Summary
In regeneration-competent vertebrates, such as salamanders, regeneration depends on the ability of various differentiated adult cell types to undergo natural reprogramming. This ability is rarely observed in regeneration-incompetent species such as mammals, providing an explanation for their poor regenerative potential. To date, little is known about the molecular mechanisms mediating natural reprogramming during regeneration. Here, we have identified the extent of extracellular signal-regulated kinase (ERK) activation as a key component of such mechanisms. We show that sustained ERK activation following serum induction is required for re-entry into the cell cycle of postmitotic salamander muscle cells, partially by promoting the downregulation of p53 activity. Moreover, ERK activation induces epigenetic modifications and downregulation of muscle-specific genes such as Sox6. Remarkably, while long-term ERK activation is found in salamander myotubes, only transient activation is seen in their mammalian counterparts, suggesting that the extent of ERK activation could underlie differences in regenerative competence between species.
Graphical Abstract
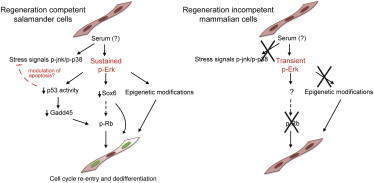
Highlights
-
•
Sustained ERK activation is required for serum reprogramming of salamander cells
-
•
Only transient ERK activation is observed in their mammalian counterparts
-
•
Constant ERK activation promotes expression of S phase genes in mammalian myotubes
-
•
The extent of ERK activation could underlie differences in regenerative competence
Unlike mammals, salamanders can regenerate complex structures such as their limbs upon amputation. This process depends on the ability of differentiated adult cells to undergo natural reprogramming. Yun and colleagues show that sustained ERK activation is critical for serum-induced reprogramming of salamander muscle cells. Their research suggests that the extent of ERK activation could underlie differences in regenerative competence between species.
Introduction
In most vertebrates, the process of myogenic differentiation entails the withdrawal of precursors from the cell cycle, followed by their fusion into myotubes. The multinucleate state is characterized by a permanent postmitotic arrest, which renders the myotubes unable to respond to proliferative cues (Pajalunga et al., 2008; Walsh and Perlman, 1997). In contrast, salamander myotubes remain responsive to such cues, being able to re-enter the cell cycle upon serum stimulation in culture (Tanaka et al., 1997) or after implantation within regenerating structures (Kumar et al., 2000).
In salamander (Notophthalmus viridescens) A1 myotubes (Ferretti and Brockes, 1988), serum stimulation induces a reprogramming process that includes partial dedifferentiation, as suggested by the downregulation of the myogenic gene Myf5 (Imokawa et al., 2004), and re-entry into the cell cycle, which is also considered an aspect of dedifferentiation. The latter depends on the phosphorylation of Rb (Tanaka et al., 1997) and the downregulation of p53 activity (Yun et al., 2013). The serum component that triggers these responses is not a conventional growth factor but an as-yet-unidentified thrombin-activated serum component that acts as a mitogen for myotubes, but not for mononucleate precursors (Lööf et al., 2007; Straube et al., 2004; Tanaka et al., 1999). Even though mammalian myotube nuclei cannot be reprogrammed upon exposure to this factor (Lööf et al., 2007), they are able to re-enter the cell cycle after forming heterokaryons with salamander myotubes (Velloso et al., 2001). This suggests that even when the initial response may be different, part of the pathway leading to serum-mediated reprogramming is conserved. Both the identity of the serum factor and the signaling pathways driving the reversal of the differentiated state in regeneration-competent salamander cells remain unknown, although extensive efforts to identify the serum factor are ongoing (Straube et al., 2004).
