A). However, when 10 mg/kg PF3845 was tested in combination with anandamide, a dose-dependent increase in responding on the THC-associated aperture occurred, with a maximum 64% responding on the drug-associated side at a dose combination of 10 mg/kg PF3845 and 10 mg/kg anandamide. Although full substitution did not occur, inspection of data for individual subjects revealed that 4 of 7 mice tested with this dose combination distributed greater than 90% of their responses on the THC-associated aperture. Limited supplies of PF3845 prevented testing of a higher dose to determine whether full substitution would occur. In addition, 10 mg/kg PF3845 produces maximal FAAH inhibition in vitro, lasting up to 24 h after i.p. administration (Ahn et al., 2009). When tested in combination with various doses of THC, 10 mg/kg PF3845 failed to shift the THC dose-effect curve (Fig. 2, panel A). In contrast with the complete lack of substitution observed with the FAAH inhibitor PF3845 alone, the MAGL inhibitor JZL184 dose-dependently increased responding on the THC-associated aperture, with a maximum 71% of the responses on the drug-associated side at the 30 mg/kg dose. The partial substitution of 30 mg/kg JZL184 was reversed by 0.3 mg/kg rimonabant [Fig. 2, panel B; F(4,24)=15.67, P<0.05], a dose combination that also significantly suppressed responding compared to vehicle and to 30 mg/kg JZL184 alone [Fig. 2, panel D; F(4,24)=7.29, P<0.05]. Response rates were not significantly decreased (compared to vehicle) with THC or either enzyme inhibitor (Fig. 2, panels C and D).
Figure 2.
Effects of THC (filled squares) on % THC-lever responding (upper panels) and response rate (lower panels) in two groups of mice trained to discriminate 5.6 mg/kg THC vs. vehicle. Left panels (A and C) also show the effects of the FAAH inhibitor PF3845 alone (unfilled squares) and the effects of 10 mg/kg PF3845 in combination with anandamide (unfilled circles) or THC (filled circles) on % THC-lever responding and response rate, respectively. Right panels (B and D) also show the effects of the MAGL inhibitor JZL184 alone (unfilled squares) on % THC-lever responding and response rate, respectively. In addition, the effects of 30 mg/kg JZL184 and 0.1 or 0.3 mg/kg rimonabant (unfilled and filled circle, respectively) are shown. Points above V and T represent the results of control tests with vehicle and 5.6 mg/kg THC conducted prior to each dose-effect curve determination. THC and anandamide were administered s.c. JZL184 and PF3845 were administered i.p. Values represent the mean (+S.E.M.) of data from 6–9 male mice in each group of discriminators unless indicated otherwise on the figure (numbers in parentheses). Asterisks (*) designate a significant (P < 0.05) decrease in response rates compared to mean rates of responding during the control test with vehicle.
3.3 Rat Drug Discrimination
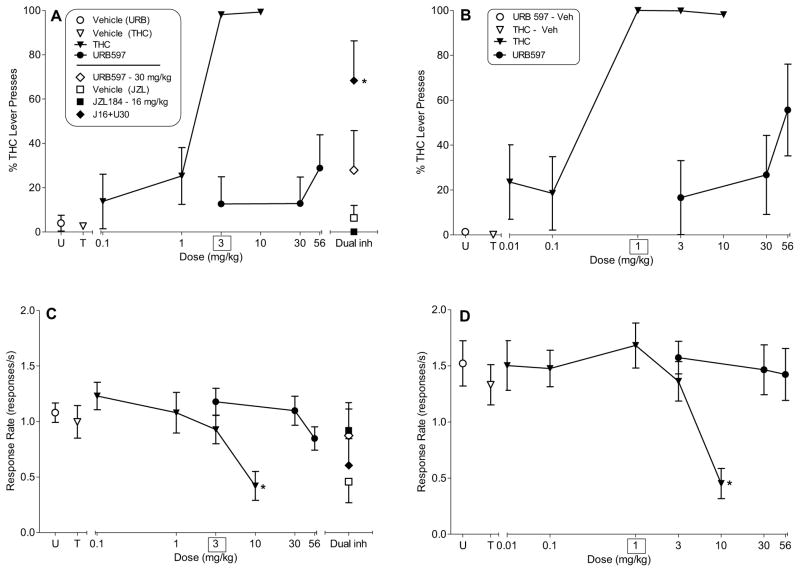
Figure 3 shows the results of tests with THC, URB597, and a combination of URB597/JZL184 in rats trained to discriminate 3 mg/kg THC from vehicle. THC produced dose-dependent substitution, with an ED50 value of 1.3 mg/kg (95% CI: 0.76 – 2.10 mg/kg) [Fig. 3, panel A]. In contrast, URB597 failed to produce significant substitution for THC at any dose tested (Fig. 3, panel A). URB597 (30 mg/kg) was then tested in combination with 16 mg/kg JZL184, a dose that did not substitute by itself. The results of this probe test showed that the combination of 30 mg/kg URB597 and 16 mg/kg JZL184 (Fig. 3, right side of panel A) significantly increased responding on the THC-associated lever compared to average responding following URB597 and JZL184 vehicles (at left and right sides of Fig. 3, panel A) [F(2,8)=4.93, P<0.05], without alteration of overall response rate. Whereas response rates following administration of 10 mg/kg THC were significantly decreased compared to rates obtained after vehicle injection [F(4,28)=13.44, P<0.05], URB597 did not affect response rates compared to vehicle (Fig. 3, panel C).
