Abstract
IMPORTANCE
Invasive candidiasis in premature infants causes mortality and neurodevelopmental impairment. Fluconazole prophylaxis reduces candidiasis, but its effect on mortality and the safety of fluconazole is unknown.
OBJECTIVE
To evaluate the efficacy and safety of fluconazole in preventing death or invasive candidiasis in extremely low-birth-weight infants.
DESIGN, SETTING, AND PATIENTS
This study was a randomized, blinded, placebo-controlled trial of fluconazole in premature infants. Infants weighing less than 750 g at birth (N = 361) from 32 neonatal intensive care units (NICUs) in the United States were randomly assigned to receive either fluconazole or placebo twice weekly for 42 days. Surviving infants were evaluated at 18 to 22 months corrected age for neurodevelopmental outcomes. The study was conducted between November 2008 and February 2013.
INTERVENTIONS
Fluconazole (6 mg/kg of body weight) or placebo.
MAIN OUTCOMES AND MEASURES
The primary end point was a composite of death or definite or probable invasive candidiasis prior to study day 49 (1 week after completion of study drug). Secondary and safety outcomes included invasive candidiasis, liver function, bacterial infection, length of stay, intracranial hemorrhage, periventricular leukomalacia, chronic lung disease, patent ductus arteriosus requiring surgery, retinopathy of prematurity requiring surgery, necrotizing enterocolitis, spontaneous intestinal perforation, and neurodevelopmental outcomes—defined as a Bayley-III cognition composite score of less than 70, blindness, deafness, or cerebral palsy at 18–22-months corrected age.
RESULTS
Among infants receiving fluconazole, the composite primary end point of death or invasive candidiasis was 16% (95% CI, 11%–22%) vs 21% in the placebo group (95% CI, 15%–28%; odds ratio 0.73 [95% CI 0.43–1.23]; P=.24; treatment difference −5% [95% CI, −13%–3%]). Invasive candidiasis occurred less frequently in the fluconazole group (3% [95% CI, 1%–6%] vs the placebo group (9% [95% CI, 5%–14%]; P=.02; treatment difference −6% [95% CI, −11%–−1%]). The cumulative incidences of other secondary outcomes were not statistically different between groups. Neurodevelopmental impairment did not differ between the groups (fluconazole 31% [95% CI, 21–41%] vs placebo, 27% [95% CI, 18–37%]; P=.60; treatment difference 4% [95% CI, −10–17%]).
CONCLUSIONS AND RELEVANCE
Among infants with a birth weight of less 750 g, 42 days of fluconazole prophylaxis compared with placebo did not result in a lower incidence of the composite of death or invasive candidiasis. These findings do not support the universal use of prophylactic fluconazole in extremely-low-birth-weight infants.
TRIAL REGISTRATION
ClinicalTrials.gov number NCT00734539
Invasive candidiasis is an important cause of late-onset infection in the premature infant; its signs are often subtle, and despite antifungal therapy, its consequences in the premature infant are severe.1,2 Following a Candida infection, nearly 70% of infants with birth weight of less than 1000 g died or experienced severe neurodevelopmental impairment, despite antifungal therapy.2 Fluconazole prophylaxis has been shown in randomized, placebo-controlled trials to reduce the incidence of invasive candidiasis in neonatal intensive care units (NICUs) with a high burden (≥15%) of candidiasis.3,4 Current recommendations include the use of fluconazole prophylaxis for infants with a birth weight of less than 1000 g who receive care in NICUs with high rates of invasive candidiasis.5
However, most NICUs in the United States and the European Union have a lower burden of disease and have not uniformly adopted prophylaxis, based on controversies regarding high-risk patients, resistance, and safety.5–7 Current evidence suggests that the incidence of invasive candidiasis among infants 1001 g to 1500 g birth weight is 1% and among infants 751 g to 1000 g birth weight, incidence is 3%.8 Surveys of NICUs in the United States and United Kingdom found that only 15% to 34% of NICUs use fluconazole prophylaxis.6,7 In addition, previous studies of fluconazole prophylaxis have mainly evaluated short-term outcomes during hospitalization, with only 1 single-center study assessing the effect of fluconazole prophylaxis on long-term neurodevelopment.9 Our goal was to evaluate the safety and efficacy of fluconazole in preventing invasive candidiasis or death among infants with a birth weight of less than 750 g in NICUs with lower incidence, and to determine the effect of fluconazole prophylaxis on neurodevelopment in surviving infants.
Methods
Sites and Patients
From November 2008 to January 2011, infants were enrolled at 32 NICUs in the United States. Study follow-up was completed in February 2013. Three sites considered for the study were using fluconazole prophylaxis as routine care in infants with a birth weight of less than 750 g and, therefore, were ineligible to participate. Seventeen additional sites lacked the infrastructure or research coordinator support to perform the trial. Infants with birth weight less than 750 g and less than 120 hours old were eligible for enrollment. Infants were excluded if they were receiving systemic antifungal therapy, were diagnosed with congenital or invasive candidiasis, or who had aspartate transaminase (AST) or alanine aminotransferase (ALT) levels greater than250 U/L or creatinine greater than 2 mg/dL.
Enrolled infants were randomized by interactive voice recognition system (Almac). Randomization was by block (n=4) and stratified by site and sibling enrollment status. Siblings were assigned to the same treatment group. Treatment group was blinded from site investigators, including clinicians performing neurodevelopmental assessments at 18- to 22- months corrected age, and from parents for the duration of the study. Enrolled infants received fluconazole (6 mg/kg twice weekly, or normal saline placebo. Study drug was administered intravenously in infants with intravenous access and enterally by orogastric tube to infants without intravenous access. Unblinded site pharmacists mixed the placebo to be the same consistency and color as study drug.