In proliferating cells, the extracellular signal-regulated kinase (ERK) family of mitogen-activated protein kinases (MAPKs) plays a critical role in driving cell-cycle progression in a variety of cell types (Albeck et al., 2013; Cook and McCormick, 1996; Murphy et al., 2002; Weber et al., 1997; Yamamoto et al., 2006). In fibroblasts, sustained ERK activation is required for successful S phase progression by promoting the downregulation of antiproliferative genes during G1 phase and controlling the state of Rb phosphorylation (Yamamoto et al., 2006). Hence, it is possible that ERK activation plays a role during the reprogramming of differentiated salamander cells. Herein, we have tested this hypothesis using the salamander A1 cell line as a model for serum-induced reprogramming.
Results and Discussion
Sustained ERK Activation in Cell-Cycle Re-entry of Salamander Myotubes
Serum stimulation of A1 myotubes triggers an early activation of the ERK pathway, which is sustained for up to 48 hr post stimulation (Figures 1A and 1B). This is accompanied by a long-term increase in the protein levels of c-FOS (Figure S1 available online), a sensor for ERK signal duration (Murphy et al., 2002). Other MAPK pathways are also activated, albeit to a lesser extent, including the stress-related MAPKs c-Jun N-terminal kinase (JNK) and p38 (Figures 1A, 1B, and S1). Therefore, we asked whether any of these pathways are required for myotube S phase re-entry following serum stimulation. The administration of specific inhibitors of ERK (U0126), JNK (SP600125), and p38 (506126) kinase activation (Figure S1), alongside serum stimulation, leads to differential disruption of both Rb phosphorylation (Figures 1C and S1) and S phase re-entry (Figures 1D and S1) depending on the targeted pathway. Inhibition of the JNK pathway leads to a 50% reduction in both myotube Rb phosphorylation and S phase re-entry, while disruption of the p38 MAPK not only does not impair these processes but also significantly enhances cell-cycle re-entry (Figure 1D). Remarkably, the inhibition of ERK signaling severely impairs both processes, suggesting that the activation of the ERK pathway is critical for the cell-cycle re-entry of salamander myotubes.
Figure 1.
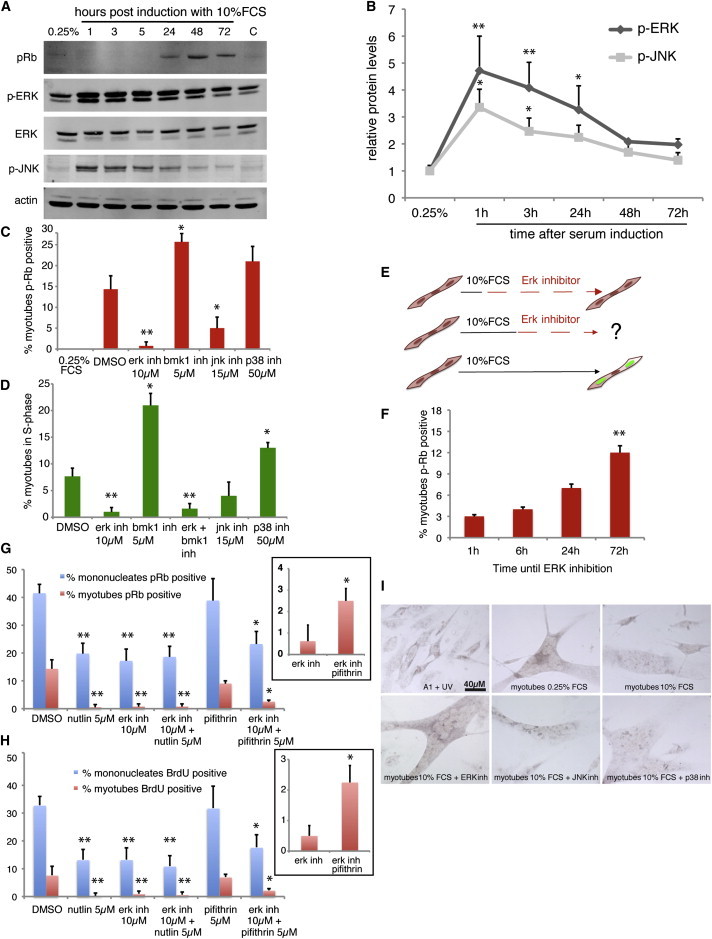
Sustained ERK Activation Is Necessary for Cell-Cycle Re-entry of Differentiated, Regeneration-Competent Salamander Myotubes
(A) Western blot analysis of A1 myotube extracts pre (0.25% FCS) or post serum induction.
