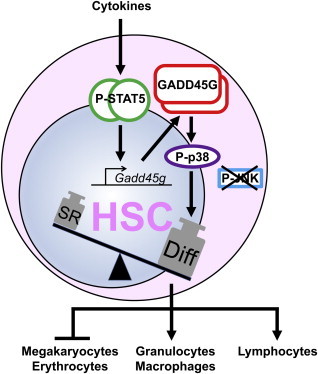
Summary
The balance of self-renewal and differentiation in long-term repopulating hematopoietic stem cells (LT-HSC) must be strictly controlled to maintain blood homeostasis and to prevent leukemogenesis. Hematopoietic cytokines can induce differentiation in LT-HSCs; however, the molecular mechanism orchestrating this delicate balance requires further elucidation. We identified the tumor suppressor GADD45G as an instructor of LT-HSC differentiation under the control of differentiation-promoting cytokine receptor signaling. GADD45G immediately induces and accelerates differentiation in LT-HSCs and overrides the self-renewal program by specifically activating MAP3K4-mediated MAPK p38. Conversely, the absence of GADD45G enhances the self-renewal potential of LT-HSCs. Videomicroscopy-based tracking of single LT-HSCs revealed that, once GADD45G is expressed, the development of LT-HSCs into lineage-committed progeny occurred within 36 hr and uncovered a selective lineage choice with a severe reduction in megakaryocytic-erythroid cells. Here, we report an unrecognized role of GADD45G as a central molecular linker of extrinsic cytokine differentiation and lineage choice control in hematopoiesis.
Graphical Abstract
Highlights
-
•
Molecular mechanism of cytokine-mediated differentiation induction in LT-HSCs
-
•
Cytokine-regulated GADD45G induces and accelerates differentiation in LT-HSCs
-
•
The absence of GADD45G increases the self-renewal capacity in LT-HSCs
-
•
GADD45G-induced program selects for myelomonocytic and lymphoid lineages
Rieger and colleagues report an unrecognized function of the tumor suppressor GADD45G as a molecular link of differentiation-promoting cytokine signaling and rapid differentiation induction in hematopoiesis. Cytokine-regulated GADD45G induces and accelerates hematopoietic stem cell differentiation and overrides the self-renewal program by specifically activating MAP3K4-mediated MAPK p38. Videomicroscopy-based single stem cell tracking further revealed a GADD45G-mediated selective lineage choice against megakaryocytic-erythroid fate.
Introduction
For an adequate quantitative production of each blood cell lineage in homeostasis and in stress conditions, the fate of hematopoietic stem cells (HSCs) to either differentiate or to self-renew must be strictly controlled (Orkin and Zon, 2008). In recent years, increasing knowledge of the many factors that contribute to the long-term maintenance of HSCs in the bone marrow (BM) niche was gained (Trumpp et al., 2010). Coordinated blood regeneration also needs HSCs to leave their quiescent state and differentiate into functional progeny, but little is known about molecules that control the initial differentiation step. Extrinsic stimuli such as cytokines have been implicated in this process (Metcalf, 2008). Cytokines are essential for blood cell generation by controlling proliferation, survival, differentiation, maturation, and function in a stage- and cell-type-specific manner (Metcalf, 2008; Rieger and Schroeder, 2009). Only in recent years it could be proved that cytokines also have instructive lineage choice capacity (Rieger et al., 2009; Sarrazin et al., 2009). Cell intrinsic factors, like transcription factors, can instruct the differentiation of distinct lineages, even across normal lineage boarders (Xie et al., 2004). However, their ability of making decisions rather than only executing them is controversial (Graf and Enver, 2009). So far, there have been rare examples that linked extrinsic stimuli with intrinsic differentiation and lineage choice mechanisms in hematopoiesis (Mossadegh-Keller et al., 2013; Sarrazin et al., 2009).
The expression of growth arrest and DNA-damage-induced 45 gamma (Gadd45g), a member of the Gadd45 family consisting of Gadd45a, b, and g, is induced by cytokines in myelomonocytic cells (Zhang et al., 1999) and dependent on signal transducer and activator of transcription (STAT) 5 (Hoffmeyer et al., 2001). GADD45G caught our interest because of its role as DNA-damage response and tumor suppressor gene that is epigenetically silenced in many malignancies (Liebermann et al., 2011). Moreover, GADD45G is upregulated in LT-HSCs of aged mice with reduced reconstitution ability (Rossi et al., 2005), and small hairpin RNA (shRNA)-mediated knockdown of GADD45G selected for aged HSCs with higher self-renewal potential (Wang et al., 2012). The Gadd45 family genes are known responders to environmental stressors such as radiation or chemicals and have been implicated in cell-cycle arrest, senescence, apoptosis, DNA repair and demethylation, as well as functional maturation in various cell systems including the hematopoietic system (Chen et al., 2014; Moskalev et al., 2012). However, the function of GADD45G in LT-HSCs has not been investigated yet.
