Abstract
Objective
To evaluate the discrepancy of endophenotypic performance between probands with schizophrenia and unaffected siblings by paternal age at proband birth, a possible marker for de novo mutations.
Methods
Pairs of schizophrenia probands and unaffected siblings (N=220 pairs) were evaluated on 11 neuropsychological or neurophysiological endophenotypes previously identified as heritable. For each endophenotype, the sibling-minus-proband differences were transformed to standardized scores. Then for each pair, the average discrepancy was calculated from its standardized scores. We tested the hypothesis that the discrepancy is associated with paternal age, controlling for the number of endophenotypes shared between proband and his or her sibling, and proband age, which were both associated with paternal age.
Results
The non-significant association between the discrepancy and paternal age was in the opposite direction from the hypothesis. Of the 11 endophenotypes only sensori-motor dexterity was significant, but in the opposite direction. Eight other endophenotypes were also in the opposite direction, but not significant.
Discussion
The results did not support the hypothesized association of increased differences between sibling/proband pairs with greater paternal age. A possible explanation is that the identification of heritable endophenotypes was based on samples for which schizophrenia was attributable to inherited rather than de novo/non-inherited causes.
Keywords: de novo mutations, copy number variants, genetic risk, familial schizophrenia, sporadic schizophrenia
1. Introduction
Genetic factors have long been implicated in schizophrenia, but identifying specific genetic causes has been difficult; it is highly likely that multiple genes of modest effect are involved, but rare de novo (i.e. non-familial) genetic variants also appear to have key roles in some cases (Gejman et al, 2011). For genetic studies seeking to isolate specific genes related to schizophrenia liability, it would be useful to identify characteristics that might separate cases with primarily familial variants from those with de novo genetic mutations.
Previous studies observed the following generalities: a) Increased age is associated with a higher rate of de novo mutations and other genomic abnormalities in male germ cells (Crosnoe and Kim, 2013; Singh et al, 2003); b) Such abnormalities are more common in schizophrenia than in normal controls (Walsh et al, 2008); and, c) Older paternal age is associated with greater risk of schizophrenia (Malaspina, 2001). A model that explains how a) and b) could imply c) is that de novo mutations and other genomic abnormalities are more frequently among the contributing factors leading to schizophrenia when the proband has an older father versus a younger one (Kong et al, 2012). It is further supported by a report that schizophrenia patients without a family history of schizophrenia have significantly older fathers than those with such a history (Malaspina et al, 2002). In this model, the proportion of schizophrenia attributable to de novo mutations is larger when the father is older. Conversely, inherited schizophrenia and its associated characteristics will be less common as the father's age increases. Although each piece of the model has been tested, the full model has not been directly tested. The present study was designed to test whether the age of the father at the birth of a schizophrenia proband – called “paternal age”– might be useful in this way for genetic studies of schizophrenia endophenotypes.
An endophenotype is a heritable characteristic associated with a disorder of interest that may be more strongly associated with some liability genes than others. Using endophenotypes may thus reduce genetic complexity, compared with the disorder itself (Gottesman and Gould, 2003), although some traits may possess their own complexity. A defining characteristic of an endophenotypic deficit is that – compared with the general population – not only will it be more prevalent in those with the disorder, but also in their relatives without the disorder, albeit perhaps smaller (Gottesman and Gould, 2003). Heritability implies that measures of endophenotypes will tend to be more similar between probands and their siblings than either would be with unrelated unaffected individuals. In contrast, when the genetic component of the disease is primarily caused by de novo genetic mutations, probands and their unaffected siblings, who are not monozygotic twins, would have more discrepant measures of endophenotypes.
We hypothesized that the endophenotypic discrepancy would increase as paternal age increased. To test this hypothesis, we used the Consortium on the Genetics of Schizophrenia (COGS) family database (Calkins et al, 2007) of schizophrenia proband families with one unaffected sibling (called here “sibling/proband pairs”). From a battery of psychophysiological and neuropsychological endophenotypes, we selected the 11 previously identified in a family-based study as heritable (Greenwood et al, 2007). For each sibling/proband pair, we summarized the differences in scores on those endophenotypes that were measured on both members of the pair, and tested whether in fact endophenotypic discrepancies increased as paternal age increased.