(B) Western blot quantification of phospho protein levels, relative to β-actin, showing the kinetics of ERK and JNK activation upon serum induction.
(C) Quantification of myotubes exhibiting phosphorylated Rb, following immunostaining 48 hr after serum induction, in the presence of the indicated compounds.
(D) Quantification of bromodeoxyuridine (BrdU)-positive myotubes, as measured by immunostaining at 72 hr post serum induction, following a BrdU pulse.
(E) Schematic representation of myotube treatments to test the effect of ERK inhibition in cell-cycle re-entry.
(F) ERK inhibition at different times after serum induction affects Rb phosphorylation in myotubes, as determined by immunostaining at 72 hr. ∗∗ refers to significant differences between 72 hr and 24 hr measurements.
(G–I) Serum-induced Rb phosphorylation (G) and myotube S phase re-entry (H) are impaired by p53 stabilization, ERK inhibition, or their combined treatment. Insets correspond to Rb phosphorylation (G) or cell-cycle re-entry (H) in myotubes exposed to the indicated treatments. (I) In situ hybridization against the p53 target gene Gadd45. ERK inhibition impairs the downregulation of Gadd45 in A1 myotubes following serum induction.
Values represent the mean ± SD (B) or mean ± SEM (C–H) (∗p < 0.05, ∗∗p < 0.01); n = 4 (B, F, and I) and n = 5 (C, D, G, and H), where n indicates the number of independent experiments. Scale bar in (I) represents 40 μm.
In a variety of mammalian contexts, it is the sustained activation of ERK signaling that promotes cell proliferation (Pagès et al., 1993; Weber et al., 1997; Yamamoto et al., 2006). To test whether sustained ERK activation mediates the cell-cycle aspects of salamander myotube reprogramming, we incubated A1 myotubes with serum and inhibited ERK signaling at different times post stimulation (Figure 1E). ERK inhibition, even at 24 hr post stimulation, has a negative effect on Rb phosphorylation (Figure 1F). This suggests that the sustained, but not transient, activation of the ERK pathway is necessary for cell-cycle re-entry of salamander myotubes.
Several isoforms of ERK MAPKs exist, with ERK1 and its close relative ERK2 mediating cell-cycle progression in a variety of cell types (Yang et al., 2013). The ERK inhibitor used in this study inhibits the activation of ERK1/2 but can also have effects on ERK5/big MAPK 1 (BMK1) signaling (Kamakura et al., 1999). Hence, we used a specific BMK1 inhibitor to dissect which of the ERK MAPK pathways mediates the myotube response to serum. As seen in Figures 1C and 1D, the inhibition of BMK1 does not impair Rb phosphorylation or S phase re-entry but significantly enhances these processes. While the general ERK inhibitor decreases S phase re-entry in both myotubes and mononucleates, the BMK1 inhibitor has a negative effect on mononucleates but a positive one on myotubes (Figures 1 and S1). This suggests that activation of both ERK1/2 and ERK5 is required for the proliferation of mononucleates but only ERK1/2 activation is necessary for S phase re-entry in myotubes. The positive effect of BMK1 inhibition on myotube cell-cycle re-entry is likely due to its negative effect on mononucleate proliferation, as it is known that the percentage of myotubes in S phase is inversely proportional to the amount of mononucleates in their vicinity (Tanaka et al., 1997). Indeed, the inhibition of BMK1 combined with the addition of 30% confluent mononucleates abrogates the effect of ERK5 inhibition and impairs myotube S phase re-entry (Figure S1).