Therefore, we decided to assess the function of GADD45G in the early HSC fate decision between self-renewal and differentiation and identified GADD45G as a rapid inducer and accelerator of HSC differentiation with selective lineage choice ability under the control of differentiation-promoting cytokines.
Results
GADD45G Is Activated by Cytokines and Immediately Induces the Differentiation in LT-HSCs
Because the expression of Gadd45 genes can be activated by various hematopoietic cytokines, we tested their ability to induce Gadd45 expression also in LT-HSCs. Stimulation of purified murine LT-HSCs (CD150+ CD48− CD34lo CD117+ Sca1+ lineage−) with the cytokine thrombopoietin (TPO) substantially increased the expression of Gadd45g, but did not change Gadd45a and Gadd45b expression (Figure 1A and Figure S1A available online). Next, we investigated if the Gadd45 genes are induced also by other cytokines in multipotent progenitors (MPPs). Whereas Gadd45a is not regulated by interleukin (IL) -3, IL-6, and TPO, Gadd45b is upregulated only upon IL-6 stimulation and Gadd45g is strongly induced by all tested cytokines (Figure 1B). Because mainly the expression of Gadd45g was regulated in immature hematopoietic stem and progenitors (HSPCs) by various cytokines, we focused on the role of Gadd45g in early hematopoietic cell-fate decisions.
Figure 1.
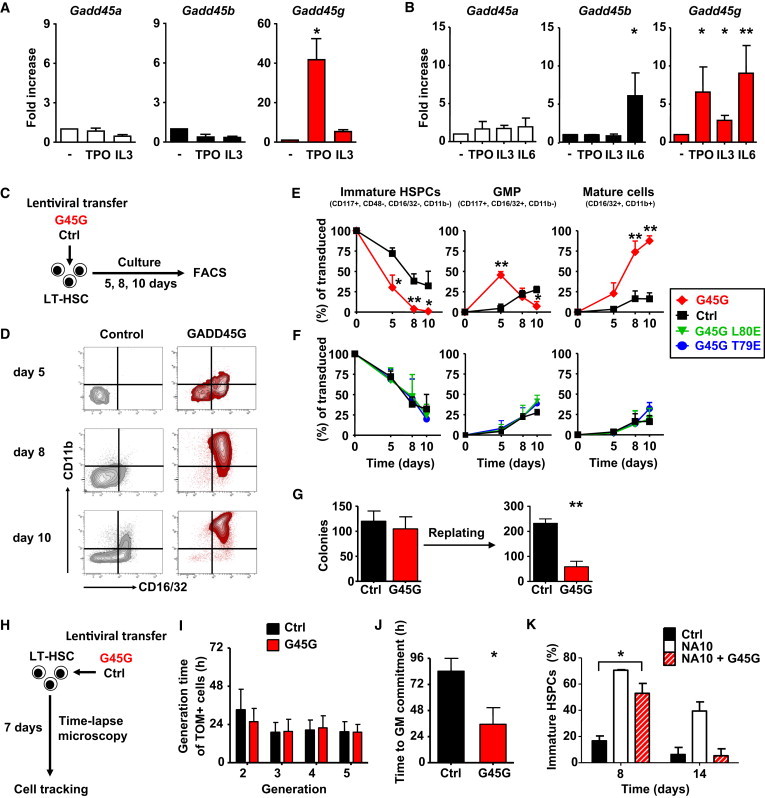
Cytokine-Stimulated GADD45G Expression Induces and Accelerates Differentiation in LT-HSCs
(A and B) Quantitative RT-PCR of Gadd45 genes in LT-HSCs (A) and MPPs (B) stimulated with cytokines. n = 3 experiments. Relative expression normalized to B2m.
(C–F) (C) Experimental scheme of (D)–(F). (D) Representative FACS blots of cultured LT-HSCs, gated for GADD45G and control-transduced cells (VENUS+).
(E and F) Quantification of FACS results after 5, 8, and 10 days of culture. Transduced cells (VENUS+) were gated for immature HSPCs, GMP-like cells and mature GM cells. n = 3 experiments.
(G) Colony formation assay of 200 LT-HSCs transduced with GADD45G and control and replating of 2,000 cells after 10 days. n = 3 experiments.
(H) Experimental scheme of (I) and (J).
(I) Generation times of transduced LT-HSCs (generation 1) and their progeny (TOMATO+) in subsequent generations.
(J) Interval of transgene expression (TOMATO+) and onset of CD16/32 expression (GMP-like stage). Data extracted from 29 (G45G) and eight pedigrees (control).
(K) Differentiation kinetics of double-transduced LT-HSCs (NA10, NUP98-HOXA10 homeodomain and GADD45G) gated on immature HSPCs by FACS. n = 3 experiments.
Data are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01. ns, not significant. See also Figure S1.