2. Methods
2.1 Subjects
Subjects were participants in the COGS-I, a multi-site family-based study of the genetics of neuro-cognitive and neurophysiological endophenotypes associated with schizophrenia. Full details of the COGS-I recruitment and assessment procedures are reported elsewhere (Calkins et al, 2007) and included standardized clinical assessment interviews of all subjects. All participants who are endophenotyped were between the ages of 18–65 and able to understand and provide informed consent. Consenting parents older than 65 had their blood drawn for genotyping purposes but did not undergo endophenotyping. The core family structure for COGS-I required at least one sibling/proband pair, i.e., the presence of a proband with schizophrenia and at least one unaffected full sibling. The probands were required to meet criteria for DSM-IV schizophrenia, following a standardized clinical assessment, medical record review, and best estimate diagnostic evaluation. Unaffected siblings never met criteria for any psychotic disorder, major affective disorder, or schizotypal personality disorder by the same clinical assessment. Subjects were screened for illicit drug or alcohol use at the time of testing and excluded if they tested positive. Also excluded were subjects who met criteria for a substance abuse disorder in the past month or a substance dependence disorder in the past 6 months. In the present study, a sibling/proband pair from each family was identified from those for whom the paternal and maternal ages at birth of the proband were known. Interviewers and endophenotypers were not blind to diagnostic group, but blind to the hypothesis of the study. An endophenotype that was tested in both the sibling and the proband from the same family was called a “shared endophenotype.” If there was more than one unaffected sibling, we used the one with the largest number – at least two – of shared endophenotypes (if tied, the oldest sibling).
2.2 Endophenotype assessment
From the COGS endophenotype assessment battery, we selected those among the primary measures previously identified as heritable in a family-based study (Greenwood et al., 2007), and described in detail elsewhere:
Antisaccade performance. In this test of oculomotor inhibition, participants are asked to fixate on a central target and respond to a peripheral cue by looking in the opposite direction at the same distance. The ratio of correct antisaccades to total interpretable saccades is measured (Radant et al., 2007).
Prepulse Inhibition of the startle response (PPI). Prepulse inhibition refers to the dampening of a response to a strong startling stimulus (pulse) when the stimulus is preceded by a weaker prestimulus (prepulse).This was measured as the percentage of inhibition of the startle reflex in response to a weak pre-stimulus using a 60-millisecond interstimulus interval (Braff et al, 1978; Braff et al, 2001).
Continuous Performance Test (CPT). The Degraded Stimulus version of the CPT (DS-CPT) is a widely used measure of deficits in sustained, focused attention with a high perceptual load. Participants detect a blurred target digit in a series of blurred other digits. DS-CPT performance was measured using the signal/noise discrimination index (d′) (Nuechterlein and Asarnow, 1999; Nuechterlein et al, 1983).
Letter-Number Span (LNS). This is a measure of working memory information storage with manipulation. It is measured as the correct reordering of intermixed numbers and letters (Horan et al, 2008; Gold et al, 1997; Lenzenweger and Gold, 2000).
California Verbal Learning Test (CVLT). The CVLT is a list-learning test that assesses verbal learning and memory. The total recall score of a list of 16 verbally presented items summed across 5 trials is measured (Stone et al, 2011).
- Penn Computerized Neurocognitive Battery (CNB). The Penn CNB is a computerized neuropsychological test battery used to assess a range of neuropsychological functions (Gur et al, 2001b; Gur et al., 2001a). Each test is measured as “efficiency,” a combination of accuracy (percentage correct) and speed (median response time in milliseconds), which is calculated as accuracy/log 10 (speed) and is expressed as standard equivalents (z scores). In the COGS modification of the Penn CNB, the following six cognitive domains were found to be heritable in the earlier family-based study and thus included in the present study:
- abstraction and mental flexibility - 4 objects are presented on a computer screen and the participant must choose the 1 that does not belong;
- face memory - participants are asked to recognize 20 previously presented target faces among 20 distracter faces;
- spatial memory - identical format as face memory except that Euclidean shapes, rather than faces, are used;
- spatial processing - two lines are presented at an angle, and the corresponding lines must be identified on a simultaneously presented array;
- sensorimotor dexterity - the participant is asked to click as quickly as possible using the computer mouse on a target that gets increasingly smaller;
- emotion processing - involves correctly identifying a variety of facial expressions of emotion.