Infants received the first dose of study drug by 120 hours of life and continued for 42 days unless the infant developed invasive candidiasis, developed evidence of liver failure (liver enzymes or conjugated hyperbilirubinemia >5-fold the upper limit of normal), or received more than 5 days of empirical antifungal therapy.
End Points
The primary end point was a composite of death or definite or probable invasive candidiasis prior to study day 49 (1 week after completion of study drug). Definite candidiasis was defined as a positive Candida culture from a normally sterile body fluid such as blood, cerebrospinal fluid, peritoneal fluid, or urine obtained via suprapubic aspiration or in/out catheterization. Probable invasive candidiasis was defined as receipt of more than 5 days of consecutive antifungal therapy and both thrombocytopenia (<150,000/103 μL) and a positive Candida culture from a non-sterile site (e.g., bag urine). Secondary end points included death prior to study day 49 and probable or definitive candidiasis prior to study day 49.
Safety end points included liver function as assessed weekly by AST or ALT, gamma-glutamyl transpeptidase (GGT), and conjugated bilirubin if collected as part of routine care; bacterial infection (positive culture from normally sterile body fluid); length of stay; intracranial hemorrhage; periventricular leukomalacia; chronic lung disease (supplemental oxygen at 36-weeks corrected gestational age); patent ductus arteriosus requiring surgery; retinopathy of prematurity requiring surgery; necrotizing enterocolitis (Bell stage II or III);10 and spontaneous intestinal perforation. Cultures from normally sterile body fluids positive for coagulase-negative staphylococci were considered bacterial infections.
Neurodevelopment was a secondary efficacy end point and was measured at 18- to 22-months corrected age. Neurodevelopmental impairment was defined as the presence of at least 1 of the following criteria: Bayley-III cognition composite score of less than 70, blindness, deafness, or diagnosis of cerebral palsy.11,12 The comprehensive neurodevelopmental evaluation included an interview with the infant’s primary caretaker, an assessment of cognition, language, and motor development using the Bayley Scales of Infant Development III, a neurologic examination, and ascertainment of hearing and vision impairment. The Bayley-III cognitive composite score has a mean (SD) value of 100 (15) with a minimum score of 55 and a maximum of 145. A Bayley-III cognitive composite score of less than 70 (>2 standard deviations below the mean) was considered significantly delayed. Cerebral palsy was defined as non-progressive disorder characterized by abnormal tone in at least 1 extremity and abnormal control of movement and posture and could be mild, moderate, or severe. Blindness was defined as no functional vision in either eye. Deafness was defined as inability to understand commands in spite of amplification, hearing aids, or cochlear implants in both ears.
Statistical Analysis
Based on prior studies,3,4 we estimated the incidence of death or candidiasis among infants with a birth weight of less than 750 g was 30%. We hypothesized that fluconazole prophylaxis would decrease the incidence of death or candidiasis in the treated patients by 15% (absolute difference). Previous studies had showed a 16% decrease in invasive candidiasis and a smaller decrease in mortality with the use of prophylaxis. With a sample size of 360, we had 92% power to observe a decrease from 30% to 15% in the primary outcome.
The full analysis dataset was defined as all randomized infants who received at least 1 dose of study drug (modified intent-to-treat analysis). All statistical tests were 2-sided with a type I error of 0.05. Demographics, baseline characteristics, and secondary end points were summarized and compared between treatment groups. Because race may affect outcomes in premature infants, maternal race and ethnicity were recorded based on maternal self-report. Ninety-five percent CIs for proportions and risk differences were calculated using asymptotic Wald CIs or exact CIs for small cell counts. A χ2 test or Fisher’s exact test was used to assess discrete variables and 1-way analysis of variance (ANOVA) or the Wilcoxon rank sum test was used for continuous variables to assess any differences between treatment groups.
In the analysis of the primary end point, we estimated that approximately 20% of the analysis population would be from multiple births. We therefore used a generalized estimating equation method to account for correlated data when analyzing the treatment effect on the primary end point. For infants with missing information for the primary end point (n=3), we analyzed the primary end point in a prespecified sensitivity analysis—assigning the infants with missing information as failures, assigning them as successes, and assigning them as missing. The results did not differ between the 3 analyses. Each of the 3 infants with missing information was transferred to a lower-acuity NICU several weeks after enrollment. We therefore present the analysis that assumed that these infants were event-free. A post hoc analysis of the primary end point was conducted to adjust for gestational age, cesarean section, and antenatal steroid use. For secondary efficacy and safety end points, no adjustments were made for multiple comparisons and all of the secondary outcome results should be considered exploratory. All analyses were performed in SAS statistical software version 9.2 (SAS Institute Inc.).
Ethical Oversight
The trial was approved by institutional review boards at all sites, and written informed consent was obtained from the parents or legal guardians.
Conduct of the study was overseen by an independent data and safety monitoring board. Upon completion of enrollment, the data and safety monitoring board chair notified the principal investigator of a potential imbalance between the 2 groups in the incidence of adverse gastrointestinal events. Under the guidance of the data and safety monitoring board, each site was instructed to review the charts of each infant with any gastrointestinal event. These events were initially recorded by the site as an adverse event or serious adverse event coded to the gastrointestinal disorder system organ class, or necrotizing enterocolitis or spontaneous intestinal perforation reported on the discharge case report form. Events recorded on the discharge case report form may have occurred before, during, or after study drug administration. The sites collected additional clinical information to determine incidence of necrotizing enterocolitis and spontaneous intestinal perforation. The presence of a patent ductus arteriosus in the week prior to the event was recorded, as was the amount of feeding. These clinical data were accompanied by a 1-page narrative and reports of all gastrointestinal radiology studies, which were reviewed centrally by 2 neonatologists from the study who were masked to intervention status. Nonconcordance between the 2 neonatologists was to be resolved by a third neonatologist. However, the 2 neonatologists reached agreement on all cases. Necrotizing enterocolitis was defined as either clinical symptoms consistent with necrotizing enterocolitis and an x-ray report describing pneumatosis intestinalis, or portal venous gas, or surgical/pathology report demonstrating bowel necrosis. Spontaneous intestinal perforation was defined as an x-ray report describing pneumoperitoneum in the absence of pneumatosis intestinalis or surgical/pathology report demonstrating isolated perforation with no evidence of necrosis.