Figure 3.
Effects of THC (filled triangles) on % THC-lever responding (upper panels) and response rate (lower panels) in rats trained to discriminate 3 mg/kg THC vs. vehicle (left panels) and re-trained to discriminate 1 mg/kg THC vs. vehicle (right panels). Left panels (A and C) show the effects of URB597 (filled circles) on % THC-lever responding and response rate, respectively. The right side of the panel shows the effects of 30 mg/kg URB597 (unfilled diamond), 16 mg/kg JZL184 (filled square) and its vehicle (unfilled square), and dual inhibition of FAAH and MAGL with 30 mg/kg URB597 and 16 mg/kg JZL184 (filled diamond). Right panels (B and D) show the effects of THC (filled triangles) and URB597 (filled circles) in rats after the THC training dose was decreased to 1 mg/kg. Points above U and T at the left side of each panel represent the results of control tests with vehicle conducted prior to the URB597 and THC dose-effect curve determinations, respectively. All compounds were administered i.p. Values on the left panels (A and C) represent the mean (±S.E.M.) of data from 8 male rats for THC and URB597 dose-effect curves, with the exception that n=6 for % drug-lever responding at the 30 mg/kg dose of THC in panel A and n=5 for the URB597 and JZL184 combination tests (panels A and C). Values on the right panels (B and D) represent the mean (±S.E.M.) of data from 6 male rats for THC and URB597 dose-effect curves. Asterisks (*) designate a significant (P < 0.05) decrease in response rates compared to mean rates of responding during the control test with vehicle.
Following re-training with 1 mg/kg THC, the dose-effect curve for THC-lever responding was shifted to the left, with an ED50 value of 0.20 mg/kg (95% CI: 0.089 – 0.43 mg/kg) [Fig. 3, panel B]. Again, URB597 failed to produce significant increases in responding on the THC-associated lever (Fig. 3, panel B) and did not affect overall response rates (Fig. 3, panel D). As previously, 10 mg/kg THC continued to produce significant decreases in response rates after rats were re-trained to discriminate 1 mg/kg THC from vehicle [F(5,25)=14.43, P<0.05] (Fig. 3, panel D).
3.4 In Vitro Assays
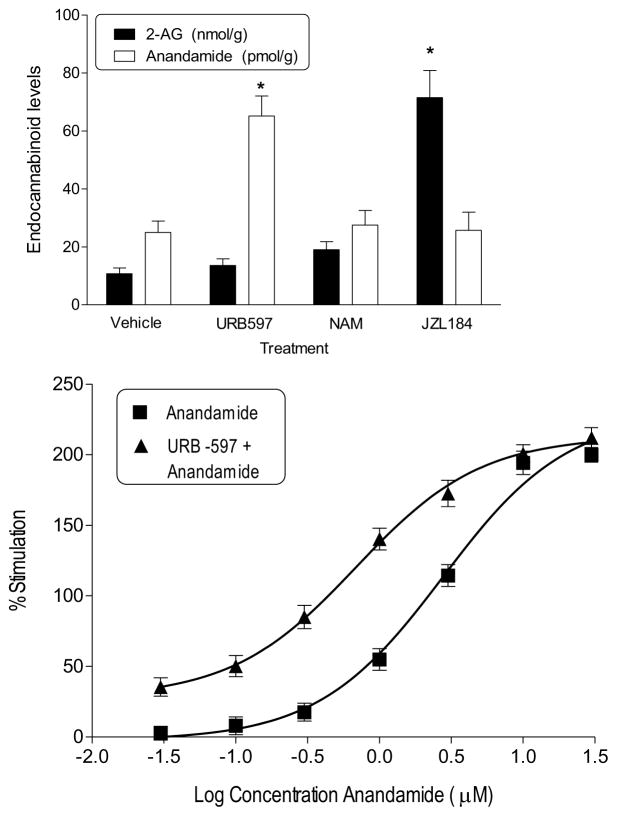
Figure 4 shows cerebellar concentrations of anandamide and 2-AG in mice following systemic injection with vehicle, 1 mg/kg URB597, 5 mg/kg NAM, or 16 mg/kg JZL184 (Fig. 4, top panel). Compared to vehicle, URB597 increased anandamide concentration without affecting concentration of 2-AG [F(3,16)=12.04, p<0.05]. In contrast, 16 mg/kg JZL184 increased 2-AG concentration without affecting anandamide concentration [F(3,16)=31.73, P<0.05] whereas 5 mg/kg NAM did not produce a significant increase in 2-AG concentration. The magnitude of increase in 2-AG was 5-fold greater for JZL184 than for NAM. Preliminary analysis also showed that rats treated with JZL184 (16 mg/kg) showed increased whole brain concentration of 2-AG (11.3 nmol/g, n=2), as compared to rats treated with vehicle (5.6 nmol/g, n=2), whereas anandamide concentration was similar in these rats (1.5 and 2.0 pmol/g in vehicle- and JZL184-treated rats, respectively).