2.3 Statistics
For each of the endophenotypes, it was hypothesized that the difference between the unaffected sibling and the proband would be larger for older paternal age. If we had complete data, the hypothesized relationships for each endophenotype could be tested by a repeated measures analysis of covariance, with the dependent variables being the sibling score minus the proband score in each family, with paternal age as the covariate. The tests for paternal age would be the association with the average across all the endophenotypes and the interaction of paternal age with the differences among the endophenotypes – i.e. the heterogeneity among the endophenotypes' associations with paternal age. However, in this dataset, only 80 families had proband/sibling pairs with all 11 endophenotypes of interest. If either the proband or the sibling is missing on any specific endophenotype, the requirement for complete data in a repeated measurements analysis excludes that pair from the entire analysis. Missing data would not preclude separate analyses for each endophenotype, on the available sibling/proband pairs, using the Bonferroni inequality. However, this would penalize for performing 11 tests of significance, even when the results are consistent across endophenotypes.
Thus, rather than primarily focusing on single endophenotypes, we chose to focus on the sibling/proband pairs, evaluating each across the available endophenotypes. In an idealized outcome with no interaction, if all endophenotypes have the same mean and variance, each sibling/proband pair would have the same difference for all endophenotypes. Insofar as the endophenotypes have different units of measurement, differences for a pair on different endophenotypes would be numerically different although comparable. Therefore, for each endophenotype, we created standardized scores (mean = 0, standard deviation = 1) from the sibling minus proband differences. Then for each sibling/proband pair, we evaluated the “average discrepancy” of the standardized differences on their shared endophenotypes, and used this as the unit of analysis. We calculated partial correlations between the average discrepancy and paternal age with relevant covariates added as described below.
We considered the following as potential covariates: the number of shared endophenotypes, sibling's and proband's ages and sexes; father's, mother's, and sibling's years of education; maternal age at proband's birth; paternal and maternal age at birth of the first unaffected sibling; proband age at onset; and multiplex vs. simplex family status. Of these, we included the variables that were significantly associated with average discrepancy in sibling/proband pairs for the shared endophenotypes. We did not consider proband's years of education, since it may be substantially associated with age at onset and the course of the disorder. An additional analysis evaluated the quadratic model for proband's paternal age, by testing the quadratic component.
3. Results
Of 274 proband families, 54 (19%) were missing either at least one paternal age (N=41) or at least 2 shared endophenotypes (N=11) or both (N=2). Standardized differences between shared endophenotypes were calculated from the remaining 220 families with sibling/proband pairs. The average number of shared endophenotypes was 9.3 ± 2.3. Among the sibling/proband pairs, there were significant associations with the average discrepancy for the number of shared endophenotypes (r = -0.323, p < 0.0005) and proband age (r = 0.179, p = 0.01), but not for the other potential covariates. Thus, in the analyses of the association of paternal age with average discrepancy and also the sibling/proband raw difference for each of the 11 endophenotypes, these two variables were used as covariates when we calculated the partial correlations. The Bonferroni adjustment for 11 endophenotypes was p = 0.05 / 11 = 0.0045. The motivation for focusing on the average discrepancy was to avoid this Bonferroni adjustment.
Table 1 presents characteristics of the 220 sibling/probands pairs, for the probands, their selected siblings, and their parents. Paternal age ranged from age 17 to 63 and, as expected, the distribution was somwwhat skewed at the upper end (skewness=0.92), as there tend to be more older father exceptions than younger ones. After controlling for the two covariates, paternal age was not associated with the average discrepancy between siblings' and probands' endophenotype scores (partial r = −0.094, df = 216, p = 0.22). For exploratory purposes, we included the maternal age of the proband as an additional covariate and the results were not significant (partial r = −0.030, df = 215, p = 0.66). As shown in Table 2, the only specific endophenotypes that even approached statistical significance (without the Bonferroni adjustment) were the cognitive domain score for sensorimotor dexterity (partial r = −0.206, df = 185, p = 0.0047) and abstraction and mental flexibility (partial r = -0.123, df = 182, p = 0.097). The remaining partial correlations ranged from −0.107 to .060. Although none of these other correlations were significant, we noted that a total of 9 of 11 were negative. The negative correlations indicated that the sibling/proband discrepancies were nominally smaller as paternal age increased, contrary to our hypothesis. There were no significant associations for the quadratic model (partial r = -0.044, df = 215, p = 0.52).
Table 1.
