Abstract
Objectives: Eriobotrya japonica belongs to the Rosaceae. Studies have shown that the flowers of this plant are rich in phenolic and flavonoid compounds. Accorrdingly, the evaluation of antioxidative effects of Eriobotrya japonica Flower Extract (EJFE) have been performed in vitro.
Material and Methods: In this study, to investigate the influences of components of EJFE on its antioxidative activity, extract was prepared using hydro-alcoholic (25:75 V/V) solvent and the antioxidative activity of the extract was evaluated based on the scavenging of various radicals (DPPH and H2O2) by spectrophotometric method and chelating of ferrous ions by ferrozine reagent.
Results: HPLC analysis of the Eriobotrya japonica Flower Extract (EJFE) revealed hesperetin and gallic acid as the major antioxidants. When the content of total flavonoid and polyphenolic compounds in the flower extract of this plant was examined, a significantly higher level of total polyphenols was found in Eriobotrya japonica flower extract.
Conclusion: Results demonstrate that the high ability to scavenge free radicals, reducing power, and Fe+2chelating activity exerted by the EJFE were due to the high content of hesperetin and gallic acid in the flowers.
Key Words: Antioxidant, Eriobotrya japonica Lindl., Flavonoids, Free radicals, Phenolic compounds
Introduction
Eriobotrya japonica (E. japonica Lindl.) is a tree with edible fruit in the Rosaceae family, indigenous to southeastern China and Japan. Now it is also cultivated in the Mediterranean areas, Australia, South Africa, South America, California, India, and North of Iran. Its white flowers turn into pale-yellow or deep-orange pomes (Vaughan and Geissler, 1997 ▶). Its leaves have been used for treatment of skin diseases and diabetes (De Tommasi. et al, 1992 ▶), chronic bronchitis, coughs, phlegm, ulcers, and cancer (Ito et al., 2000 ▶). Reactive oxygen species (ROS) in the forms superoxide (•O2-), hydrogen peroxide (H2O2), and hydroxyl radical (•OH) are by-product of normal cellular metabolism and attack biological systems leading to destruction and peroxidation of cell membranes, many age-related disease, and susceptibility to cancer and infection (Yen and Chen, 1995 ▶). Flavonoids and phenolic compounds are secondary metabolites found to be abundant in the plant kingdom and effective in human health and decrease the risk of several diseases by reducing oxidative stress (Peluso, 2006 ▶). In this study, we report the separation of the main phenolic and flavonoid constituents from the flower extracts of E. japonica, which have not been previously reported.
Materials and Methods
Hesperetin was purchased from Sigma (Steinheim, Germany), Gallic acid from Acros (Geel, Belgium), and HPLC grade methanol from Fluka (Buchs, Switzerland). Ethanol and BHA were from Merck (Darmstadt, Germany). Napthylethylenediamide dihydrochloride, Sodium nitroprusside (SNP), Sulfanilamide, FeCl2, FeCl3, 1,1-Diphenyl-2-Picryl Hydrazyl radical (DPPH), 2,4,6-Tri- Pyridyl-S-Triazine (TPTZ), Butylated hydroxy anisole (BHA) and EDTA were purchased from Sigma-Aldrich (St. Louis, MO, USA). All Millipore syringe filters (0.22 and 0.45μm) were purchased from Millipore Company (JET BIOFIL® Syringe Driven Filters). The double distilled water was used. Other chemicals were purchased from Sigma-Aldrich. The highest commercially available purity reagents were used.
Preparation of the extract for HPLC
Extraction procedure was performed according to the method proposed by Hertog et al. (1992) ▶. Briefly 0.5g of sample was refluxed using 50ml of 50% aqueous methanol at 90°C for two hours. This solution contains 1.5 g L-1 of butylated hydroxy anisol(BHA) in order to prevent the analytes from oxidation. The obtained extracts were cooled, filtered to remove solid particles, then filtered by a 0.45 µm syringe Millipore filters and injected into the HPLC system.
HPLC system
The chromatographic measurements were carried out with HPLC system consisted of a model 515 solvent delivery system equipped with model 7725i injector fitted with a 20 μL loop. Column used was Spherisorb C18 (250×4.6 mm, 5 μm) all from Waters (Milford, MA, USA). The UV detector was model LC-95 set at 260 nm. The mobile phase used for separation and determination of analytes was methanol: 0.4% phosphoric acid (42.5 / 57.5 v/v) containing 0.5% THF as organic modifier with flow rate of 1.0 ml min-1 at 30 °C. Determination of gallic acid and hesperetin in EJFE was performed using standard addition method with HPLC at λ = 260 nm. Identification of each compound was performed by its retention time and spiking with the standard.