Figure 4.
Top panel: Endocannabinoid concentrations in the cerebellum of male ICR mice following i.p. injection with vehicle, 5 mg/kg NAM, 1 mg/kg URB597 or 16 mg/kg JZL184. Bottom panel: Concentration effect curves for anandamide-stimulated [35S]GTPγS binding with and without addition of 50 nM URB597 in tissues from cerebellum of adult male ICR mice.
Figure 4 also shows the effects of anandamide alone and in combination with URB597 (10 – 50 nM) on [35S]GTPγS binding (Fig. 4, bottom panel). Emax values for anandamide-induced stimulation were similar with and without addition of URB597 (229.00 ± 6.66 % and 212.90 ± 3.49 %, respectively). In contrast, adding URB597 shifted the anandamide concentration curve to the left, resulting in EC50 values of 2.76 μM for anandamide alone and 0.69 μM for anandamide plus URB597.
4.0 Discussion
THC served as a discriminative stimulus in both rats and mice, as has been shown previously (Järbe and McMillan, 1979; McMahon et al., 2008; Vann et al., 2009; Wiley et al., 1993). In the three groups of THC-trained mice, initial dose-effect curves for THC-associated responding showed considerable overlap across the entire dose range tested. This remarkable similarity occurred despite substantial diversity in several procedural variables between Experiments 1 and 2. While the overall shape of the dose-effect curves for THC-associated responding was also similar across species, two major differences were apparent. The first was the higher training doses used in mice than in rats, with 5.6 to 30 mg/kg used for mice (McMahon et al., 2008; Vann et al., 2009; Wiley et al., 2011) and 1.8 to 5.6 mg/kg used for rats (Järbe et al., 2001; Järbe et al., 2006; Wiley et al., 2004). A second major species difference was the lower baseline response rate in mice than in rats, which occurred regardless of whether the response for mice was a lever press (Vann et al., 2009; Wiley et al., 2011) or a nose poke. Because training dose affects substitution in drug discrimination and because drug-induced suppression of response rates may interfere with choice behavior, these factors may serve as alternative explanations for differences that may otherwise appear to be due to species.
When its metabolism was not inhibited, anandamide failed to substitute for THC in mice (Vann et al., 2009; Wiley et al., 2011), as is consistent with most THC discrimination studies in rats (Burkey and Nation, 1997; Järbe et al., 2001; Wiley et al., 1998). The two FAAH inhibitors, PF3845 (Ahn et al., 2009) and URB597, produced different effects on anandamide substitution. Whereas PF3845 enhanced substitution of anandamide, URB597 did not. Neither compound produced THC-like effects on its own. The results with PF3845 are consistent with those of previous studies, in which inhibition of anandamide metabolism with URB597 (Solinas et al., 2007) or with phenylmethyl sulfonyl fluoride (PMSF) (Vann et al., 2009) also enhanced anandamide substitution. Cross-substitution of THC for anandamide has also been demonstrated in FAAH(−/−) mice trained to discriminate anandamide from vehicle (Walentiny et al., 2011). Together, these results suggest an overlap in the discriminative stimulus effects of anandamide and THC when the rapid metabolism of anandamide is inhibited, although the present URB597 results are at odds with this interpretation (however, see next paragraph). Interestingly, PF3845 did not shift the THC dose-effect curve, as did PMSF in a previous study (Vann et al., 2012), suggesting that PMSF may have produced the observed shift through greater magnitude of inhibition of endogenous anandamide (see PMSF in vitro data; Vann et al., 2012) or through inhibition of a non-FAAH pathway.