In order to simulate the consequences of cytokine-induced GADD45G expression in LT-HSCs, we ectopically expressed GADD45G in LT-HSCs by lentiviral transduction (Figure S1B) and determined the status of differentiation after 5, 8, and 10 days in culture by fluorescence-activated cell sorting (FACS) (Figure 1C). The transduced cells were distinguished by coexpression of a fluorescent protein. The differentiation process was initiated and strongly accelerated in LT-HSCs once GADD45G was expressed (Figures 1D and 1E). The percentage of immature HSPCs decreased rapidly after induction of GADD45G expression (Figure 1E, left). Although only 5% of the control-transduced LT-HSCs expressed markers for granulocyte-macrophage (GM) progenitors (GMPs) at day 5, already 45% of the GADD45G-expressing LT-HSCs reached the GMP stage of differentiation (Figure 1E, middle), and the majority of cells have differentiated into mature GM cells (CD11b+/CD16/32+) already at day 8 (Figure 1E, right). Dimerization of GADD45G is necessary for many of its functions, and point mutations either at amino acid position 79 (T79E) or 80 (L80E) of GADD45G inhibit dimerization (Schrag et al., 2008). Both mutants lost their ability to induce differentiation in LT-HSCs (Figure 1F), and we included L80E as additional negative control in our assays. Next, we confirmed our findings at the clonal functional level by colony formation assays. Although the potential of LT-HSCs to form colonies was unchanged by GADD45G, their ability to form secondary colonies after replating was severely decreased, indicating that GADD45G accelerates their differentiation (Figure 1G). To assess the differentiation kinetics of LT-HSCs into GMPs induced by GADD45G at the single-cell level, we continuously observed individual LT-HSCs and their progeny by time-lapse microscopy and cell tracking, a technology that records individual cell behavior in ontogeny pedigrees (Kimura et al., 2009; Rieger et al., 2009). LT-HSCs were transduced with lentiviruses coding for GADD45G or a control and were immediately administered to time-lapse microscopy (Figure 1H). GADD45G expression did not alter the cell-cycle time in LT-HSCs or their progeny in subsequent generations (Figure 1I). We measured the time interval between the onset of transgene expression (determined by nuclear membrane tdTOMATO) and expression of surface CD16/32 indicating the GMP-like stage of development. On average, LT-HSCs differentiated in only 36 hr into committed cells of the GMP stage when GADD45G was expressed, whereas control-transduced LT-HSCs required more than 84 hr (Figure 1J).
Based on these findings, we concluded that GADD45G is a rapid inducer of LT-HSC differentiation. Next we addressed the question whether GADD45G can also overcome an enhanced self-renewal program in LT-HSCs. We cotransduced LT-HSCs with lentiviral vectors coding for GADD45G (VENUS+) and NUP98-HoxA10 homeodomain (referred as NA10, TOMATO+), a potent inducer of self-renewal divisions (Ohta et al., 2007). When both programs were expressed at the single-cell level, initially the differentiation was prolonged, but, after 14 days, all cells finally were differentiated, whereas 39% of single NA10-expressing cells remained immature (Figures 1K and S1C).
GADD45G Switches HSCs from Their Self-Renewal Program into Differentiation In Vivo
GADD45G rapidly initiates the differentiation program in LT-HSCs in vitro, but it was unclear whether LT-HSCs maintain their self-renewal program under optimal in vivo conditions. Hence, we competitively transplanted LT-HSCs either expressing GADD45G or control (VENUS+) in lethally irradiated recipients and determined the reconstitution of T, B, and myeloid cells by FACS (Figure 2A). Donor LT-HSCs of both groups successfully homed and engrafted into the BM, as almost equal numbers of mature blood cells were generated 14 days after transplantation (Figure 2B). However, although in the control group the blood reconstitution increased over time, GADD45G-expressing cells were almost undetectable in the peripheral blood (PB) already 4 weeks after transplantation, and no long-term reconstitution was measured in PB or in BM (Figure 2B). In secondary transplantations, where the long-term self-renewal ability of LT-HSCs is further challenged, no GADD45G-positive cells engrafted, indicating a lack of remaining LT-HSCs in the primary recipients (Figure 2B). To examine the influences of GADD45G expression on cell cycle or viability in vivo, we transplanted GADD45G-expressing HSPCs, and determined both cell fates after 7 and 14 days in BM (Figure S2A). No changes in the rate of proliferating (bromodeoxyuridine [BrdU]+) or viable cells were seen in GADD45G-expressing cells (Figures S2B and S2C). These data clearly show that GADD45G induces differentiation at the expense of LT-HSC maintenance and self-renewal also in vivo. GADD45G is neither inhibiting cell proliferation nor survival and allows the generation of mature progeny.
Figure 2.