Sustained activation of ERK signaling has been shown to promote the downregulation of Gadd45, a classical p53 target (Yamamoto et al., 2006). Furthermore, we have recently shown that the downregulation of p53 activity is a critical step during A1 myotube cell-cycle re-entry (Yun et al., 2013). To test whether p53 downregulation is promoted by ERK signaling, we compared the effects on Rb phosphorylation and S phase re-entry caused by the stabilization of p53 using nutlin3a (Vassilev et al., 2004), the inhibition of ERK activation, or a combination of both. All three treatments affected both processes equally (Figures 1G and 1H) at the mononucleate or myotube level, suggesting that the downregulation of p53 activity and the activation of ERK signaling form part of the same pathway. We additionally observed that while treatment of myotubes with the p53 inhibitor α-pifithrin did not affect Rb phosphorylation or cell-cycle re-entry, the combined inhibition of ERK signaling and p53 had a negative effect on this processes. However, the joint inhibition of ERK and p53 significantly increased the percentage of myotubes positive for Rb phosphorylation and re-entering the cell cycle, when compared with the inhibition of ERK signaling on its own (Figures 1G and 1H). This suggests that ERK signaling is required for cell-cycle re-entry by, at least in part, promoting the downregulation of p53 activity. To test this further, we investigated the effect of inhibiting different MAPKs on the regulation of Gadd45 gene expression by in situ hybridization. ERK inhibition abrogated the downregulation of Gadd45 induced by serum stimulation (Figure 1I), suggesting that the activation of the ERK pathway promotes cell-cycle re-entry in salamander myotubes by downregulating p53 activity.
ERK Activation Promotes Phenotypic Changes in Salamander Myotubes
A second aspect of the response to serum stimulation in salamander myotubes is the promotion of transcriptional changes that lead to partial dedifferentiation. As ERK activation is required at such an early stage of the response, it is plausible that it contributes to the promotion of dedifferentiation as well as cell-cycle progression. To address this possibility we cloned the newt homolog of the muscle-specific gene Sox6 (Hagiwara et al., 2007) (Figure S2) and examined its expression in purified myotube cultures. While serum stimulation leads to a 50% reduction of the relative levels of Sox6 (Figure 2A), ERK inhibition abrogates this effect, suggesting that the downregulation of Sox6 upon serum stimulation is dependent on ERK activation. This observation is consistent with a previous study suggesting that ERK activation drives the dedifferentiation of rodent Schwann cells (Harrisingh et al., 2004). While the expression of Sox6 changed upon serum stimulation, we did not detect any changes in the protein levels of muscle-specific myosin heavy chain under similar conditions (Figure 2C). This is consistent with the notion that serum promotes a limited dedifferentiation process in regeneration-competent salamander myotubes.
Figure 2.
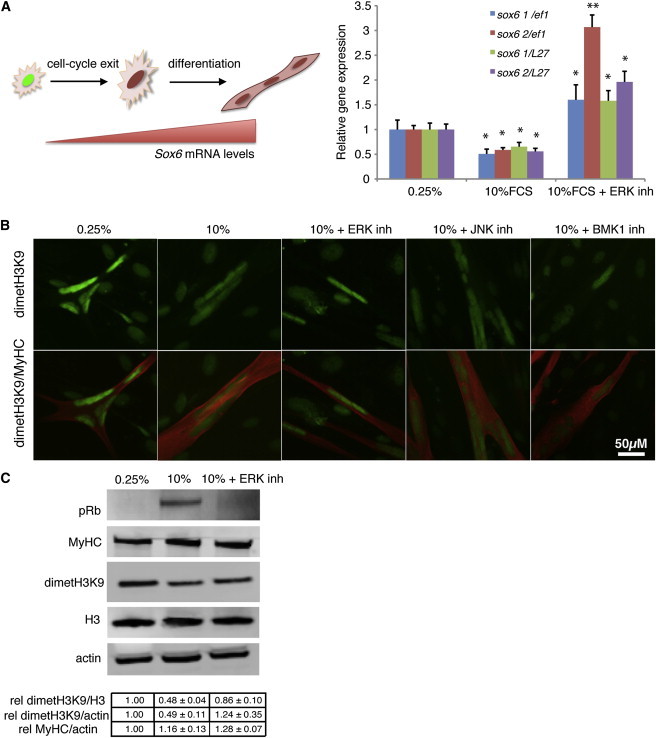
ERK Activation Leads to Downregulation of Muscle-Specific Gene Expression and Promotes Epigenetic Changes during Reprogramming of Salamander Myotubes
(A) Left: schematic representation of the variation in Sox6 mRNA levels upon myogenic differentiation. Right: quantitative RT-PCR analysis of Sox6 (with two different primers, Sox6 1 and Sox6 2) in RNA extracts from purified A1 myotubes, normalized to Ef1-α or L27.
