Abstract
We evaluated in vivo the μ-opioid system during spontaneous episodic migraine headaches. Seven patients were scanned at different phases of their migraine using positron emission tomography with the selective μ-opioid receptor (μOR) radiotracer [11C]carfentanil. In the ictal phase, there was μOR activation in the medial prefrontal cortex, which was strongly associated with the μOR availability level during the interictal phase. Furthermore, μ-opioid binding changes showed moderate negative correlation with the combined extension and severity of the attacks. These results indicate for the first time that there is high μOR activation in the migraineurs' brains during headache attacks in response to their pain.
Introduction
Currently, 11.1% of episodic and 34.3% of chronic migraine patients routinely use opioids according to the American Migraine Prevalence and Prevention Study.1 The numbers of opioid-users can even skyrocket to even 72% when derived from migraineurs admitted to tertiary care pain clinics.2 Although the endogenous opioid system has long been implicated in regulating pain nociceptive signals,3 frequent use of opioids increases the risk of chronification of the migraine attacks and even allodynia.4 Hence, the status quo of the endogenous μ-opioid release and μ-opioid receptor (μOR) concentrations during headaches are critical elements for the understanding of the neurobiology of migraine and, most importantly, its clinical alleviation or aggravation.
Recent advances in positron emission tomography (PET) with [11C]carfentanil (CFN), a selective radiotracer for μORs, have demonstrated that there is a faulty μOR availability (nondisplaceable binding potential, BPND) in chronic trigeminal pain patients in vivo,5 but information is still lacking regarding migraine. In this study, notwithstanding the PET-radiotracer complex logistics, cost, radiation limits, and the spontaneous nature of the attacks, we examined for the first time in vivo changes in μOR activity in the brains of episodic migraine patients at baseline and during nondrug-induced headaches.
Patients and Methods
The University of Michigan Institutional Review Board and the Radioactive Drug Research Committee approved the study. After initial screening by phone, the patients were thoroughly examined by a pain specialist to confirm the episodic migraine diagnosis following the International Headache Society classification6 (Table 1). Subjects were excluded in cases of opioid and hormonal contraceptive use during the past 6 months, pregnancy, and concomitant chronic pain conditions. The protocol was divided into one screening appointment, one magnetic resonance imaging (MRI) session, and two PET sessions: one during headache (ictal) and another during nonheadache (interictal) phases of their migraine. Interictal phase also required participants to be headache free for at least 48 h prior to the scan, and to have abstained from the use of any migraine medication during the same period. Both PET scans were scheduled a priori, and the patients had to confirm in the early morning of those days the occurrence, or not, of the attacks. For females, the PET sessions were arranged during separate mid-late follicular phases (5–10 days after menstrual bleeding) with the assistance of a gynecologist.
Table 1.
Clinical profile of episodic migraine participants enrolled in this study. Sequence of subjects follows figure 1, left image, from left to right
| Subjects | Gender | Age | Diagnosis1 | Pain intensity2 | Pain frequency3 | Pain duration (h) | Chronicity in years | Usual abortive medication4 |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 21 | With aura | 6 | 2 | 12 | 7 | Ibuprofen |
| 2 | Female | 21 | Without aura | 8 | 4 | 12 | 5 | None |
| 3 | Female | 26 | Without aura | 6 | 8 | 12 | 15 | Acetaminophen |
| 4 | Female | 38 | With aura | 6.2 | 6 | 72 | 20 | Acetaminophen |
| 5 | Male | 22 | With aura | 6.7 | 8 | 24 | 6 | Acetaminophen |
| 6 | Male | 26 | With aura | 5 | 2 | 5 | 2 | None |
| 7 | Female | 36 | With aura | 8.6 | 12 | 72 | 20 | Acetaminophen |
Based on ICHD-3 beta (However, none of the participants reported visual aura preceding or during the ictal PET scan).
Pain intensity during ictal PET scan.
Average days per month.
Preventive medication was an exclusion criteria, and abortive medication was not allowed 48hr prior to interictal and ictal PET scans.
Ictal and interictal PET sessions
PET sessions with CFN, a selective and specific μOR radioligand7 were performed for 90 min. PET scans were acquired with a Siemens HR+ scanner in 3D mode (reconstructed FWHM resolution 5.5 mm in-plane and 5.0 mm axially) with septa retracted and scatter correction. Subjects were positioned in the PET scanner gantry and two intravenous (antecubital) lines were placed. CFN was produced using a cycloton in the vicinity,8 and each dose (15 ± 1 mCi, ≤0.03 μg/kg) was administered 50% as a bolus with the remainder continuously infused over the course of the scan to achieve steady-state tracer levels ∼35 min after tracer administration.