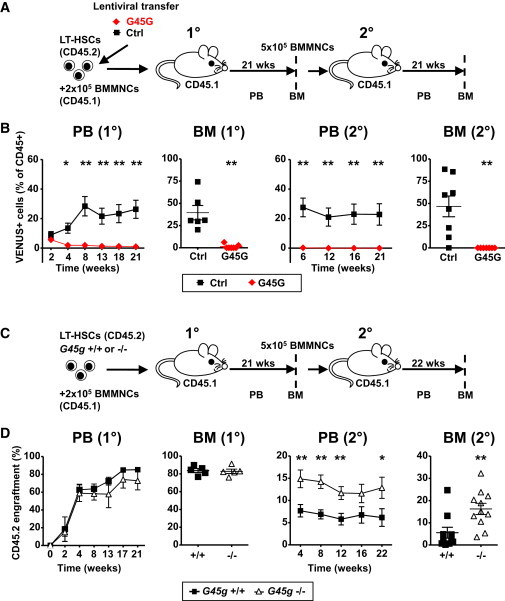
GADD45G Controls Self-Renewal and Differentiation of LT-HSCs In Vivo
(A) Experimental scheme of (B). BMMNCs, bone marrow mononuclear cells.
(B) Transduced multilineage donor cell reconstitution (VENUS+) was determined by FACS in peripheral blood (PB) and in bone marrow (BM) of primary (1°) and secondary (2°) recipients (six and eight mice per condition for 1° and 2° transplantation, respectively). Initial transduction efficiency in LT-HSCs was 45.4% (GADD45G) and 52.1% (control).
(C) Experimental scheme of (D).
(D) LT-HSCs from adult Gadd45g−/− and +/+ littermates were transplanted into lethally irradiated recipients (five and 10–11 mice per genotype for 1° and 2° transplantation, respectively), and multilineage donor cell reconstitution was measured by FACS in PB and in BM of 1° and 2° recipients.
Data are represented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01. See also Figure S2.
We then hypothesized that the absence of GADD45G may lead to an enhanced self-renewal and a delayed differentiation in LT-HSCs. Gadd45g has been germline deleted in a mouse model (Lu et al., 2001), with no major consequences for steady-state hematopoiesis (Figures S2D–S2F). To enforce enhanced self-renewal, we isolated LT-HSCs from Gadd45g−/− and Gadd45g+/+ littermates and transplanted them competitively in lethally irradiated recipients (Figure 2C). Although LT-HSCs from both groups equally well reconstituted primary recipients, the Gadd45g−/− BM cells from primary recipients were superior in repopulating secondary recipients (Figures 2D, S2G, and S2H). Therefore, the absence of GADD45G increases the stemness of LT-HSCs, most likely due to increased self-renewal.
GADD45G Selects for Lymphoid and Myelomonocytic Differentiation at the Expense of Megakaryocytic-Erythroid Lineage Fate
Megakaryocytes arise after several days culturing LT-HSCs, but they were almost absent, when LT-HSCs were transduced with GADD45G (Figure 3A). Next, we determined the megakaryocytic-erythroid (ME) potential in a colony formation assay with LT-HSCs expressing GADD45G in permissive cytokine conditions. Although 36% of colonies contained megakaryocytes and/or erythroid cells from control or L80E-transduced LT-HSCs, we only scored 6.2% ± 2.5% of colonies with ME potential when GADD45G was expressed (Figure 3B). Further, we addressed whether LT-HSCs expressing GADD45G were able to develop into ME progenitors (MEP) in vivo. We transplanted LSK (lineage− CD117+ Sca1+) cells transduced with GADD45G or control in lethally irradiated recipients and determined the differentiation into committed progenitors in the BM after 11 days (Figure 3C). Although we did not detect a difference in the LSK or GMP compartment, MEPs expressing GADD45G were absent, demonstrating a lineage bias (Figure 3D). GADD45G might instruct a program of lymphoid and GM lineage choice in multipotent cells. Alternatively, ME committed cells are unable to mature when GADD45G is expressed, which will lead to a selective elimination of these lineages. To discriminate both scenarios, we analyzed the development of LT-HSCs into GMPs by continuous cell tracking (Figure 3E; Movie S1). As expected, LT-HSC derived pedigrees containing any megakaryocytic progeny were almost absent when GADD45G was expressed (Figure 3F). We counted the death of cells that express GADD45G, but are not already committed to the GM lineage, thereby resembling an uncommitted or ME committed stage. Substantially more cell-death events appeared when GADD45G was expressed, in comparison to the control, suggesting a selective mechanism of cells that are committed to the ME lineage (Figure 3G). Indeed, when we transduced prospectively isolated MEPs with GADD45G, then colony formation was inhibited (Figure 3H). This inhibition did not appear in transduced GMPs (Figure 3I). Furthermore, when we cultured MEPs in cytokine conditions that promote megakaryo-/erythropoiesis, GADD45G transduction resulted in the disappearance of all transduced cells, whereas GADD45G-expressing GMPs in GM supporting cytokine conditions developed normally (Figure S3). This clearly indicates that the differentiation program that is induced by GADD45G is not compatible with ME cell fate.