Characteristics of sibling/proband pairs.
| Sibling | Proband | Within Pair Difference | Test Statistic (df), p | |
|---|---|---|---|---|
| Age: mean (SD) | 33.3 (10.1) | 32.8 (9.8) | 0.4 (4.7) | t (219) = 1.36, 0.174 |
| Male: N (%) | 104 (47) | 164 (75) | 114 (52) | Chi-square (1) = 0.027, 0.87 |
| Years of education: mean (SD) | 15.5 (2.4) | 13.6 (2.1) | 1.9 (2.7) | t (218) = 10.16, <0.0005 |
| Onset age: mean (SD) | NA | 20.9 (5.0) | ||
| Shared Family Characteristics | ||||
| Paternal age at proband's birth: mean (SD) | NA | 30.1 (6.7) | ||
| Maternal age at proband's birth: mean (SD) | NA | 28.2 (5.2) | ||
| Father's years of education: mean (SD) | NA | 15.3 (3.3) | ||
| Mother's years of education: mean (SD) | NA | 14.6 (3.0) | ||
| Multiplex: N (%) | NA | 14 (6) |
Table 2.
Partial correlations of paternal age with the endophenotypic average difference and individual endophenotypes, controlling for proband age and number of shared endophenotypes.
| Endophenotype | Paternal Age partial r (df), p |
|---|---|
| Average Difference | -0.09 (216), 0.22 |
| Antisaccade | -0.11 (165), 0.17 |
| PPI | 0.03 (127), 0.75 |
| CPT | -0.03 (181), 0.66 |
| LNS | -0.02 (210), 0.82 |
| CVLT | 0.06 (204), 0.40 |
| CNB-abstraction & mental flexibility | -0.123 (182), 0.10 |
| CNB-face memory | -0.08 (193), 0.27 |
| CNB-spatial memory | -0.01 (188), 0.88 |
| CNB-spatial processing | -0.09 (174), 0.22 |
| CNB-sensori-motor dexterity | -0.21 (185), 0.005 |
| CNB-emotion processing | -0.08 (188), 0.28 |
4. Discussion
Excluding more likely de novo schizophrenia cases would enhance studies of inherited genetic variants, so we performed this study of schizophrenia endophenotypes to assess whether paternal age might serve as a proxy for de novo mutations. The results did not support the hypothesis that endophenotypic average discrepancies in sibling/proband pairs increases as paternal ages of probands with schizophrenia increases. For each sibling/proband pair, we summarized the average discrepancy of up to 11 primary endophenotypes in COGS previously found to be heritable among schizophrenia proband families (Greenwood et al, 2007). The relationship between paternal age and the summary was non-significant and in the direction opposite from the hypothesis. Additionally, when endophenotypes were examined individually, the only significant endophenotype – but not significant by the Bonferroni adjustment for 11 endophenotypes – was in the opposite direction from that hypothesized, as were 8 of the other 10 endophenotype results.
In contrast to our model, paternal age may be a poor proxy for de novo mutations in schizophrenia. No study to our knowledge has specifically examined the effect of paternal age on the rate of de novo mutations in schizophrenia compared with the general population. It is possible that paternal age is a risk factor for schizophrenia more from underlying sociological causes than from biological or genomic ones. Paternal age might be a risk factor for schizophrenia because older fathers are more likely to have greater genetically mediated schizophrenia related personality traits, such as aloofness and inappropriate rapport (Kendler et al, 1995). The presence of these traits might both indicate a greater genetic load and tend to delay mating in the social world. Matching the Danish national psychiatric registry with the Danish Civil Registry System, risk for schizophrenia was associated with late fatherhood (age at the birth of the first child), and controlling for this nullified the association of risk for schizophrenia with paternal age of the proband (Petersen et al, 2011). This suggests that late fatherhood underlies the increased risk for schizophrenia observed with older paternal age. Consistent with this possibility, we cautiously note that for the individual endophenotypes most of the discrepancies were nominally reduced with increasing paternal age, which would be expected if older fathers were carrying a greater familial/genetic load.
However, two comparisons of probands with and without a family history for schizophrenia do not support this late fatherhood model. In the Danish study, risk for schizophrenia was similarly associated with later fatherhood in probands with and without a family history of schizophrenia (Petersen et al, 2011). Contrary to the late fatherhood model, probands with a positive family history have been observed to have relatively younger fathers than probands without a family history (Malaspina et al, 2002). It must be noted that in both of these studies, proband family history does not ensure paternal family history of schizophrenia which would be a basis for the late fatherhood model.