(B) Representative images of myotubes following immunostaining against dimethylated H3K9 and myosin heavy chain (MyHC) 1.5 days after the indicated treatments. Scale bar represents 50 μm.
(C) Western blot analysis of purified myotube extracts. Note the decrease in dimetH3K9 upon serum induction and its impairment when ERK signaling is inhibited. The relative amount of dimethylated H3K9 was quantified for the indicated treatments.
Values represent the mean ± SEM (∗p < 0.05); n = 4 (A–C), where n indicates the number of independent experiments.
Furthermore, we noticed that there are large-scale epigenetic changes in the A1 myotubes following serum stimulation. Immunostaining against the repressive histone mark dimethyl H3K9 (Jenuwein and Allis, 2001) revealed a decrease in signal intensity in myotubes upon serum stimulation, consistent with previous observations (Lööf et al., 2007). Interestingly, this decrease is abrogated only by ERK, but not by JNK or BMK1 inhibition (Figure 2B). Western blot analysis of purified myotube extracts indicates that there is a 50% decrease in dimethyl H3K9 upon serum stimulation but only a 20% decrease when ERK signaling is inhibited (Figure 2C). This is particularly interesting for two reasons. First, the demethylation of H3K9 at E2F-regulated promoters is required for the progression into S phase (Ogawa et al., 2002). Second, H3K9 demethylation is required for the expression of pluripotency-associated genes and the maintenance of embryonic stem cells (Loh et al., 2007).
In the light of these observations, it is possible that the changes in H3K9 demethylation, which we show to be dependent on ERK activation, generate a favorable environment for altering gene expression in the direction of partial dedifferentiation and cell-cycle progression in regeneration-competent cells.
Lack of Sustained ERK Activation in Regeneration-Incompetent Mammalian Myotubes
While salamander myotubes are able to respond to serum stimulation by promoting a signaling cascade leading to partial dedifferentiation and cell-cycle re-entry, mammalian myotubes are unable to do so (Tanaka et al., 1997). Nevertheless, the latter appear to have retained some responsiveness, as serum stimulation (Tiainen et al., 1996) or incubation with partially purified serum factor preparations (Lööf et al., 2007) leads to the upregulation of immediate-early proliferative response genes such as c-fos. However, the subsequent steps in the pathway leading to reprogramming do not take place. In the light of our observations, this suggests that altered or absent ERK activation could underlie the lack of responsiveness found in mammalian myotubes.
We explored this possibility using the murine myogenic cell line Pmi28 (Figure 3A), which can be induced to form myotubes that cannot dedifferentiate or re-enter the cell cycle upon serum stimulation (Duckmanton et al., 2005). Remarkably, we found that serum stimulation of Pmi28 myotubes leads to transient ERK1/2 activation, which peaks at 1 hr post induction and returns to baseline in approximately 3 hr (Figures 3B and 3C). In this system, the intensity of ERK activation is considerably lower than in the induced salamander myotubes (Figure 3C). Furthermore, unlike A1 myotubes, stimulated Pmi28 myotubes do not activate JNK and do not exhibit any changes in the total amounts of the repression marker dimethyl H3K9 (Figure 3D). However, serum stimulation promotes the upregulation of c-FOS and an increase in cyclin D1 and D3, which depend on the transient activation of ERK (Figures 3D and 3E). This suggests that the transient response to serum previously observed in mammalian myotubes is mediated by ERK signaling. Nonetheless, consistent with previous observations (Lööf et al., 2007; Tiainen et al., 1996), we did not observe any changes in late proliferative genes such as Cyclin E2 and PCNA (Figure 3E). Together, these observations suggest that the extent of ERK signaling could underlie differences in regenerative capabilities between salamander and mammalian cells.