Results
Patients
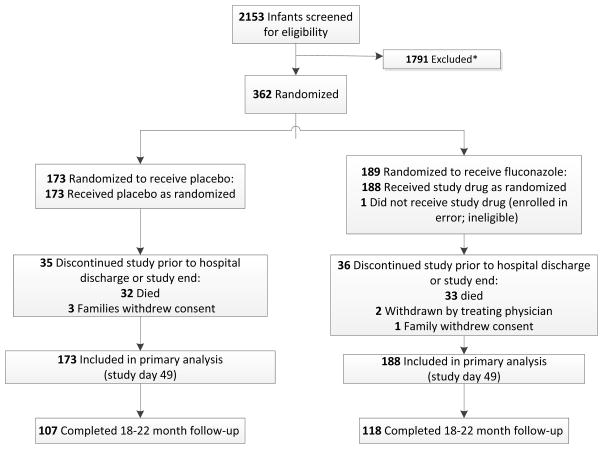
Of 2153 infants screened, 362 infants were enrolled; 188 received fluconazole, and 173 received placebo (Figure). One of the infants randomized to the treatment did not receive study drug due to a randomization error. Of the infants randomized to placebo, 3 families withdrew consent. Of the infants randomized to receive fluconazole, 1 family withdrew consent and 2 infants were withdrawn from the study by the treating physician. The median gestational age was 25 weeks (Table 1), and a majority of the infants were female (57%), non-white (60%), inborn (87%), and intubated at randomization (78%). More infants randomized to placebo were delivered by cesarean section (78% vs. 67%) and received antenatal steroids (87% vs. 75%).
Figure 1.
Enrollment and outcomes for receipt for fluconazole vs placebo. *Data for reasons of exclusion are not available.
Table 1.
Participant Characteristics for Receipt of Fluconazole vs Placeboa
| Fluconazole (N=188) | Placebo (N=173) | |
|---|---|---|
| Male | 79 (42) | 75 (43) |
| Maternal race | ||
| Black | 102 (54) | 94 (54) |
| White | 73 (39) | 70 (40) |
| American Indian or Alaskan Native | 10 (5) | 7 (4) |
| Asian | 3 (2) | 0 |
| Other | 0 | 2 (1)b |
| Hispanic or Latino ethnicity (maternal) | 21 (11) | 25 (15) |
| Gestational age, weeks, median (25th–75th percentile), wk | 25 (24–26) | 25 (24–26) |
| Birth weight, median (25th–75thpercentile), g | 653 (570–700) | 640 (573–700) |
| Inborn | 160 (85) | 155 (90) |
| Cesarean delivery | 125 (67) | 134 (78) |
| Intubated at randomization | 150 (80) | 132 (76) |
| Antenatal steroid use | 140 (75) | 150 (87) |
| Postnatal age at treatment onset, median (25th–75th percentile), d | 4 (3–5) | 4 (3–4) |
| Multiples | 29 (15) | 24 (14) |
Data are reported as No. (%), unless otherwise indicated.
Due to small percentages, 2 individuals were classified as Other; they self-reported race/ethnicity as Egyptian and Indian.
Primary and Secondary Efficacy End Points
The primary composite end point of death or invasive candidiasis by study day 49 was not statistically different between the 2 groups (fluconazole, 16% [95% CI, 11%–22%] vs placebo, 21% [95% CI, 15%–28%]; absolute difference −5% [95% CI, −13%–3%]; odds ratio [OR] 0.73, [95% CI 0.43–1.23]; P=.24). In an exploratory analysis, absence of statistical difference between the 2 groups continued through hospital discharge (for fluconazole, 21% [95% CI, 15%–27%], vs placebo, 26% [95% CI, 19%–33%]; P=.29; treatment difference −5% [95% CI, −14%–4%]) (Table 2). The post hoc analysis of the primary end point, adjusted for gestational age, cesarean delivery, and antenatal steroids, was not statistically different between treatment groups (OR=0.59, [95% CI 0.33–1.05]; P=.08).
Table 2.