Figure 3.
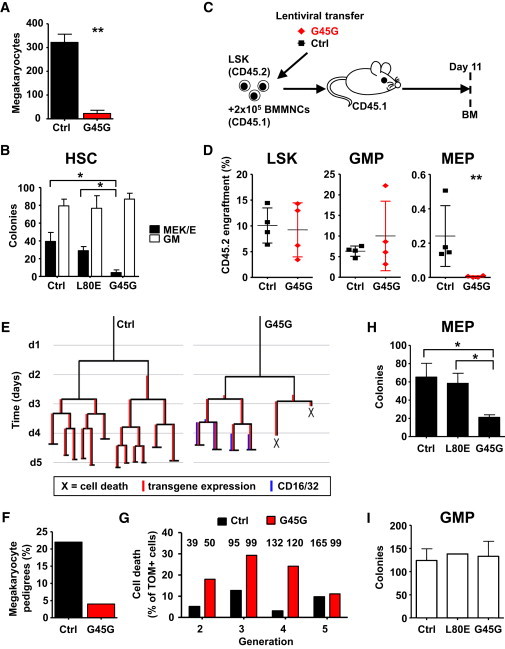
GADD45G Is Not Compatible with Megakaryocyte-Erythroid Differentiation and Thereby Selects for Lymphomyeloid Lineages
(A) TdTOMATO+ megakaryocytes after a 7 day culture of 100 LT-HSCs. Transduction efficiency was equal for both conditions. n = 3 experiments.
(B) Colony formation assay of 200 LT-HSCs transduced with GADD45G or control under permissive cytokine conditions. n = 3 experiments.
(C) Experimental scheme of (D).
(D) Transplantation of transduced LSKs in lethally irradiated recipients (four mice per condition) and BM FACS after 11 days. Transduction efficiency was 59% (GADD45G) and 72% (control).
(E) Example pedigrees illustrating the filiation of continuously tracked LT-HSCs (at the apex, generation 1) and all their progeny in subsequent generations.
(F) Percentage of pedigrees with megakaryocyte development.
(G) Percentage of cell-death events in each generation, before cells have differentiated into GMP-like cells (CD16/32+). Analyzed cell numbers are indicated.
(H and I) Colony formation assay of 400 MEPs or 200 GMPs transduced with GADD45G or control. n = 3 experiments.
Data are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01. See also Movie S1. See also Figure S3.
GADD45G Activates MAPK p38, but Not JNK, for Differentiation Induction
GADD45G collaboratively operates with a variety of other factors to orchestrate its various functions (Moskalev et al., 2012). One pathway that has been uncovered is the ability of GADD45G to activate the mitogen-activated protein kinase (MAPK) p38 pathway (Mita et al., 2002). Dimeric GADD45G binds to MAP3K4 near the autoinhibitory domain, which results in a conformational change relieving autoinhibition (Mita et al., 2002). Activated MAP3K4 phosphorylates MKK3/MKK6, which leads to p38 and/or JNK phosphorylation. To elucidate the involvement of this pathway in the differentiation induction by GADD45G, we cultured LT-HSCs transduced with GADD45G in the presence of four p38 inhibitors with distinct pharmacokinetics. The used concentrations highly restricted the selectivity of these inhibitors to the p38α isoform. In the absence of any inhibitor, only 26% of immature HSPCs expressing GADD45G remained in culture after 5 days, whereas more than 58% of cells resembled immature HSPCs in the control conditions (Figure 4A). With any of the four inhibitors, the fast differentiation induction by GADD45G was inhibited, and the cells behaved similar to conditions without ectopic GADD45G (Figure 4A). Along the same line, whereas already 17% mature GM cells appeared by GADD45G expression after 5 days without inhibitors, the differentiation was completely abrogated by p38 inhibition (Figure 4B).
Figure 4.