Figure 3.
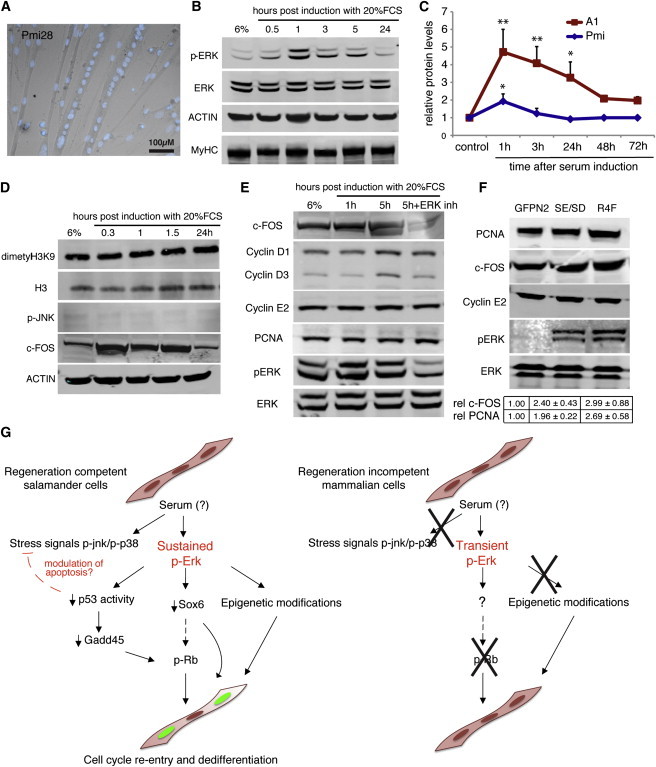
Regeneration-Incompetent Mammalian Myotubes Do Not Trigger Sustained ERK Activation upon Serum Stimulation
(A) Representative image of Pmi28 myotubes 3 days after myogenesis. Nuclei are stained with Hoechst 33258 (blue). Scale bar represents 100 μm.
(B, C, and E) Western blot analysis of Pmi28 myotube extracts pre (6% HS) or at different times after induction with 20% FCS.
(D) Differential kinetics of ERK activation in mammalian versus salamander myotubes at different times after serum induction following western blot quantification of relative levels of phosphorylated ERK.
(F) Western blot analysis of whole-cell extracts from myotubes overexpressing pnGFPN2 (GFPN2), MEK1-R4F (R4F), and MEK1S218E/S222D(SE/SD). The relative amount of c-fos and PCNA was quantified for the indicated treatments.
(G) A model for the role of ERK1/2 activation during serum-induced reprogramming of regeneration-competent salamander myotubes compared to their mammalian counterparts.
Values represent the mean ± SD (∗p < 0.05, ∗∗p < 0.01); n = 4 (B–F), where n indicates the number of independent experiments.