Primary and Secondary End Points and Neurodevelopmental End Points for Receipt of Fluconazole vs Placebo
| No. (%) [95% CI]
|
Treatment Difference (95% CI)a | P-value | ||
|---|---|---|---|---|
| Fluconazole (N=188) | Placebo (N=173) | |||
| Primary | ||||
|
| ||||
| Prior to day 49 | ||||
|
| ||||
| Death or candidiasis | 31 (16) [11 to 22] | 37 (21) [15 to 28] | −5 [−13 to 3] | 0.24 |
| Death | 27 (14) [9 to 19] | 25 (14) [9 to 20] | 0 [−7 to 7] | 0.98 |
| Invasive candidiasis | 6 (3) [1 to 6] | 16 (9) [5 to 14] | −6 [−11 to −1] | 0.02 |
| Definite candidiasis b | 5 (3) [0 to 5] | 12 (7) [3 to 11] | −4 [−9 to 0] | |
| Probable candidiasis b | 1 (1) [0 to 3] | 7 (4) [2 to 8] | −4 [−14 to 7] | |
|
| ||||
| Before hospital discharge | ||||
|
| ||||
| Death or candidiasis | 40 (21) [15 to 27] | 45 (26) [19 to 33] | −5 [−14 to 4] | 0.29 |
| Death | 34 (18) [13 to 24] | 33 (19) [13 to 25] | −1 [−9 to 7] | 0.84 |
| Invasive candidiasis | 8 (4) [1 to 7] | 19 (11) [6 to 16] | −7 [−12 to −1] | 0.02 |
|
| ||||
| Neurodevelopmental | ||||
|
| ||||
| Neurodevelopmental impairment composite end pointc | 27/87 (31) [21 to 41] | 23/84 (27) [18 to 37] | 4 [−10 to 17] | 0.60 |
| Cognition composite score, median (25th%tile, 75th%tile) | 90 (70 to 100) | 85 (75 to 95) | 0.59 | |
| Language composite score, median (25th%tile, 75th%tile) | 79 (65 to 93) | 79 (68 to 91) | 0.87 | |
| Motor composite score, median (25th%tile, 75th%tile) | 85 (64 to 97) | 88 (70 to 97) | 0.63 | |
| Bayley-III cognition composite score <70c,d | 17/95 (18) [10 to 26] | 13/95 (14) [7 to 21] | 4 [−6 to 15] | 0.43 |
| Bayley-III cognition composite score <85c,d | 29/95 (31) [21 to 40] | 39/95 (41) [31 to 51] | −11 [−24 to 3] | 0.13 |
| Cerebral palsyc | 12/112 (11) [5 to 16] | 12/107 (11) [5 to 17] | −1 [−9 to 8] | 0.91 |
| Mild | 4 (4) [1 to 9] | 5 (5) [2 to 11] | −1 [−14 to 12] | |
| Moderate | 3 (3) [1 to 8] | 7 (7) [3 to 13] | −4 [−17 to 10] | |
| emsp;Severe | 5 (4) [1 to 10] | 0 (0) [0 to 3] | 4 [−9 to 18] | |
| Deafness, both earsc | 5/95 (5) [2 to 12] | 3/90 (3) [1 to 9] | 2 [−12 to 16] | 0.72 |
| Blindness, both eyesc | 1/107 (1) [0 to 5] | 1/97 (1) [0 to 6] | 0 [−14 to 14] | >0.99 |
Data represent the difference in percentages for fluconazole minus placebo (95%CI for that difference). Differences may not sum due to rounding.
Definite candidiasis was defined as a positive Candida culture from a normally sterile body fluid such as blood, cerebrospinal fluid, peritoneal fluid, or urine obtained via suprapubic aspiration or in-out catheterization. Probable invasive candidiasis was defined as receipt of more than 5 days of consecutive antifungal therapy and both thrombocytopenia (<150,000/103 3L) and a positive Candida culture from a nonsterile site (e.g., bag urine).
Values for fluconazole and placebo are reported as No./No. Total (%) [95%CI].
The Bayley-III cognition composite score ranges from 55 to 145 with a lower score being defined as neurodevelopmental impairment.
The fraction of infants who died prior to study day 49 was not statistically different between the 2 groups (for fluconazole, 14% [95% CI, 9%–19%] vs placebo, 14% [95% CI, 9%–20%]; P=.98; treatment difference 0% [95% CI, −7%–7%]). Fewer infants developed definite or probable candidiasis by study day 49 in the fluconazole group (3% [95% CI, 1%–6%] vs in the placebo group 9% [95% CI, 5%–14%]; P=.02; treatment difference −6% [95% CI, −11%–−1%]). In an exploratory analysis, this statistically significant protective effect persisted through the remainder of the hospital stay, with fewer infants in the fluconazole group developing invasive candidiasis before discharge (4% [95% CI, 1%–7%] vs in the placebo group 11% [95% CI, 6%–16%]; P=.02; treatment difference −7% [95% CI, −12–−1%]). Positive Candida cultures among the 6 infants with invasive candidiasis who received fluconazole included endotracheal (n=1), cerebrospinal fluid (n=1), and blood (n=4). Positive Candida cultures among the 16 infants with invasive candidiasis who received placebo included endotracheal (n=4), sterile urine (n=5), cerebrospinal fluid (n=1), peritoneal (n=2), and blood (n=10). Five infants had positive cultures from different sites or methods: blood and peritoneal (n=2), blood and urine (n=1), endotracheal and urine (n=1), and blood, cerebrospinal fluid, and urine (n=1).
Mortality was high among infants with candidiasis. Among those with definite candidiasis, death occurred in 7 of 17 infants (41%) (95% CI, 18%–67%) and among those with probable candidiasis, death occurred in 3 of 8 (38%; 95% CI, 9%–76%). Mortality among patients with definite or probable candidiasis in the fluconazole group occurred in 2 of 6 (33%;95% CI, 4%–78%) and in the placebo group, death occurred in 6 of 16 (38%;95% CI, 15%–65%).
Safety
Seven infants (4 in the fluconazole group and 3 in the placebo group) had increases in AST or ALT of greater than250 U/L; 1 of these infants who received placebo had elevation in both AST and ALT. There were no between-group differences in the number of infants with GGT greater than100 U/L (fluconazole group, 20% [95% CI, 14%–26%] vs the placebo group, 21% [95% CI, 15%–28%]; P=.78; treatment difference −1% [95% CI, −10%–7%]) or with conjugated hyperbilirubinemia (Table 3). There were no differences in the incidence of invasive bacterial infections or length of stay. The fraction of infants in each group who developed intracranial hemorrhage, periventricular leukomalacia, chronic lung disease, patent ductus arteriosus requiring surgery, and retinopathy of prematurity requiring laser surgery were not statistically different.
Table 3.