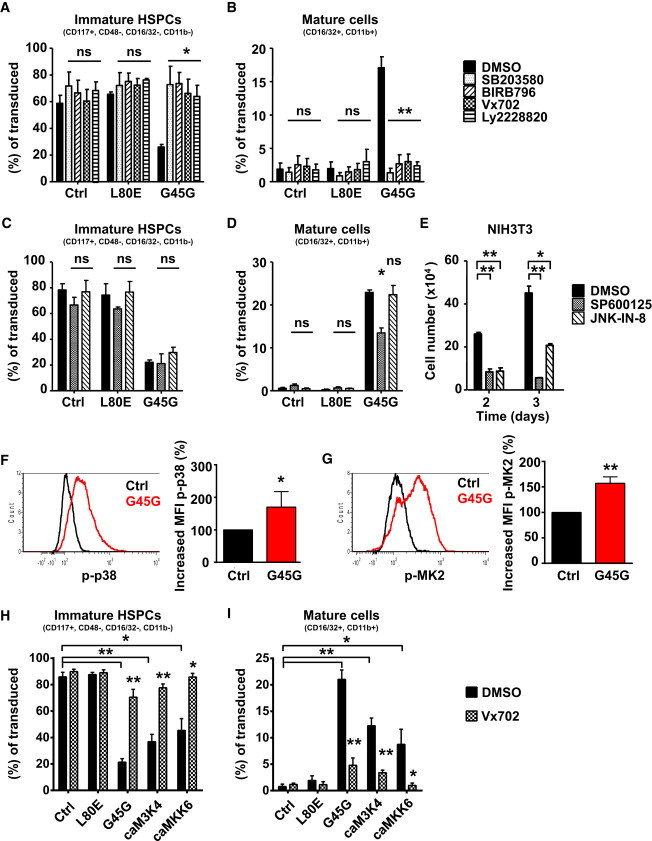
GADD45G Induces Differentiation by Specifically Activating MAPK p38
(A–D) Five day culture of transduced LT-HSCs in the presence of p38 (A and B) or JNK inhibitors (C and D). FACS for immature HSPCs (A and C) and mature GM cells (B and D). n = 3 experiments.
(E) Positive control for JNK inhibitor activity by reduced NIH 3T3 expansion. n = 3 experiments.
(F and G) (F) FACS histogram and quantification of phosphorylated p38 and (G) phosphorylated MK2 in 5 day-cultured MPPs after GADD45G or control transduction by phosphoflow cytometry. MFI, mean fluorescence intensity (n = 3 experiments).
(H and I) Five day culture of transduced LT-HSCs in the absence/presence of p38 inhibitor Vx702. FACS for immature HSPCs (H) and mature GM cells (I). n = 4 experiments.
ca, constitutively active; M3K4, MAP3K4. Stars above inhibitor conditions indicate significance versus respective DMSO control. Data are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01. ns, not significant. See also Figure S4.
The activation of MAP3K4 also mediates phosphorylation of JNK. However, the differentiation induction by GADD45G was not blocked by two JNK inhibitors, SP600125 and JNK-IN-8 (Figures 4C–4E), suggesting that GADD45G selectively leads to p38 activation. Next, we tested the phosphorylation status of p38 and one of its common downstream targets, MAPK-activated kinase 2 (MK2), after GADD45G expression in MPPs by phosphoflow cytometry. Indeed, cells expressing GADD45G showed a remarkable increase in p38 (Figure 4F) and MK2 phosphorylation (Figure 4G). To further investigate the molecular link between GADD45G and p38 phosphorylation, we tested potential GADD45G downstream molecules, namely, MAP3K4 and MKK6, for their ability to phenocopy GADD45G-mediated differentiation induction. We could show that lentivirally expressed constitutively active mutants of MAP3K4 and MKK6 induce and accelerate the differentiation in LT-HSCs similar to GADD45G (Figures 4H and 4I), that p38 is activated in all cases (Figures S4A and S4B), and that the accelerated differentiation is blocked by p38 inhibition (Figures 4H and 4I). These results strongly suggest that GADD45G activates a cascade of MAP3K4-MKK6-p38 leading to differentiation induction in HSPCs.
Discussion
We determined GADD45G as a molecular link between differentiation-promoting cytokine signaling and the rapid differentiation induction in murine LT-HSCs (Figure S4C). GADD45G acts like a toggle switch in HSCs. Although the mRNA and the protein are expressed only at low levels in steady state, GADD45G expression can rapidly be triggered by cytokine stimulation via the transcription factors STAT5A/B (Hoffmeyer et al., 2001). Dimeric GADD45G initiates an activation cascade leading to phosphorylated p38 and thereby to differentiation induction. Once GADD45G is upregulated in LT-HSCs, the differentiation commitment has occurred, and the cells are unable to maintain self-renewal. In the presence of IL-3 and IL-6, both potent inducers of GADD45G, single LT-HSCs lose their stem cell capacity within 3 days in culture, whereas the cells rapidly expand (Ema et al., 2000).
As recently reported, the inactivation of p38 activity by small molecule inhibitors maintained stemness ex vivo in human and murine HSCs (Baudet et al., 2012; Zou et al., 2012). Furthermore, an increase in reactive oxygen species results in the activation of p38 and finally in HSC exhaustion (Ito et al., 2006). We could completely abrogate the GADD45G induced differentiation by inhibiting activated p38. Phosphorylated p38 activates GATA-3 and leads to GATA-3 nuclear translocation specifically in LT-HSCs resulting in an exit of their self-renewal program (Frelin et al., 2013). Here, we provide the missing upstream molecular link between p38-mediated GATA3 activation (Frelin et al., 2013) and cytokine-induced differentiation induction in LT-HSCs, by our findings of GADD45G-mediated activation of p38. Hence, these results may open avenues to uncouple the cytokine-induced cell fates of differentiation from cell-cycle progression, for advanced ex vivo expansion of HSCs.