Preparation of the extract for evaluation of antioxidant properties (in vitro)
E. Japonica flowers were collected freshly from gardens of Ghaemshar, Mazandaran, Iran in october 2012. In outset, the flowers were exposed in the shadow for two weeks, then kept in 37ºC Oven for one day and were finally pulverized. About 81.5 grams of the flowers dry powder was shaken and extracted exhaustively with hydro-alcoholic (25:75 v/v). Next, it was filtered with Whatman filter paper and concentrated under reduced pressure in a rotary evaporator in 30 ºC for 6h to yield dried hydro-alcoholic extract, which was 9.5% of the dry weight of the plant flower.
Determination of total phenolic content in EJFE
The amount of total phenolic compounds in extracts was determined spectrophotometrically using Folin-Ciocalteu Reagent(FCR) with small modifications (Singleton et al, 1999 ▶, Fukumoto and Mazza., 2000 ▶). One hundred milligrams of the extract was extracted with 10 ml of ethanol/water (75:25, v/v, 0.3% HCl) and filtered through a 0.22 μm Millipore filter. Two hundred and fifty microliter filtrate was mixed with 1.25ml of FCR (0.2 M) and 1ml of sodium carbonate (7.5 g dl-1). The mixture was incubated in the dark at room temperature for 2h to complete the reaction. Then, absorbance of the solution was measured at 760 nm with a T80+ UV-VIS spectrophotometer using distilled water as the blank. Evaluation was based on the standard curve of gallic acid (concentration range of 0-2 mg ml-1), which was dissolved in ethanol/water (75:25, v/v, 0.3% HCl). The concentration of total phenolic compounds was expressed in mg gallic acid equivalents (GAE) per gram of dried extract. All measurements were replicated three times.
Estimation of total flavonoid content in EJFE
Total flavonoid content in the extracts was determined spectrophotometrically using AlCl3 reagent (Jia et al., 1999 ▶) with minor changes. One hundred milligrams of the extract was extracted with 10 ml of ethanol/water (75:25 v/v, 0.3% HCl) and filtered through a 0.22 μm Millipore filter. 250 μL filtrate was mixed with 750 μL of methanol and 50 μL of AlCl3.6H2O (10% ethanolic) plus 50 μL of potassium acetate (1M) and 1.4 ml of double distilled water. After incubation at room temperature for 30 min, the absorbance of the reaction mixtures was measured at 415 nm. The blank sample was a 1:1 mixture of the examined extracts and double distilled water. Flavonoid content was expressed in mg hesperetin equivalents (HE) per gram of dried extract by using a standard curve of hesperetin(concentration range 0-1 mg ml-1). All measurements were replicated three times.
Determination of reducing power in EJFE
The reducing power of the Eriobotrya japonica Lindl. flower extract was determined according to Yen and Chen method (1995) ▶. In this method, about 0.5 ml of different amounts of the extract (25-800 μg ml-1) in water were mixed with phosphate buffer (1.25 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe (CN)6] (1.25 ml, 1%).
The mixtures were incubated for 20 minutes at 50°C. 1.25 ml of TCA (10%) was added to the mixture to stop the reaction, and then it was centrifuged at 2500g for 10 min. The supernatant (1.25 ml) was mixed with distilled water (1.25 ml) and FeCl3 (250 μL, 0.1%), and absorbance was measured at 700 nm against a blank solution. All measurements were replicated three times. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid (Vit-C) was used as positive control.
Metal chelating activity by EJFE
Metal ions play an important role as catalysts of oxidative processes, leading to the formation of hydroxyl radicals by decomposing hydrogen peroxide. The chelating of ferrous ions by EJFE was estimated using ferrozine reagent (Dinis et al., 1994 ▶, Ebrahimzadeh et al., 2009 ▶) with minor modifications. One milliliter of the extract (concentration range of 10-100 μg ml-1) was added to a solution of 2 mM FeCl2 (0.05 ml).