Electronic mobile pain data entry
Headache and facial pain intensity and area were analyzed using a free and interactive Apple mobile application developed in-house, PainTrek (University of Michigan) (Fig. 1 – left). PainTrek provides a 3D head and facial map based on a squared grid system with vertical and horizontal coordinates using anatomical landmarks. Each quadrangle frames well-detailed craniofacial and cervical areas and was filled out by the patients to express his or her exact migraine headache location and intensity, as well as other pain characteristics. Entries in the facial map were then translated into appropriately colored regions on the 3D head for viewing. In addition, the application allowed us to calculate and display the rating of average pain intensity and extension for all patients together, which consisted of the total sum of patient(s)' pain severity in each anatomical location, divided by the number of responses in the area (Mild:1/Moderate:2/Severe:3). Anatomical regions without pain were considered null responses and not counted in the rating average. We also accounted the overall pain for each participant by calculating the Pain Area and Intensity Number Summation (P.A.I.N.S) of all rated squares together. This approach showed the precise anatomical distribution and intensity of the migraine attacks studied across all our patients or individually, providing a more objective and detailed sensory-discriminative information of the attacks.
Figure 1.
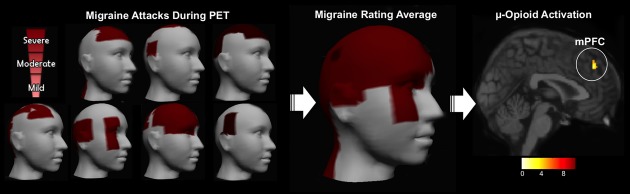
Migraine headache severity and μ-opioid ictal activation during PET. Left: Headache and facial pain intensity and area were recorded and analyzed from the seven migraine patients at the ictal PET sessions. We used a free and interactive Apple mobile application developed in-house (PainTrek, University of Michigan). Center: The 3D image represents the average rating of the pain intensity and location of the migraine headache attacks of all patients at the time of the ictal PET sessions. The average pain intensity was moderate (6.6 ± 1.6; VAS [1–10]) and pain extension was 39 ± 26.7 square units for the headache attacks. Right: μ-Opioid activation during spontaneous migraine headache attack. The image shows decrease in the μOR BPND of the medial prefrontal cortex region of the seven migraine patients during attack as compared with the interictal phase (P = 0.000).
MRI acquisition
MRI scans were acquired on a 3T scanner (General Electric, Milwaukee, WI). These images provide anatomical information for structure identification and were utilized for the anatomical standardization to the ICBM/MNI atlas coordinate system. This established the linear and nonlinear warping transformation matrices applied to the coregistered receptor binding PET maps. The acquisition sequence was axial T1 FAST SPGR MR (TE = 3.4, TR = 10.5, TI = 200, flip angle 25°, FOV 24 cm, 1.5-mm thick slices, NEX = 1), acquisition matrix 256 × 256, 60 slices.
Neuroimaging analysis
T1-weighted MR and PET images of each subject were coregistered to each other using a mutual information algorithm.9 For this purpose, K1 ratio images were first aligned to the MR, and the transformation matrix applied to the coregistered BPND scans of the same image set. The MR scans were then anatomically standardized to ICBM brain atlas stereotactic coordinates by nonlinear warping, and the resulting transformation matrix applied to both K1 ratio and BPND image sets.10,11
Subsequently, dynamic image data for each of the receptor scans were transformed on a voxel-by-voxel basis into three sets of parametric maps, which were coregistered to each other. These were (1) a tracer transport measure (K1 ratio, proportional to cerebral blood flow; tracer transport = blood flow × tracer extraction) and (2) receptor-related measures (BPND), encompassing data from 10–40 min (baselines). These parametric images were calculated using a modified Logan graphical analysis12 with the occipital cortex (a region devoid of μORs) as the reference region.
Results
Of the 12 episodic migraine patients scanned during their interictal phase, seven patients (four females/three males) confirmed by phone, upon awakening, the occurrence of their spontaneous migraine when scheduled a priori for their potential ictal PET scans. Clinical characteristics of the migraine headache are summarized in Table 1. Participants managed to tolerate the headache attacks until the end of the scan sessions without any abortive pharmacotherapy. The average intensity of the headache attacks was moderate (6.6 ± 1.6; VAS [1–10]) and pain extension was 39 ± 26.7 square units (Fig. 1 – center). With the exception of patient 1, all other patients had migraine predominantly on the right side. For clinical and neuroimaging analysis, patient 1's data were flipped. No additional migraine attacks were reported by the patients during the 3 days that preceding or following the ictal phase scanned. Their average frequency of attacks was 6 ± 3.6 per month, and history of 11.1 ± 7.1 years of migraine suffering.