In contrast with increased substitution produced by anandamide and PF3845, URB597 failed to enhance substitution of anandamide for THC in mice and did not substitute on its own in either mice or rats, regardless of THC training dose. Given that URB597 has been shown to enhance anandamide’s substitution for THC in rats (Solinas et al., 2007), the present results may represent a species difference. Of note, however, 0.3 mg/kg URB597 increased cerebellar concentration of anandamide (but not 2-AG) and accentuated anandamide-stimulated CB1 receptor activation, confirming that it was an effective inhibitor of FAAH. Hence, a more likely explanation URB597’s failure to potentiate anandamide substitution for THC is the exquisite sensitivity to anandamide-induced decreases in response rates observed in THC-trained mice in Experiment 1, an effect that was not observed in Experiment 2. A response rate criterion (≥ 0.16 responses/s) implemented in the acquisition training of mice may have prevented a floor effect in response rate data in Experiment 2. Enhanced sensitivity of the mice in Experiment 1 appeared to be selective for endocannabinoids rather than for all cannabinoid agonists, as the mice did not exhibit a similar decrease in response rate when lower doses of THC were administered. While the reason for the potentiated potency of anandamide in decreasing responding in these mice remains unclear, it could be related to procedural variables such as route of administration or to the absence of a response rate criterion during acquisition. Whatever the cause, this anomaly may have interfered with adequate assessment of anandamide’s THC-like effects in this group of mice. Further, it may account for the different effects of the two FAAH inhibitors, URB597 and PF3845. PF3845 increased substitution of anandamide in THC-trained mice in Experiment 2, but only at doses of anandamide that could not be tested in combination with URB597 due to suppression of overall response rate.
2-AG also did not substitute for THC in mice when it was administered in the absence of a metabolic inhibitor, although it reduced response rates at higher doses. When co-administered with 1 mg/kg of the MAGL inhibitor NAM (Saario et al., 2005), a dose that was shown to potentiate the cannabimimetic effects of 2-AG in the tetrad tests in mice (Burston et al., 2008), 2-AG still did not substitute for THC. At 5 mg/kg, NAM failed to produce a significant increase in cerebellar concentration of 2-AG, suggesting that 1 mg/kg NAM may have been insufficient to inhibit MAGL despite the observed decreases in response rates. This hypothesis is consistent with the relatively poor potency and selectivity of NAM for MAGL (Matuszak et al., 2009). In contrast, the more potent and selective MAGL inhibitor JZL184 (Long et al., 2009a; Matuszak et al., 2009) increased nose pokes in the THC-associated aperture in mice even in the absence of 2-AG co-administration (Long et al., 2009c). Cannabinoid CB1 receptor mediation of this effect is suggested by its rimonabant reversal, as well as by the pronounced increase in brain 2-AG concentration produced by JZL184 (Long et al., 2009c). While JZL184 did not fully substitute for THC in wildtype mice or in rats in the present study, full substitution was observed in FAAH(−/−) mice trained to discriminate THC from vehicle and in wildtype mice treated with the dual FAAH/MAGL inhibitor JZL 195 (Long et al., 2009c). Similarly, maximal responding on the THC-associated lever in rats in the present study (68%) was engendered by co-administration of the FAAH and MAGL inhibitors, URB597 (30 mg/kg) and JZL184 (16 mg/kg), respectively.
Together with previous results, the present results suggest a complex interplay between anandamide and 2-AG in producing THC-like discriminative stimulus effects. Anandamide typically does not substitute for THC unless its rapid metabolism by FAAH is inhibited through pharmacological or genetic means (Solinas et al., 2007; Vann et al., 2009; Walentiny et al., 2011). Notably, however, flooding of the system by exogenous administration of anandamide is generally necessary for this substitution to occur; i.e., FAAH inhibitors increase anandamide concentration in the brain, but when administered alone, do not substitute for THC. Further, FAAH(−/−) mice have elevated brain concentration of anandamide, but do not show a distinct phenotype suggestive of a persistent THC-like state (Cravatt et al., 2001). In contrast with the anandamide profile, endogenous increases in 2-AG (e.g., through administration of JZL184) produced substantial substitution for THC even in the absence of exogenous flooding of the system with 2-AG. Further, MAGL(−/−) mice have both elevated brain concentrations of 2-AG and an altered phenotype suggestive of chronic stimulation of CB1 receptors (Schlosburg et al., 2010). These results suggest that endogenous increases in 2-AG may be more effective in inducing THC-like discriminative stimulus effects in mice than are endogenous increases in anandamide. Nevertheless, full substitution required addition of endogenous increases in anandamide via FAAH inhibition [through a dual inhibitor, JZL195, or JZL184 in FAAH(−/−) mice] (Long et al., 2009c). The lack of significant substitution of JZL184 alone in rats may be related to a species difference in compound potency (e.g., see Long et al., 2009b), to an insufficient dose (e.g., see mouse data), and/or to differences in training dose. Alternatively, JZL184 also inhibits FAAH, though its acute administration does not elevate whole brain anandamide concentrations (Long et al., 2009b). Consistent with this hypothesis, a novel MAGL inhibitor with increased selectivity (KML29) produced minimal substitution for THC in mice trained to discriminate THC (Ignatowska-Jankowska et al., 2014), but fully substituted for anandamide in FAAH (−/−) mice, an effect that was rimonabant reversible (Ignatowska-Jankowska et al., 2014).