The reaction was initiated by addition of 5 mM ferrozine (1 ml), then it was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was then measured at 562 nm. The percentage inhibition of ferrozine- Fe2+ complex formation was calculated as [(Ab-At or (As)/ Ab] × 100, where Ab was the absorbance of the blank, At was the absorbance of the extract and As was the absorbance of positive control, EDTA.
DPPH radical scavenging activity by EJFE
The stable 1, 1-diphenyl-2-picryl hydrazyl radical (DPPH) scavenging ability in the flowers of the E. japonica was evaluated by spectrophotometric method (Yamaguchi et al., 1998 ▶). In this method, 1 ml of different concentrations of EJFE (concentration range of 0-60 mg ml-1) was added to a methanolic solution of DPPH (100 μM) at an equal volume. After 20 min at room temperature and in darkness, the absorbance was measured at 517 nm against a blank solution. The experiment was repeated three times. Vit-C, BHA and Hesperetin were used as standard controls. IC50 values denote the concentration of the sample which is required to scavenge 50% of DPPH free radicals.
Scavenging of hydrogen peroxide by EJFE
Scavenging of H2O2 was determined by the method of Ruch et al. (1989) ▶ with minor modification. A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (pH 7.4). Approximately 2 ml the EJFE (concentration range of 0.1-1 mg ml-1) in methanol were added to a hydrogen peroxide solution (0.6 ml, 40 mM). The absorbance of hydrogen peroxide at 230 nm was determined after ten minutes against a blank solution containing phosphate buffer without H2O2. Ascorbic acid, BHA and Hesperetin were used as standards. The percentage of hydrogen peroxide scavenging by the EJFE and the standard compounds were calculated as follows:
% scavenged (H2O2) = [(Ab–At or As)/ Ab] × 100 where Ab absorbance of the control, At absorbance of the extract and As was the absorbance standard. The experiment was repeated three times.
Results
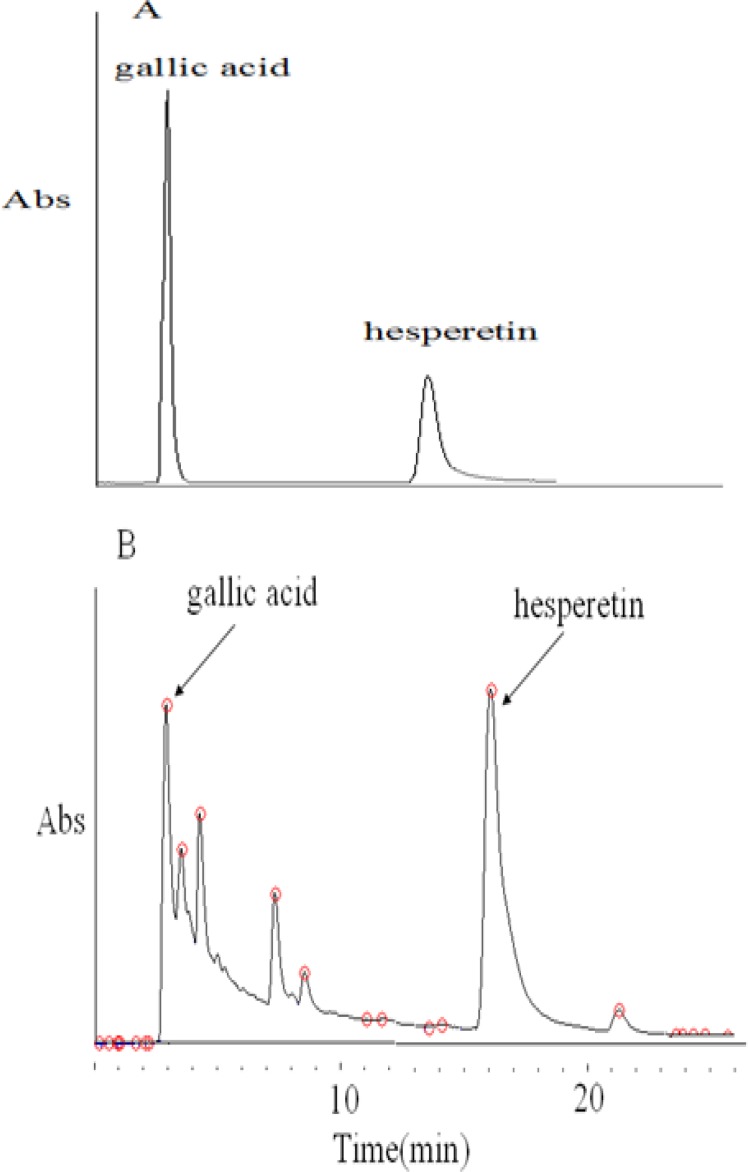
In our research, HPLC analysis of standards such as gallic acid, hesperetin (A) and E. japonica Extract (B) is shown in Figure 1. Several peaks were observed in the HPLC chromatogram. A major peak was obtained at a retention time of 3.4 min and was identified as gallic acid and other polar compounds. Another prominent peak was obtained at retention time of 16.6 and was identified as hesperetin (Figure 1).