Safety End Points and Other Secondary End Points for Receipt of Fluconazole vs Placebo
| No. (%) [95% CI]a
|
Treatment Difference (95% CI)b | P-value | ||
|---|---|---|---|---|
| Fluconazole (N=188)a | Placebo (N=173)a | |||
| Aspartate aminotransferase >250 U/L | 4 (2) [1 to 5] | 3 (2) [0 to 5] | 0 [−10 to 10] | >0.99 |
| Alanine aminotransferase >250 U/L | 0 (0) [0 to 2] | 1 (1) [0 to 3] | −1 [−11 to 10] | 0.48 |
| Gamma-glutamyl transpeptidase >100 U/L | 38 (20) [14 to 26] | 37 (21) [15 to 28] | −1 [−10 to 7] | 0.78 |
| Conjugated bilirubin >5 mg/dL | 15 (8) [4 to 12] | 20 (12) [7 to 16] | −4 [−10 to 3] | 0.25 |
| Bacterial infectionc | 109 (58) [51 to 65] | 100 (58) [50 to 65] | 0 [−10 to 10] | 0.97 |
| Length of stay, days, median (25th%tile, 75th%tile)d | 113 (90 to 148) | 107 (88 to 128) | 0.08 | |
| Intracranial hemorrhage (grade III or IV) | 33 (18) [12 to 23] | 31 (18) [12 to 24] | 0 [−8 to 8] | 0.93 |
| Periventricular leukomalacia | 10 (5) [3 to 10] | 6 (3) [1 to 7] | 2 [−8 to 12] | 0.39 |
| Chronic lung diseasee | 114 (61) [54 to 68] | 93 (54) [46 to 61] | 7 [−3 to 17] | 0.19 |
| Patent ductus arteriosus requiring surgery | 46 (24) [18 to 31] | 43 (25) [18 to 31] | 0 [−9 to 9] | 0.93 |
| Retinopathy of prematurity requiring laser surgery | 29 (15) [10 to 21] | 25 (14) [9 to 20] | 1 [−6 to 8] | 0.80 |
| Stage II or III necrotizing enterocolitise | 25 (13) [8 to 18] | 23 (13) [8 to 18] | 0 [−7 to 7] | >0.99 |
| Spontaneous intestinal perforatione | 16 (9) [5 to 13] | 9 (5) [2 to 9] | 3 [−2 to 8] | 0.22 |
Applies to categories except length of stay.
Data represent the difference in percentages for fluconazole – placebo [95% CI for that difference].
Positive cultures from a sterile site.
Participants who died or terminated from the study prior to discharge home were excluded from the length-of-stay analysis.
These numbers reflect adjudicated results; see Table 4.
In the original reporting of data from the sites, the number of infants in each group who developed stage II or III necrotizing enterocolitis was not statistically different (fluconazole group, 18% [95% CI, 12%–23%] vs 20% in the placebo group [95% CI, 14%–26%]; P=0.61; treatment difference −2% [95% CI, −10%–6%]); however, a higher fraction of the infants randomized to receive fluconazole developed spontaneous intestinal perforation: (23 of 188 [12%] in the fluconazole group (95% CI, 8%–17%) vs. 9 of 173 [5%] in the placebo group [95% CI, 2%–9%]; P=.02; treatment difference 7% (95% CI, 1%–13%). After the difference was detected following the database lock and adjudicated by 2 neonatologists who were blinded to group allocations, the incidences of necrotizing enterocolitis and spontaneous intestinal perforation were not statistically different in the 2 study groups (Table 4). In a number of instances, a participant was noted to have experienced both an episode of necrotizing enterocolitis and spontaneous intestinal perforation, and, on adjudication, was determined to only have 1 or the other.
Table 4.
Gastrointestinal Secondary Outcomes for Receipt of Fluconazole vs Placebo
| End Point | All Participants (N=361) | Fluconazole (N=188) | Placebo (N=173) | Treatment Difference (95% CI)a | P-value |
|---|---|---|---|---|---|
| Original site designation | |||||
| Stage II or III necrotizing enterocolitis | 67 (19) [15 to 23] | 33 (18) [12 to 23] | 34 (20) [14 to 26] | −2 [−10 to 6] | 0.61 |
| Spontaneous intestinal perforation | 32 (9) [6 to 12] | 23 (12) [8 to 17] | 9 (5) [2 to 9] | 7 [1 to 13] | 0.02 |
| Adjudicated reviewer designation | |||||
| Stage II or III necrotizing enterocolitis | 48 (13) [10 to 17] | 25 (13) [8 to 18] | 23 (13) [8 to 18] | 0 [−7 to 7] | >0.99 |
| Spontaneous intestinal perforation | 25 (7) [4 to 10] | 16 (9) [5 to 13] | 9 (5) [2 to 9] | 3 [−2 to 8] | 0.22 |
Data represent the difference in percentages for fluconazole – placebo [95% CI for that difference].
Mortality and Neurodevelopmental Follow-up
Of the infants who were enrolled in the study and for whom consent was retained 84% completed the neurodevelopmental assessment or died. Of the 188 infants who received fluconazole, 3 were withdrawn early and 33 died, leaving 152 who were followed until hospital discharge. Of these, 118 completed neurodevelopmental follow-up. Of the 173 infants who received placebo, 3 were withdrawn early and 32 died, leaving 138 who were followed until hospital discharge. Of these, 107 completed neurodevelopmental follow-up (Figure). There was no difference in gestational age between infants who completed neurodevelopmental follow-up (mean gestational age at birth, 25 weeks [25th– 75th percentiles 24– 26 weeks]) vs. infants who were lost to follow-up (mean gestational age at birth, 25 weeks [25th– 75th percentiles 24– 26 weeks]; P=.88) or incidence of candidiasis between infants who completed neurodevelopmental follow-up (11 of 225 [5%] [95% CI, 2%–8%] vs infants who were lost to follow-up (3 of 57 [5%] [95% CI, 1%–11%]; P>.99).