In summary, elicitation of THC-like discriminative stimulus effects requires a complex interplay between anandamide and 2-AG. Although anandamide has been shown to substitute fully for THC, exogenous administration plus inhibition of its metabolism by FAAH is necessary. Endogenous increase in anandamide concentration via FAAH inhibition is not sufficient. In contrast, endogenous increase in 2-AG concentration may be more effective in promoting THC-like discriminative stimulus effects, as suggested by the partial substitution observed with JZL184 in THC-trained mice. Most effective, however, appears to be endogenous increases in both anandamide and 2-AG. The variable results across specific FAAH and MAGL inhibitors emphasizes the need for optimization of selectivity of pharmacological tools as well as testing of new models (e.g., MAGL knockout mice) in the discrimination paradigm.
Acknowledgments
Research supported by NIH/NIDA Research Grants DA-026449, DA-03672, and DA-09789, Center Core Grant DA-033934, and Training Grant DA-07027. The authors thank Anu Mahadevan (Organix, Inc.) for providing anandamide and 2-AG, Jonathan Long (Scripps Institute, La Jolla, CA) for providing JZL184, and Merck & Co., Inc., for providing URB597.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta-9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Sim-Selley LJ, Harloe JP, Mahadevan A, Razdan RK, Selley DE, Wiley JL. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–553. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids. 2002;121:135–148. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Boulaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Amer Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Gold L, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the discriminative stimulus properties of Δ9-THC and CP-55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther. 1992;262:479–486. [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabionoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwan BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-Psychopharmacol Biol Psychiatr. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O’Neal ST, Walentiny DM, Wiley JL, Cravatt BF, Lichtman AH. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: Antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of delta(9)-THC and (R)-methanandamide in rats. Psychopharmacology (Berl) 2006;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, McMillan DE. Discriminative stimulus properties of tetrahydrocannabinols and related drugs in rats and pigeons. Neuropharmacology. 1979;18:1023–1024. doi: 10.1016/0028-3908(79)90171-0. [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochem. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nature Chem Biol. 2009a;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009b;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA. 2009c;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Moller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Matuszak N, Muccioli GG, Labar G, Lambert DM. Synthesis and in vitro evaluation of N-substituted maleimide derivatives as selective monoglyceride lipase inhibitors. J Med Chem. 2009;52:7410–7420. doi: 10.1021/jm900461w. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington, D.C: 2003. [PubMed] [Google Scholar]
- Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Jarvinen T, Niemi R. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vann RE, Walentiny DM, Burston JJ, Tobey KM, Gamage TF, Wiley JL. Enhancement of the behavioral effects of endogenous and exogenous cannabinoid agonists by phenylmethyl sulfonyl fluoride. Neuropharmacology. 2012;62:1019–1027. doi: 10.1016/j.neuropharm.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of Delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner JA, Nguyen TK, Grainger DB, Wiley JL, Vann RE. The endogenous cannabinoid anandamide shares discriminative stimulus effects with (9)-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol. 2011;656:63–67. doi: 10.1016/j.ejphar.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J, Ryan W, Razdan R, Martin B. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Britt DT, Balster RL, Martin BR. Discriminative stimulus effects of delta 9-tetrahydrocannabinol and delta 9-11-tetrahydrocannabinol in rats and rhesus monkeys. Neuropharmacology. 1993;32:359–365. doi: 10.1016/0028-3908(93)90157-x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Matthew Walentiny D, Vann RE, Baskfield CY. Dissimilar cannabinoid substitution patterns in mice trained to discriminate Delta(9)-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol. 2011;22:480–488. doi: 10.1097/FBP.0b013e328348eced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]