Figure 1.
HPLC chromatogram of standards of gallic acid and hesperetin (A) and Eriobotrya japonica flower extract (EJFE) (B).
Statistical analysis was performed for validation of determination of analytes in E. japonica. Limit of detection (LOD) was calculated according to “3Sb/m” where Sb is the standard deviation of the blank and is equal to p-p Noise when only mobile phase was passing through the column for 45 minutes and “m” is the steepness of calibration curve. Linear dynamic range(LDR) was obtained by plotting the peak areas versus concentration and the relative standard deviation(RSD or %RSD) was determined by analyzing standard solutions (20 µg ml-1) repeated five times (Table 1).
Table 1.
Statistical results for validation of determination of gallic acid and hesperetinin Eriobotrya japonica
| Compound | LOD(20μg ml -1 ) |
LDR
(20μg ml -1 ) |
R 2 | % RSD (n=5) |
|---|---|---|---|---|
| gallic acid | 0.07 | 0.07-35 | 0.999 | 1.22 |
| Hesperetin | 0.03 | 0.03-50 | 0.999 | 2.2 |
On the other hand, quantitative determination of total phenolic and flavonoids has shown that the EJFE contains 97.8 ± 0.7 mg g-1 of total polyphenols expressed as gallic acid equivalent (GAE, mg g-1 of extract) and 36.5± 0.3 mg g-1 of total flavonoids expressed as hesperetin equivalent.
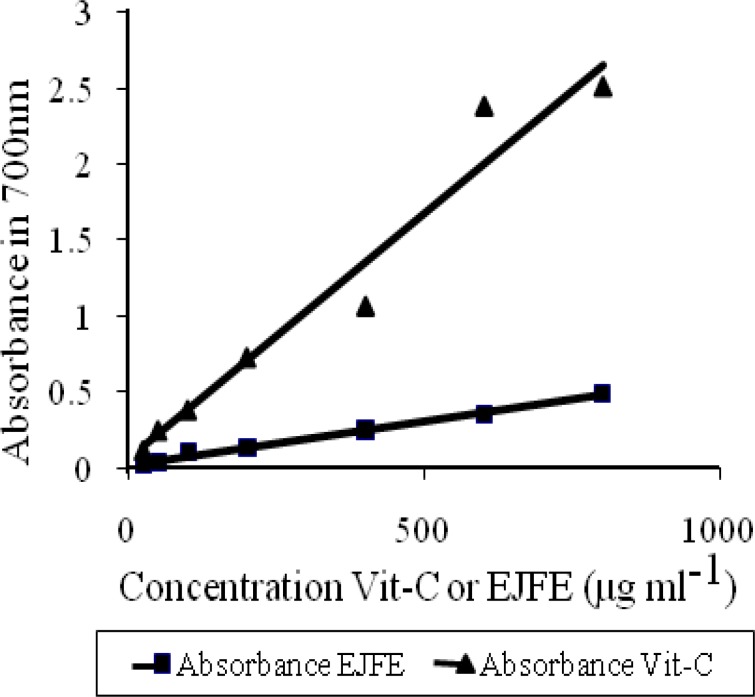
Another part of the research evaluated the reducing power of EJFE in comparison to ascorbic acid (Vit-C). The reducing power of EJFE was found to be significant, but in comparison to Vit-C is lower dose-dependently (Figure 2).
Figure 2.
Reducing power of hydro-alcoholic extract of Eriobotrya japonica flower in comparison to Vitamine C (Vit-C). In concentration 800 μg ml-1, the reducing power of EJFE in comparison to Vit-C is lower about 19.52%. Each value is mean±SE. (n = 3).