We found reductions in μOR BPND during a spontaneous migraine attack compared to the baseline in the medial prefrontal cortex (mPFC) ipsilateral to the headache (MNI coordinates with a center of mass at right: x = 2; y = 43; z = 42; P = 0.000) (Fig. 1 – right). These results indicate the acute activation of the endogenous opioid neurotransmission interacting with μOR due to the pain of the migraine attack. The μOR BPND in the mPFC cluster during the ictal migraine phase was positively correlated with the μOR BPND levels during interictal phase (r = 0.74) (Fig. 2). No correlations were found with the averages of attack intensity, extension, or frequency separately. However, when intensity and extension of the current headache attacks were accounted for together (P.A.I.N.S.) there was a moderate negative correlation with mPFC activation (r = −0.61).
Figure 2.
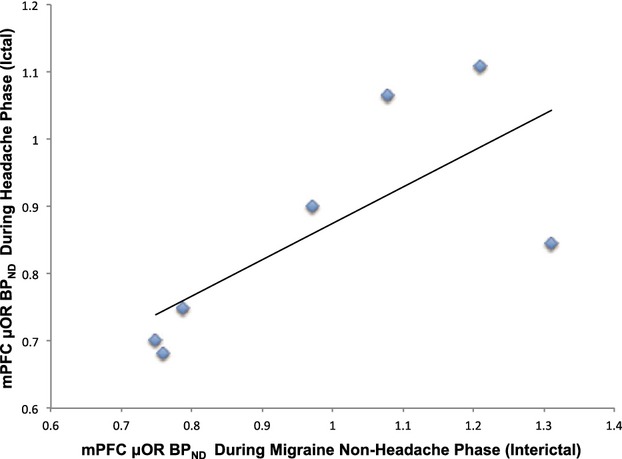
Baseline medial prefrontal cortex receptor density is associated with μ-opioid system activation at the time of the migraine headache attack. The μOR BPND in the mPFC cluster during the ictal migraine phase was positively correlated with the μOR BPND levels during interictal phase (r = 0.74). This result indicates that μ-opioid system activity during spontaneous chronic pain attacks (migraine headaches) is forecasted by the opioid-receptor availability in the nonheadache phase.
Discussion
Our study demonstrated for the first time in vivo that there was reduced μOR BPND in the central modulatory pain system of migraine patients during spontaneous headache, compared to their nonheadache phase. There were less μORs available for binding the specific PET radiotracer CFN in the ipsilateral mPFC during the ictal phase, possibly due to the increased endogenous μ-opioid neurotransmission interacting with μORs. This implies that the migraine headache attack induced the release of the endogenous μ-opioids to fight the ongoing pain. However, due to the continuation of the migraine throughout the scan it can be inferred that the higher endogenous μ-opioid activation was ineffective to control the barrage of nociceptive inputs associated with the migraine headache pain. The continuation of pain along with the decreased BPND of the CFN during the ictal phase in the mPFC as compared to the interictal headache phase shows an association between endogenous μ-opioid release and migraine headache pain in this area of the brain.
The mPFC region, including the rostral anterior cingulate cortex, had been linked, although indirectly, to migraine attacks by other animal and human studies. It processes the cognitive–emotional and spatio-temporal variables associated with spontaneous clinical pain.13 The μOR activation of that region increases connectivity with the periaqueductal gray matter (PAG) in analgesia,14 another region rich in μOR and involved in migraine pathophysiology.15 In migraine, functional activation in the prefrontal region has been previously noticed in spontaneous and triggered migraine attacks.16,17 In addition, meningeal neurogenic inflammation associated with migraine can be modulated in animal studies by morphine, and afterward, overturned by naloxone, a μ-opioid antagonist.18 Nevertheless, based on our preliminary findings, the imbalance between the faulty descending inhibition and the facilitation of the ascending trigeminal sensory inputs must both be present during the occurrence of the migraine symptomatology. Otherwise, only the acute increase in the release of endogenous μ-opioid we observed at the time of the attacks would be enough to cease the patients' suffering, which was not the case. Furthermore, the level of this μ-opioid activation fluctuates depending on the migraine experience, as it weakens with the progression of the area and severity of the migraine attack, showing a moderate negative correlation with the pain summation (P.A.I.N.S).