Neurodevelopmental impairment was not statistically different between the fluconazole group—(31%; 95% CI, 21%–41%) and the placebo group (27% [95% CI, 18%–37%]; P=.60; treatment difference 4% (−10%–17%) (Table 2). This finding remained after controlling for infant exposure to antenatal steroids, cesarean section, and level of maternal education (P=0.87). The composite scores for language, cognition, and motor development were not statistically different between the groups, and the fraction of infants with a Bayley-III cognition composite score of <70 was not statistically different (18% [95% CI, 10–26%] in the fluconazole group and 14% [95% CI, 7–21%] in the placebo group; P=.43; treatment difference 4% [−6–15%]). The fraction of infants who developed cerebral palsy, blindness, or deafness in each group was also not statistically different (Table 2).
Discussion
Fluconazole prophylaxis compared with placebo was not associated with a statistically significant difference in the composite primary end point—death or definite or probable invasive candidiasis— although it was associated with a statistically significant reduction in the incidence of definite or probable candidiasis alone. This study adds new evidence regarding the efficacy of fluconazole prophylaxis but raises the question of whether prevention of invasive candidiasis translates into substantial improvements in the outcomes of prematurity.
These discrepant results may be related to the lack of effect on mortality of fluconazole prophylaxis. There was no difference in overall mortality between the fluconazole group (14%; 95% CI, 9%–19%]) and the placebo group (14%; 95% CI, 9%–20%]). We estimated an incidence of death or invasive candidiasis of 30% in the placebo group, but our placebo group had an incidence of only 21% (95% CI, 15%–28%). Although not underpowered for the primary outcome, the overall lack of mortality benefit resulted in a nonsignificant finding for the primary outcome of death or invasive candidiasis, despite the significant decrease in invasive candidiasis associated with fluconazole prophylaxis. The incidence of culture-proven candidiasis was lower in the placebo group of our study compared with placebo groups in previous trials.3,4 This may be due to decreased use of broad-spectrum antibiotics (e.g., third-generation cephalosporins), increased use of line bundles, and improved handwashing.8 There was also a high inborn population (87%) enrolled in this study, which is associated with improved outcomes. In addition, we anticipated a mortality benefit by preventing cases of invasive candidiasis that might be missed due to the low sensitivity of blood cultures.13 Relatively high-volume blood cultures in adults have a less than 30% sensitivity for invasive candidiasis, and blood volumes for culture in premature infants are frequently 1 mL or less.13
Fluconazole prophylaxis was also not associated with the secondary end point of neurodevelopmental impairment in this cohort of infants from NICUs with low-to-moderate incidence of candidiasis. Our study was not powered for differences in neurodevelopmental impairment alone. For these extremely premature infants, multiple factors affected neurodevelopmental impairment. Additionally, neurodevelopmental outcomes were not statistically different between fluconazole and placebo patients in a cohort from 1 of the previous single-center, randomized controlled trials.9
Fluconazole was associated with a statically significant reduction in the incidence of probable or definite candidiasis, consistent with several,3,4 but not all,14,15 randomized trials of fluconazole prophylaxis in infants. This finding is also consistent with studies conducted in older patients.16–20 Fluconazole has been very effective in preventing candidiasis in studies with a high incidence (13%3 and 20%4 in the placebo groups compared with <2% in nurseries in North America and Europe2). The placebo group in the present study had a 9% (95% CI, 5%–14%) incidence of definite or probable invasive candidiasis and a 7% (95% CI, 3%–11%) incidence of definite invasive candidiasis. Thus, overall, the effect of fluconazole prophylaxis on the incidence of candidiasis from randomized and non-randomized studies appears largely to be independent of baseline incidence.
We did not find any statistically significant safety concerns associated with fluconazole prophylaxis in this study, although power was low for these outcomes. After blinded review of operative notes and radiograph reports, the adjudicated data did not demonstrate an association with spontaneous intestinal perforation and fluconazole prophylaxis. Spontaneous intestinal perforation is a different disease entity from necrotizing enterocolitis.21 Differentiating spontaneous intestinal perforation from necrotizing enterocolitis is difficult, and the diagnosis is often not confirmed until laparotomy. An association between fluconazole and spontaneous intestinal perforation was also not observed in previous fluconazole prophylaxis studies.
The incidence of invasive candidiasis has decreased in the United States over the last decade.8 As the incidence of candidiasis in a NICU decreases, the benefit of prophylaxis relative to overall harm (effect on Candida resistance patterns, potential safety concerns, drug cost) becomes lower. Based on both the results of our study in NICUs with a low incidence of invasive candidiasis, and previous prophylaxis trials in high-incidence NICUs,3,4 the routine use fluconazole prophylaxis should be limited to units with moderate-to-high incidence of invasive candidiasis. However, additional research is needed to precisely define the incidence at which the benefits of fluconazole prophylaxis outweigh the risks.
Limitations
Our study has some limitations. After randomization, there were imbalances between the 2 treatment groups. A higher proportion of infants in the placebo group were born via cesarean section. This may have decreased Candida colonization in the infants in this group.22 Additionally, more infants in the placebo group were exposed to antenatal steroids, which may have decreased mortality and morbidities in the placebo group. Both of these imbalances may have biased the results toward the null. However, on post hoc analysis of the primary end point adjusted for gestational age, cesarean section, and antenatal steroids, the result remained similar to the primary analysis. We included probable candidiasis in our primary end point. We acknowledge that fluconazole prophylaxis may have resulted in eradication of Candida from nonsterile sites, potentially biasing the results away from the null. Secondary analyses were not adjusted for multiple comparisons.
Conclusions
Among infants with a birth weight of less than 750 g, fluconazole prophylaxis compared with placebo was not associated with a statistically significant difference in the incidence of the composite of death or invasive candidiasis. These findings do not support the universal use of prophylactic fluconazole in extremely low-birth-weight infants.