By continuously observing hundreds of individual HSCs and their progeny during differentiation, we can exclude a change in cell-cycle length in HSPCs upon GADD45G expression, which was unexpected, because studies on solid tissue cells reported a G2/M cell-cycle arrest mediated by GADD45G (Vairapandi et al., 2002). Also, the activation of p38 has been described as a decision point between senescence and apoptosis (Tront et al., 2006). None of these fates were observed in HSCs upon GADD45G expression. Intriguingly, the differentiation kinetics permitted by GADD45G is very fast: HSCs are driven to differentiate into GMPs within 36 hr. During GADD45G-accelerated differentiation, the metastable progenitor stages seem unchanged but are temporally shortened to a minimum. This finding opens a new perspective of our current understanding of sequential differentiation programs in hematopoiesis.
Stressed cells with an inability to repair their damage are committed to undergo apoptosis to prevent transformation. Alternatively, the induction of terminal differentiation preserves the integrity of the blood system. This safety mechanism was proposed as a function of the transcription factor BATF in aged HSCs to eliminate damaged HSCs from the system (Wang et al., 2012). The enhanced expression of GADD45G in aged HSCs, and the finding that aged GADD45G-depleted HSCs show an increased self-renewal and accumulation (Wang et al., 2012), may indicate a role of GADD45G in HSC life span control. Further, the epigenetic inactivation of Gadd45g in many malignancies explains the inability of tumor cells to terminally differentiate (Liebermann et al., 2011). Further studies are warranted to utilize the GADD45G-mediated pathway to therapeutically switch misregulated self-renewal in cancer-initiating cells into differentiation.
Experimental Procedures
Mice
C57BL/6, B6.SJL, and Gadd45g−/− (Gadd45γtm1Flv [Lu et al., 2001]), all maintained in C57BL/6 background, were used at 10–14 weeks of age. Animal experiments were approved by the regional council.
Ex Vivo Differentiation
One hundred LT-HSCs were lentivirally transduced (MOI 100) and cultured in SFEM (STEMCELL Technologies) supplemented with 100 ng/ml SCF and TPO (PeproTech). Cells were analyzed by FACS (antibodies against CD48, CD117, CD16/32, CD11b). Inhibitors were used as follows: BIRB796 (0.4 μM), SB203580 (10 μM), VX-702 (1 μM), LY2228820 (0.1 μM, all from Selleck), SP600125 (12.5 μM), and JNK-IN-8 (1 μM, from Merck).
FACS
BM cells were subjected to lineage depletion (EasySep Biotin Selection Kit, STEMCELL Technologies) and HSPC populations were sorted with a FACS Aria (BD Biosciences) after staining with antibodies against CD117, Sca1, CD150, CD48, CD16/32, CD34, and Streptavidin (Figure S1A; Table S1). Viable sorted cells were counted with trypan blue exclusion.
Colony Formation Assay
LT-HSCs, GMPs, and MEPs were lentivirally transduced (MOI 100), seeded 24 hr later in M3434 medium (STEMCELL Technologies), and scored microscopically after 9–12 days for transduction and colony formation.
Time-Lapse Imaging and Cell Tracking
Microscopy and tracking of LT-HSCs were performed as described (Rieger et al., 2009), until the fate of all progeny in the fifth cell generation was determined.
Statistics
Significance was determined by a t test (two-tailed, unpaired, unequal).
Author Contributions
F.B.T. performed experiments, analyzed the data, and helped to write the manuscript. S.W., P.G., N.H., M.R., and B.B. performed experiments and analyzed the data, F.J.T. provided bioinformatic analyses, L.H. advised the study and commented on the manuscript, T.S. developed the tracking software and commented on the manuscript. M.A.R. conducted and supervised the study, interpreted the data, and wrote the manuscript.