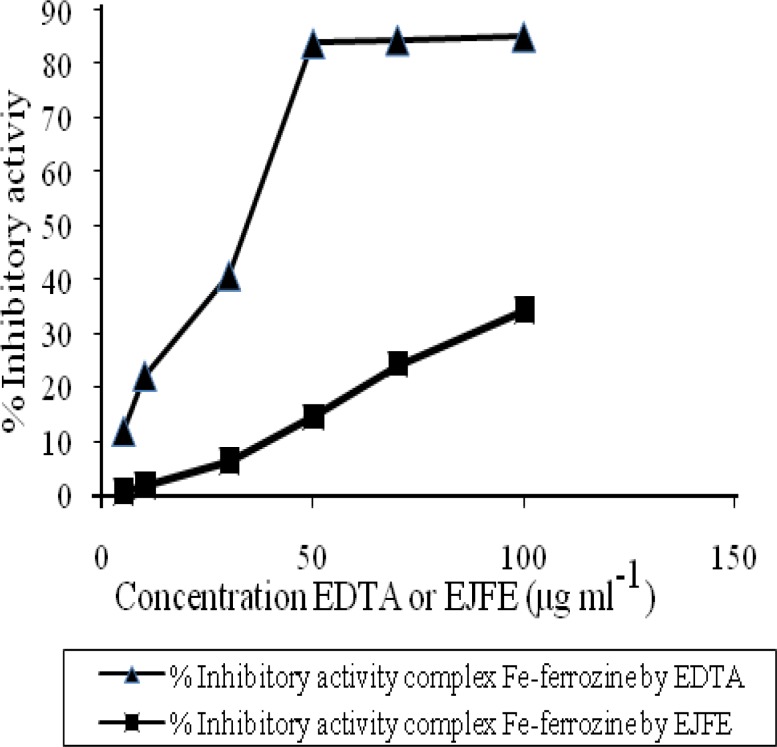
In experiments related to the chelating activities of the EJFE, Ferrozine can quantitatively form complexes with Fe+2 to make a purple color. But, in the presence of other chelating agents such as plant extracts, the complex formation is reduced resulting in a decrease of the purple color of the complexes (Figure 3).
Figure 3.
Chelating activity of EJFE in comparison to EDTA. The absorbance of the Fe+2-ferrozine complex decreased dose-dependently. In concentration 50 μg ml-1, chelating activity of EJFE in comparison to EDTA is lower about 17.29%. Each value is mean±SE. (n=3).
The antioxidant activity of EJFE was evaluated in a series of in vitro tests. Each of these assays was based on one feature of antioxidant activity, such as the ability to scavenge various free radicals. In 20 mg ml-1 concentration, the EJFE significantly scavenged DPPH radicals in comparison to Vit-C, but less than BHA and Hesperetin (Table 2). Also, the neutralization of H2O2 by the E. japonica flower extract was measured spectrophotometrically. The ability of the assessed extract to neutralize H2O2 was dose dependent and it significantly scavenged H2O2 with the IC50 values of 3128.91 in 1000 μg ml-1 concentration (Table 3).
Table 2.
Percentage of neutralization of the DPPH radical by EJFE in comparison to Vit-C, hesperetin and BHA
| Concentration(20mg ml -1 ) | %Inhibitory activity DPPH(Mean±SD) | IC 50 (mg ml -l ) |
|---|---|---|
| Vit-C | 51.03±2.98 | 19.59 |
| Hesperetin | 63.81±2.76 | 15.76 |
| BHA | 86.77±4.31 | 11.52 |
| EJFE | 53.82±1.49 | 18.58 |
Table 3.