Acknowledgments
Sources of Funding
This work was funded by grants from NIH 5R01HD057956-05, the USFDA (5R01FD003519-04), the Thrasher Research Fund, and the Best Pharmaceuticals for Children Act under the guidance of the NICHD (HHSN2752010000031) for the Pediatric Trials Network. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the NIH (UL1TR001117).
Fluconazole Prophylaxis Study Team Investigators and Study Coordinators
University of Miami Miller School of Medicine, FL (28 patients enrolled): Karina Lifschitz (site coordinator [SC]); UF Health, Jacksonville, FL (25 patients enrolled): Michele Burke (SC), Renee Prince (SC); University of Alabama–Birmingham (25 patients enrolled): Robert L. Schelonka (principal investigator [PI]), Claire Roane (SC); Duke University Medical Center, Durham, NC (24 patients enrolled): Elizabeth Bradsher (SC); Kings County Hospital, Brooklyn, NY (23 patients enrolled): Sukhvinder Ranu (co-investigator), Subhatra Limbu (SC); Pennsylvania Hospital, Philadelphia (20 patients enrolled): Toni Mancini (SC); Wayne State University, Detroit, MI (18 patients enrolled): Seetha Shankaran (PI), Melanie Lulic (SC); Memorial Hospital of South Bend, IN (16 patients enrolled): Mashelle Monhaut (SC); University of Florida Health Shands Hospital, Gainesville (16 patients enrolled): Matthew Saxonhouse (PI), Cindy Miller (SC); University of Texas Medical Branch, Galveston (16 patients enrolled): Karen E. Smith (co-PI), Kristin Pollock (SC); Columbia University, New York, NY (14 patients enrolled): Erin Humel-Amadori (SC), Glen Bona (SC); Brookdale University Hospital, Brooklyn, NY (12 patients enrolled): Chika Iwuchukwu (SC); University of Minnesota Amplatz Children’s Hospital, Minneapolis (12 patients enrolled): Marla Mills (SC), Nichole Birge (SC); University of Louisville, KY (11 patients enrolled): Karen Kernen (SC); University of California–San Diego Medical Center (11 patients enrolled): Wade Rich (SC); Wolfson Children's Hospital, Jacksonville, FL (11 patients enrolled): Michele Burke (SC), Renee Prince (SC); Children's Hospital of Orange County, Orange, CA (9 patients enrolled): Ofelia Vargas-Shiraishi (SC), Kathy Shea (SC); Children's Hospital Medical Center of Akron, OH (8 patients enrolled): Anand Kantak (PI), Judy Ohlinger (SC); University of Tennessee Health Science Center, Memphis (7 patients enrolled): Ramasubbareddy Dhanireddy (PI), Sheila Dempsey (SC); University of Texas Health Science Center at Houston (7 patients enrolled): Kathleen Kennedy (PI), Georgia McDavid (SC), Peggy Robichaux (SC); University of Texas Southwestern Medical Center, Dallas (7 patients enrolled): Pablo Sanchez (PI), Deborah McElroy (SC), Luz Muniz (SC); Cook Children’s Health Care System IRB, Fort Worth, TX (6 patients enrolled): Jonathan Nedrelow (PI), Barbara Austin (SC), Sara Scott (SC); East Carolina University, Greenville, NC (6 patients enrolled): Scott MacGilvray (PI), James Cummings (PI), Sherry Moseley (SC); University of Arkansas for Medical Sciences, Little Rock (5 patients enrolled): Ashley Ross (PI), Michelle Hart (SC), Howard Lee (SC); Virtua West Jersey Hospital, Voorhees, NJ (5 patients enrolled): Paresh Pandit (PI), Christine Catts (SC); Texas Children’s Hospital, Houston (4 patients enrolled): Mohan Pammi (PI), Eric Eichenwald (PI), Teresa Falk (SC); Riley Hospital for Children at Indiana University, Indianapolis (4 patients enrolled): Brenda Poindexter (PI), Leslie Wilson (SC); SUNY Downstate Medical Center, Brooklyn, NY (4 patients enrolled): Agnes Perenyi (PI), Susan Sullivan (SC), Sara Higgerson (SC); University of Nevada School of Medicine, Reno (3 patients enrolled): Echezona Ezeanolue (PI), Aaron Hunt (SC); Tulane University School of Medicine, New Orleans, LA (2 patients enrolled): Phillip Gordon (PI); Jane Reynolds (SC); Wesley Medical Center, Wichita, KS (2 patients enrolled): Barry Bloom (PI), Paula Delmore (SC); Arkansas Children's Hospital, Little Rock (1 patient enrolled): Ashley Ross (PI), Michelle Hart (SC), Howard Lee (SC); Duke Clinical Research Institute, Durham, NC: Jamie Gao (project leader), Debbe Blackwell (clinical research associate), Kristy Hwang (data manager), Elizabeth Vandyne (safety associate), Debbie Hendrick (clinical trials assistant), Tedryl Gentry Bumpass (lead clinical research associate).
Footnotes
Role of the Sponsor
The NIH, US FDA, and the Thrasher Research Fund had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Additional Contributions
Oversight of the work of the study team was provided by The Best Pharmaceuticals for Children Act—Pediatric Trials Network Administrative Core Committee:Jeffrey Barrett, PhD, Children's Hospital of Philadelphia, Philadelphia, PA; Edmund Capparelli, Pharm-D, University of California–San Diego, San Diego, CA; Michael Cohen-Wolkowiez, MD, PhD, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, PharmD, PhD, Children's Mercy Hospital, Kansas City, MO; Matthew Laughon, MD, MPH, University of North Carolina at Chapel Hill; Andre Muelenaer, MD, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O'Shea, MD, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, MD, MSc, Penn State College of Medicine, Hershey, PA; John van den Anker, MD, PhD, George Washington University School of Medicine and Health, Washington, DC; Thomas J. Walsh, MD, Weill Cornell Medical College of Cornell University, New York, NY. The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor-Zapata, MD, Anne Zajicek, MD, PharmD, Alice Pagan, BBA. The EMMES Corporation (Data Coordinating Center): Ravinder Anand, PhD, Traci Clemons, PhD, Gina Simone, BS.