Interestingly, in a study by Maarrawi and colleagues with eight refractory pain patients, motor cortex stimulation induced decreases in [11C]diprenorphine binding, a “nonselective” opioid PET radiotracer, in the anterior cingulate cortex and PAG, which were significantly correlated with pain relief.19 In a subsequent article, the same authors showed that the treatment efficacy in those patients was predicted by their preoperative opioid-receptor availability.20 Here, our brief report unravels the role of μ-opioid system activity during spontaneous chronic pain attacks (migraine headaches), not treatment, as forecasted by the opioid-receptor availability in the nonheadache phase. Another difference in our study is that we used μOR BPND, a “selective” measurement in vivo of endogenous μOR availability,10 and its immediate reduction reflects the activation of this neurotransmitter system during migraine headache suffering.
In summary, there was an ineffective release of endogenous μ-opioids acting on μOR in the right mPFC to fight the ongoing migraine pain. Conceivably, this crucial descending inhibition was not able to acutely counteract the ascending trigeminal sensory inputs in their plenitude, and the other general symptoms and mechanisms associated or not with the migraine attack. These preliminary findings could explain the limitations of abortive opioid therapy for migraine patients4, since there is already high brain occupancy of μOR during the headache phase and the treatment efficacy is also dictated by the patients' baseline opioid-receptor availability (nonheadache phase). Further studies should be performed to develop novel and more effective therapeutic and prophylactic modulation of the endogenous μ-opioid system before the migraine headache phase initiation.21,22
Acknowledgments
This work was supported by the following grants (A. F. DaSilva): National Institute of Health – National Institute of Neurological Disorders and Stroke – K23 NS062946, Dana Foundation's Brain and Immuno-Imaging Award, and the Migraine Research Foundation Research Grant Award. The authors acknowledge the PET Center Nuclear Medicine Technologists (Jill M. Rothley, Edward J. McKenna, Andrew R. Weeden, Paul Kison, and Caitlin Hendriks) and the personnel of Functional MRI Laboratory (Scott Peltier and Keith Newnham). Alexandre DaSilva, the principal investigator, designed the study and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors declare no conflicts of interest related to this study. PainTrek is a free University of Michigan Apple mobile application created by A. F. DaSilva and E. Maslowski.
Conflict of Interest
None declared.
References
- 1.Bigal ME, Borucho S, Serrano D, Lipton RB. The acute treatment of episodic and chronic migraine in the USA. Cephalalgia. 2009;29:891–897. doi: 10.1111/j.1468-2982.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- 2.Nijjar SS, Gordon AS, Clark MD. Entry demographics and pharmacological treatment of migraine patients referred to a tertiary care pain clinic. Cephalalgia. 2010;30:87–91. doi: 10.1111/j.1468-2982.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 3.Sora I, Takahashi N, Funada M, et al. Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton RB, Bigal ME. Opioid therapy and headache: a cause and a cure. Neurology. 2004;62:1662–1663. doi: 10.1212/wnl.62.10.1662. [DOI] [PubMed] [Google Scholar]
- 5.DosSantos MF, Martikainen IK, Nascimento TD, et al. Reduced basal ganglia mu-opioid receptor availability in trigeminal neuropathic pain: a pilot study. Mol Pain. 2012;8:74. doi: 10.1186/1744-8069-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 7.Titeler M, Lyon RA, Kuhar MJ, et al. Mu opiate receptors are selectively labelled by [3H]carfentanil in human and rat brain. Eur J Pharmacol. 1989;167:221–228. doi: 10.1016/0014-2999(89)90582-7. [DOI] [PubMed] [Google Scholar]
- 8.Jewett DM. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28:733–734. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- 9.Meyer CR, Boes JL, Kim B, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1:195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 10.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 11.Zubieta JK, Smith YR, Bueller JA, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight YE, Goadsby PJ. The periaqueductal grey matter modulates trigeminovascular input: a role in migraine? Neuroscience. 2001;106:793–800. doi: 10.1016/s0306-4522(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 16.Denuelle M, Fabre N, Payoux P, et al. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47:1418–1426. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 17.Afridi SK, Matharu MS, Lee L, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128(Pt 4):932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- 18.Williamson DJ, Shepheard SL, Cook DA, et al. Role of opioid receptors in neurogenic dural vasodilation and sensitization of trigeminal neurones in anaesthetized rats. Br J Pharmacol. 2001;133:807–814. doi: 10.1038/sj.bjp.0704136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maarrawi J, Peyron R, Mertens P, et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69:827–834. doi: 10.1212/01.wnl.0000269783.86997.37. [DOI] [PubMed] [Google Scholar]
- 20.Maarrawi J, Peyron R, Mertens P, et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. 2013;154:2563–2568. doi: 10.1016/j.pain.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 21.DaSilva AF, Mendonca ME, Zaghi S, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012;52:1283–1295. doi: 10.1111/j.1526-4610.2012.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DosSantos MF, Love TM, Martikainen IK, et al. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psychiatry. 2012;3:93. doi: 10.3389/fpsyt.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]