Percentage of neutralization of H2O2 by EJFE in comparison to Vit-C,hesperetin and BHA
| Concentration (1000μg ml -1 ) | %Inhibitory activity H 2 O 2 (Mean±SD) | IC 50 (μgr ml -1 ) |
|---|---|---|
| Vit-C | 41.42± 6.54 | 1207.14 |
| Hesperetin | 19.51± 2.31 | 2562.78 |
| BHA | 24.57± 5.83 | 2035.01 |
| EJFE | 15.98± 3.66 | 3128.91 |
Discussion
In this Study, the E. japonica extraction yield was about 9.5% of the dry weight of the plant flower with hydro-alcoholic extract (alcohol 96°). We performed HPLC analysis of EJFE to identify some of flavonoids and phenolic compounds and we showed that Hesperetin as bioflavonoid and gallic acid to be the major antioxidants in it. In quantitive determination of total polyphenols and flavonoids in EJFE, our study has shown that the extracts were the high contents of phenolic and flavonoid compounds. Based on the reports of Federico Ferreres et al. (2009) ▶, total phenolic content of E. japonica leaves, peel and flesh was about 64, 1337 and 1668 mg kg-1, respectively. Also, Lu et al. (2009) ▶ reported that total flavonoid content of leaves of E .japonica was 615 g kg-1, but our study showed that concentrations of gallic acid and hesperetin in the flower of this plant was 7200 and 15600 g kg-1, respectively. There is a relationship between antioxidant activities with the content of total phenolics or flavonoids. A high yield of polyphenols in the extract indicated that it possesses a high antioxidant activity.
In chelating activities tests, both plants extract and EDTA interfered with the formation of Fe+2 and ferrozine complexes, suggesting that it has chelating activity and captures ferrous ions before ferrozine. Chelation therapy may reduce iron-related complications in some diseases such as thalassemia major, cancer, HIV or Wilson’s disease (Grazul and Budzisz., 2009 ▶).
Also, scavenging of H2O2 and DPPH by EJFE may be attributed to their phenolic compounds which can donate electrons to be, thus neutralizing it to water and neutral compounds. Since, H2O2 and free radicals can pass membranes and oxidize a number of cell compounds, thus elimination of hydrogen peroxide and DPPH, as well as the OH radicals is important for human health, food and drug.
In this study, it was also described for the first time that EJFE has antioxidative activity through free radicals scavenging, metal chelatory, reducing power and detoxification.
Acknowledgment
The authors want to express their gratitude to Dr Vaheid Sharifi of Mazandaran University for his technical assistance in performance of HPLC.
Conflict of interest
There is not any conflict of interest in this study.
References
- De Tommasi N, Aquino R, De Simona F, Pizza C. Plant metabolites new sesquiterpene and ionona glycosides from Eryobotrya japonica. J Nat Prod. 1992;55:1025–1032. [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh MA, Nabavi SM, Nabavi SF. Correlation between the in vitro iron chelating activity and poly phenol and flavonoid contents of some medicinal plants. Pakis J Biol Sci. 2009;12:934–938. doi: 10.3923/pjbs.2009.934.938. [DOI] [PubMed] [Google Scholar]
- Ferreres F, Gomes D, Valentao P, Goncalves R, Pio R, Chagas EA, Seabra RM, Andrade PB. Improved loquat (Eriobotrya japonica Lindl ) cultivars: Variation of phenolics and antioxidative potential. Food Chem. 2009;114:1019–1027. [Google Scholar]
- Fukumoto L, Mazza G. Asessing antioxidant and prooxidant activities of phenolic compounds. J Agri Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Grazul M, Budzisz E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord Chem Rev. 2009;253:2588–2598. [Google Scholar]
- Hertog MC, Hollman PCH, Venema DP. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J Agri Food Chem. 1992;40:1591–1598. [Google Scholar]
- Ito H, Kobayashi E, Takamatsu Y, Li SH, Hatano T, Sakagami H, et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem Pharm Bull. 2000;48:687–693. doi: 10.1248/cpb.48.687. [DOI] [PubMed] [Google Scholar]
- Jia Z, Tang M, Wu J. The determination of flavonoids content in mulberry and their scavenging effect on superoxide radicals. Food Chem. 1999;64:555–599. [Google Scholar]
- Lu H, Li CWL, Wu RJL, Zhang HQ. Hypoglycemic effect of the total flavonoid fraction from Folium Eribotrya. Phytomedicine. 2009;16:967–971. doi: 10.1016/j.phymed.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Peluso MR. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase and modulate lipid homeostasis in adipose tissue and liver. Exp Biol Med. 2006;231:1287–1299. doi: 10.1177/153537020623100802. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Vaughan JG, Geissler CA. The New Oxford Book of food plants. New York: Oxford University Press; 1997. pp. 110–111. [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao Y. HPLC method for evaluation of the free radical-scavenging activity of food by using 1,1-diphenil,-2-picrylhydrazil. Biosci Biotech Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agri Food Chem. 1995;43:27–32. [Google Scholar]