Conflicts of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Benjamin reports receipt of support from the National Institutes of Health (NIH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the US Food and Drug Administration (USFDA), and the National Center for Advancing Translational Sciences of the NIH for work in pediatric and neonatal clinical pharmacology, and the nonprofit organization Thrasher Research Fund for work in neonatal candidiasis; and research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr Smith reports receipt of salary support for research from the NIH, the US Department of Health and Human Services, and the National Center for Advancing Translational Sciences of the NIH; and support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). No other disclosures were reported.
Author Contributions
- Study concept and design: Benjamin, Wade, Kaufman, Berezny, Smith
- Acquisition, analysis, or interpretation of data: Hudak, Duara, Randolph, Bidegain, Mundakel, Natarajan, Burchfield, White, Shattuck, Neu, Bendel, Kim, Finer, Stewart, Arrieta, Wade, Kaufman, Manzoni, Prather, Testoni, Smith.
- Drafting of the manuscript: Benjamin, Smith
- Critical revision of the manuscript for important intellectual content: Hudak, Duara, Randolph, Bidegain, Mundakel, Natarajan, Burchfield, White, Shattuck, Neu, Bendel, Kim, Finer, Stewart, Arrieta, Wade, Kaufman, Manzoni, Prather, Testoni, Berezny, Smith
- Statistical Analysis: Prather.
- Obtained funding: Benjamin, Wade, Kaufman, Smith.
- Administrative, technical, or material support: Testoni, Berezny.
- Study supervision: Benjamin, Berezny, Smith.
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality, and neurodevelopmental outcomes at 18–22 months. Pediatrics. 2006;117(1):84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 3.Manzoni P, Stolfi I, Pugni L, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356(24):2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345(23):1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burwell LA, Kaufman D, Blakely J, Stoll BJ, Fridkin SK. Antifungal prophylaxis to prevent neonatal candidiasis: a survey of perinatal physician practices. Pediatrics. 2006;118(4):e1019–1026. doi: 10.1542/peds.2006-0446. [DOI] [PubMed] [Google Scholar]
- 7.Clerihew L, McGuire W. Antifungal prophylaxis for very low birthweight infants: UK national survey. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F238–239. doi: 10.1136/adc.2007.121830. [DOI] [PubMed] [Google Scholar]
- 8.Aliaga S, Clark RH, Laughon M, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133(2):236–242. doi: 10.1542/peds.2013-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman DA, Cuff AL, Wamstad JB, et al. Fluconazole prophylaxis in extremely low birth weight infants and neurodevelopmental outcomes and quality of life at 8 to 10 years of age. J Pediatr. 2011;158(5):759–765. doi: 10.1016/j.jpeds.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams-Chapman I, Bann CM, Das A, et al. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr. 2013;163(4):961–967. e3. doi: 10.1016/j.jpeds.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayley N. Manual for the Bayley Scales of Infant Development III. San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- 13.Berenguer J, Buck M, Witebsky F, Stock F, Pizzo PA, Walsh TJ. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Disseminated versus single-organ infection. Diagn Microbiol Infect Dis. 1993;17(2):103–109. doi: 10.1016/0732-8893(93)90020-8. [DOI] [PubMed] [Google Scholar]
- 14.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics. 2001;107(2):293–298. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]
- 15.Parikh TB, Nanavati RN, Patankar CV, et al. Fluconazole prophylaxis against fungal colonization and invasive fungal infection in very low birth weight infants. Indian Pediatr. 2007;44(11):830–837. [PubMed] [Google Scholar]
- 16.Gotzsche PC, Johansen HK. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst Rev. 2002;2(2):CD000026. doi: 10.1002/14651858.CD000026. [DOI] [PubMed] [Google Scholar]
- 17.Laverdiere M, Rotstein C, Bow EJ, et al. Impact of fluconazole prophylaxis on fungal colonization and infection rates in neutropenic patients: the Canadian Fluconazole Study. J Antimicrob Chemother. 2000;46(6):1001–1008. doi: 10.1093/jac/46.6.1001. [DOI] [PubMed] [Google Scholar]
- 18.Ho KM, Lipman J, Dobb GJ, Webb SA. The use of prophylactic fluconazole in immunocompetent high-risk surgical patients: a meta-analysis. Crit Care. 2005;9(6):R710–717. doi: 10.1186/cc3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castagnola E, Machetti M, Bucci B, Viscoli C. Antifungal prophylaxis with azole derivatives. Clin Microbiol Infect. 2004;10(Suppl 1):86–95. doi: 10.1111/j.1470-9465.2004.00847.x. [DOI] [PubMed] [Google Scholar]
- 20.Playford EG, Webster AC, Sorrell TC, Craig JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother. 2006;57(4):628–638. doi: 10.1093/jac/dki491. [DOI] [PubMed] [Google Scholar]
- 21.Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65(2):138–144. doi: 10.1203/PDR.0b013e31818c7920. [DOI] [PubMed] [Google Scholar]
- 22.Waggoner-Fountain LA, Walker MW, Hollis RJ, et al. Vertical and horizontal transmission of unique Candida species to premature newborns. Clin Infect Dis. 1996;22(5):803–808. doi: 10.1093/clinids/22.5.803. [DOI] [PubMed] [Google Scholar]