Acknowledgments
We thank C. Jourdan, S. Bothur, T. Merovci, C. Weiser, C. Molenda, K. Pesek, P. Schwab, and A. Eller for excellent technical assistance and J. Lausen and Y. Feuermann for critically reading the manuscript. R. Davis and A. Schambach kindly provided the plasmids Addgene plasmid #13518 and pRRL.PPT.SFFV.IRES.eGFP.wPRE, respectively. Funding was provided by the Intramural Research Program of NIDDK/NIH to L.H. and by the LOEWE Center for Cell and Gene Therapy (HMWK III L 4- 518/17.004 [2013]) to M.A.R.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Baudet A., Karlsson C., Safaee Talkhoncheh M., Galeev R., Magnusson M., Larsson J. RNAi screen identifies MAPK14 as a druggable suppressor of human hematopoietic stem cell expansion. Blood. 2012;119:6255–6258. doi: 10.1182/blood-2012-01-403949. [DOI] [PubMed] [Google Scholar]
- Chen Y., Ma X., Zhang M., Wang X., Wang C., Wang H., Guo P., Yuan W., Rudolph K.L., Zhan Q., Ju Z. Gadd45a regulates hematopoietic stem cell stress responses in mice. Blood. 2014;123:851–862. doi: 10.1182/blood-2013-05-504084. [DOI] [PubMed] [Google Scholar]
- Ema H., Takano H., Sudo K., Nakauchi H. In vitro self-renewal division of hematopoietic stem cells. J. Exp. Med. 2000;192:1281–1288. doi: 10.1084/jem.192.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Herrington R., Janmohamed S., Barbara M., Tran G., Paige C.J., Benveniste P., Zuñiga-Pflücker J.C., Souabni A., Busslinger M., Iscove N.N. GATA-3 regulates the self-renewal of long-term hematopoietic stem cells. Nat. Immunol. 2013;14:1037–1044. doi: 10.1038/ni.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer A., Piekorz R., Moriggl R., Ihle J.N. Gadd45gamma is dispensable for normal mouse development and T-cell proliferation. Mol. Cell. Biol. 2001;21:3137–3143. doi: 10.1128/MCB.21.9.3137-3143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y., Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Kimura A., Rieger M.A., Simone J.M., Chen W., Wickre M.C., Zhu B.M., Hoppe P.S., O’Shea J.J., Schroeder T., Hennighausen L. The transcription factors STAT5A/B regulate GM-CSF-mediated granulopoiesis. Blood. 2009;114:4721–4728. doi: 10.1182/blood-2009-04-216390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann D.A., Tront J.S., Sha X., Mukherjee K., Mohamed-Hadley A., Hoffman B. Gadd45 stress sensors in malignancy and leukemia. Crit. Rev. Oncog. 2011;16:129–140. doi: 10.1615/critrevoncog.v16.i1-2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Yu H., Chow C., Li B., Zheng W., Davis R.J., Flavell R.A. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita H., Tsutsui J., Takekawa M., Witten E.A., Saito H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 2002;22:4544–4555. doi: 10.1128/MCB.22.13.4544-4555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalev A.A., Smit-McBride Z., Shaposhnikov M.V., Plyusnina E.N., Zhavoronkov A., Budovsky A., Tacutu R., Fraifeld V.E. Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res. Rev. 2012;11:51–66. doi: 10.1016/j.arr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossadegh-Keller N., Sarrazin S., Kandalla P.K., Espinosa L., Stanley E.R., Nutt S.L., Moore J., Sieweke M.H. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Sekulovic S., Bakovic S., Eaves C.J., Pineault N., Gasparetto M., Smith C., Sauvageau G., Humphries R.K. Near-maximal expansions of hematopoietic stem cells in culture using NUP98-HOX fusions. Exp. Hematol. 2007;35:817–830. doi: 10.1016/j.exphem.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S.H., Zon L.I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M.A., Schroeder T. Analyzing cell fate control by cytokines through continuous single cell biochemistry. J. Cell. Biochem. 2009;108:343–352. doi: 10.1002/jcb.22273. [DOI] [PubMed] [Google Scholar]
- Rieger M.A., Hoppe P.S., Smejkal B.M., Eitelhuber A.C., Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Zahn J.M., Ahlenius H., Sonu R., Wagers A.J., Weissman I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S., Mossadegh-Keller N., Fukao T., Aziz A., Mourcin F., Vanhille L., Kelly Modis L., Kastner P., Chan S., Duprez E. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell. 2009;138:300–313. doi: 10.1016/j.cell.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Schrag J.D., Jiralerspong S., Banville M., Jaramillo M.L., O’Connor-McCourt M.D. The crystal structure and dimerization interface of GADD45gamma. Proc. Natl. Acad. Sci. USA. 2008;105:6566–6571. doi: 10.1073/pnas.0800086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tront J.S., Hoffman B., Liebermann D.A. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–8454. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- Trumpp A., Essers M., Wilson A. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- Vairapandi M., Balliet A.G., Hoffman B., Liebermann D.A. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J. Cell. Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- Wang J., Sun Q., Morita Y., Jiang H., Gross A., Lechel A., Hildner K., Guachalla L.M., Gompf A., Hartmann D. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Xie H., Ye M., Feng R., Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Zhang W., Bae I., Krishnaraju K., Azam N., Fan W., Smith K., Hoffman B., Liebermann D.A. CR6: A third member in the MyD118 and Gadd45 gene family which functions in negative growth control. Oncogene. 1999;18:4899–4907. doi: 10.1038/sj.onc.1202885. [DOI] [PubMed] [Google Scholar]
- Zou J., Zou P., Wang J., Li L., Wang Y., Zhou D., Liu L. Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells. Ann. Hematol. 2012;91:813–823. doi: 10.1007/s00277-011-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