Our model assumed that the traits assessed were associated with schizophrenia, regardless of whether schizophrenia was attributable to inherited or de novo/non-inherited causes. An alternative endophenotypic model would explain the lack of significance by assuming that these traits are only found in the inherited form. In both the original and the alternative model, for inherited schizophrenia, siblings would average between unrelated, unaffected individuals and probands, since the sibling would tend to have an incomplete version of the genetic load of the proband. In the original model, for de novo schizophrenia, the sibling would not carry the deficit, and instead be like individuals in families without schizophrenia, very different from the proband. In the alternative model, both the de novo proband and the sibling would not have any inherited endophenotypic deficits, and thus the pair would not be substantially different from each other. In this regard, in a separate study from our consortium, the association between paternal age and the severity of endophenotypic performance was recently assessed in both primary and secondary endophenotypes (Tsuang et al, 2014). Only one (of sixteen) endophenotypes was significantly associated with paternal age (a secondary endophenoytype in the COGS, the CPT-IP, and thus not included in this study and not previously included in our heritability study). The specific result indicated better performance was associated with older fathers, consistent with observed results in this study, contrary to the original hypothesis. However, this result did not remain significant after adjusting for sixteen comparisons.
Our sample was relatively large, but limited by having few probands with truly late paternal ages, for which the effect of paternal age is stronger (Malaspina et al, 2001). Of the 220 sibling/proband pairs, only 21 had paternal ages at or beyond age 40. The dearth of older fathers might have been exacerbated by the ascertainment methods in the COGS-I family study, which focused on recruiting schizophrenia proband families with a minimum of two offspring and living parents who could be recruited for a blood draw and, if younger than age 65, endophenotypic testing. This recruitment goal might have reduced ascertainment of families with older fathers. Nevertheless, we do regard this group as constituting “older fathers” with respect to schizophrenia risk. A recent study of the Danish population found that, compared with rates for schizophrenia for offspring of fathers aged 25-29, the incidence rate ratios (IRR) for offspring of fathers at greater ages, categorized by five year intervals, were all significantly higher. The IRRs rose from 1.08 to 1.21 to 1.40, as paternal age groups increased from 30-34 to 35-39 to 40-44, but for fathers aged 45+ the IRR rose less, to 1.47 (McGrath et al, 2014), despite the open age range.
Another limitation was that by using only one sibling/proband pair per family we avoided concerns about lack of independence between multiple pairs within the same family, but at the same time we did not exploit all the information in the family that might have been present in some of the families. Finally, in autism, another disorder in which paternal age has been found to be a risk fact (McGrath et al., 2014), we note that the presence of a child with autism may lead a reduced number of subsequent children (stoppage rules), but the diagnosis of schizophrenia is usually substantially later. Nonetheless, if the first child has schizophrenia, there may be premorbid traits that could inhibit having additional children afterwards, which would make the family ineligible for our study.
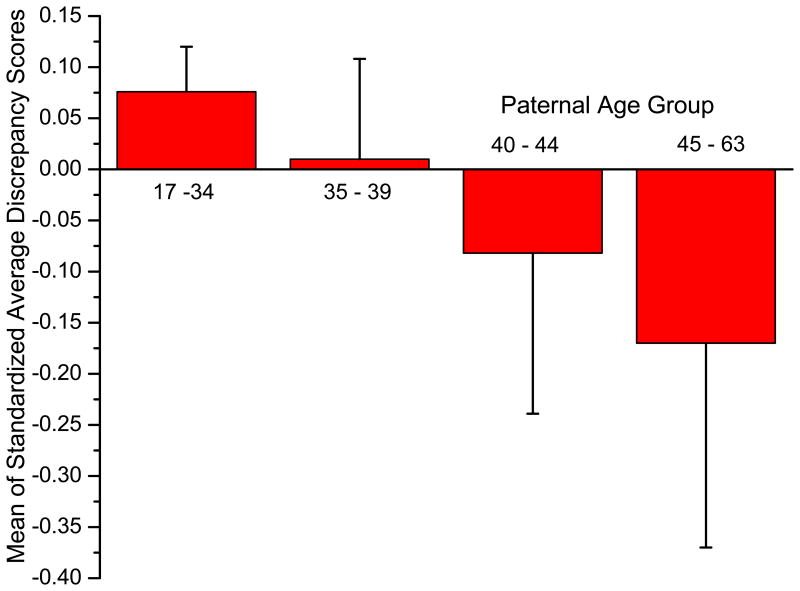
To present descriptively the impact of proband age after age 40 on the average discrepancy, we created four categories, 34 and below (n = 165), 35 to 39 (n= 34), 40 to 44 (n = 13), and 45 to 63 (n= 8). Figure 1 shows their estimated average discrepancy, after controlling for the number of shared endophenotypes and proband age in an analysis of covariance. Although there was no statistical significance (F [3, 214] = 0.809, p = 0.49), not surprising in view of the small sample sizes for probands over age 40, this result confirms the failure of the original hypothesis.
Figure.
The mean sibling/proband average discrepancy by age group, covarying for proband age and the number of shared endophenotypes, with standard deviation bars.
Contrary to our hypothesis, we did not find that the average discrepancy between sibling and proband performance was associated with paternal age. Moreover, the only significant association among the 11 endophenotyes, and the majority of associations, were in the opposite direction. A possible explanation is that the endophenotypes included in this study were identified due to their relevance to genetically-transmitted schizophrenia, but they are not relevant to schizophrenia arising from de novo mutations.
Acknowledgments
This material is based upon work supported by NIMH grants R01 MH65571, R01 MH042228, R01 MH65588, R01 MH65562, R01 MH65707, R01 MH65554, R01 MH65578, R01 MH086135, and R01 MH65558. We are grateful to all the subjects who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. The consortium on the genetics of endophenotypes in schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophrenia Bulletin. 2007;33:33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnoe LE, Kim ED. Impact of age on male fertility. Current Opinion in Obstetrics and Gynecology. 2013;25:181–185. doi: 10.1097/GCO.0b013e32836024cb. [DOI] [PubMed] [Google Scholar]
- Gejman PV, Sanders AR, Kendler KS. Genetics of schizophrenia: new findings and challenges. Annual Review of Genomics and Human Genetics. 2011;12:121–144. doi: 10.1146/annurev-genom-082410-101459. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Archives of General Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001a;25:777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001b;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Horan WP, Braff DL, Nuechterlein KH, Sugar CA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Green MF. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophrenia Research. 2008;103:218–228. doi: 10.1016/j.schres.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Archives of General Psychiatry. 1995;52:296–303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A, Wong WS, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, Thorsteinsdottir U, Stefansson K. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, Gold JM. Auditory working memory and verbal recall memory in schizotypy. Schizophrenia Research. 2000;42:101–110. doi: 10.1016/s0920-9964(99)00121-8. [DOI] [PubMed] [Google Scholar]
- Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizohrenia Bulletin. 2001;27:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, Goetz D, Goetz R, Harlap S, Gorman J. Paternal age and sporadic schizophrenia: evidence for de novo mutations. American Journal of Medical Genetics. 2002;114:299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Archives of General Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A Comprehensive Assessment of Parental Age and Psychiatric Disorders. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2013.4081. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Asarnow RF. Degraded Stimulus Continuous Performance Test (DS-CPT): Program for IBM-Compatible Microcomputers. Version 8.11. Los Angeles, CA: 1999. [Google Scholar]
- Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science. 1983;220:327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. American Journal of Psychiatry. 2011;168:82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, Freedman R, Green MF, Greenwood TA, Gur RE, Light GA, Meichle SP, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang MT, Turetsky BI, Tsuang DW. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophrenia Research. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertility and Sterility. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Stone WS, Giuliano AJ, Tsuang MT, Braff DL, Cadenhead KS, Calkins ME, Dobie DJ, Faraone SV, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Roe AH, Schork NJ, Siever LJ, Silverman JM, Swerdlow NR, Thomas AR, Tsuang DW, Turetsky BI, Seidman LJ. Group and site differences on the California Verbal Learning Test in persons with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia (COGS) Schizophrenia Research. 2011;128:102–110. doi: 10.1016/j.schres.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang D, Esterberg M, Braff D, Calkins ME, Cadenhead K, Dobie DJ, Freedman R, Green MF, Greenwood TA, Gur RC, Gur RE, Horan WP, Lazzeroni LC, Light GA, Millard SP, Olincy A, Nuechterlein KH, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sprock J, Sugar CA, Swerdlow NR, Tsuang MT, Turetsky BI, Radant AD. Is there an association between advanced paternal age and endophenotype deficit levels in schizophrenia? PLoS One. 2014 doi: 10.1371/journal.pone.0088379. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]