Finally, we tested whether the artificial promotion of ERK activation could promote cell-cycle re-entry in this system. Overexpression of two different constitutively active ERK activator kinase 1 (MEK1) mutants, MEK1-R4F and MEK1S218E/S222D (Mansour et al., 1994), does not lead to S phase re-entry in Pmi28 myotubes. Such failure in promoting cell-cycle progression could be attributed to the lack of coactivation of other MAPKs (e.g., JNK), the presence of epigenetic factors in mammalian myotubes that stabilize the differentiated state, or the intrinsic limitations of the overexpression system, which does not permit an exact mimicry of the required kinetics of ERK activation. However, constitutive ERK activation by overexpression of the MEK1 mutants does promote an increase in the protein levels of c-FOS and, importantly, the late proliferative gene PCNA (Figure 3F). These changes could result from either the sustained activation of ERK signaling or the transient elevation of phosphorylated ERK (pERK) levels upon constitutive expression of the mutants. The induction of untransfected Pmi myotubes with 20% serum leads to a pERK/ERK ratio of 0.87 ± 0.13. In contrast, the overexpression of MEK1-R4F and MEK1S218E/S222D leads to lower pERK/ERK ratios (0.34 ± 0.05 and 0.53 ± 0.07, respectively, corrected for total protein load); however, these are sustained over time. Hence, our results suggest that the promotion of sustained ERK activation can further the progression of the cell-cycle re-entry program in mammalian myotubes.
A Model for the Role of ERK Signaling during the Reprogramming of Postmitotic Differentiated Cells
We propose that in regeneration competent salamander myotubes—and possibly other cell types—an as-yet-unidentified thrombin-activated serum factor triggers sustained activation of ERK signaling (Figure 3G). Such activation leads to the downregulation of p53 activity, which facilitates cell-cycle re-entry via Rb phosphorylation, and promotes alterations in the gene expression landscape that could favor both partial dedifferentiation and cell-cycle re-entry. ERK activation could also crosstalk with the JNK and p38 stress-related signaling cascades, for example by modulating the apoptotic response mediated by these kinases. In contrast, regeneration-incompetent mammalian cells are incapable of inducing sustained ERK activation, most likely due to a lack of an upstream receptor or signaling component, and are therefore unable to undergo reprogramming.
In this study, we show that sustained ERK activation is required for the promotion of phenotypic changes suggestive of partial dedifferentiation and, critically, for cell-cycle re-entry in salamander myotubes. It remains an issue whether other muscle-specific genes are downregulated upon serum stimulation and whether this partial dedifferentiation is dependent on cell-cycle re-entry. In this regard, an earlier study showed that cellularization (Velloso et al., 2000), another index of dedifferentiation, proceeds in the absence of cell-cycle re-entry. Further studies should determine the connection between these processes.
It is particularly interesting that a similar mechanism to that used by proliferating cells following standard growth factor stimulation (sustained ERK signaling) is activated during salamander myotube reprogramming, as the serum factor responsible for triggering this process is not one of the conventional growth factors known to promote proliferation in undifferentiated cells (Straube et al., 2004; Tanaka et al., 1999). Indeed, the inhibition of fibroblast growth factor (FGF) and VEGF, growth factors that elicit sustained ERK1/2 signaling in a range of proliferating cell types, does not perturb either ERK1/2 or Rb phosphorylation in purified myotubes (Figure S3). Nevertheless, the use of a general receptor tyrosine kinase inhibitor abrogates ERK, JNK, and Rb phosphorylation following serum stimulation, suggesting that a receptor tyrosine kinase is required for the initiation of the reprogramming response. This unidentified RTK may be a target, direct or indirect, of the thrombin-activated serum factor. Further efforts should focus on defining the nature of both the serum factor as well as the RTK, so as to gain a better understanding of the mechanisms regulating the plasticity of the differentiated state in regeneration-competent cells and eventually apply this knowledge in a clinical setting.
Conclusions
Together, our results suggest that the ability to trigger sustained ERK activation may underlie species-specific differences in the generation of cells of regenerative potential. Furthermore, they suggest that current approaches to induce regenerative potential in mammalian systems should consider the manipulation of MAPK signaling, with a focus on the promotion of ERK activation.
Experimental Procedures
Cell Culture
A1 cells were previously derived from newt (N. viridescens) limb mesenchyme (Ferretti and Brockes, 1988). The cells were grown on 0.75% gelatine-coated plastic dishes in minimal essential medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), 25% H2O, 2nM L-glutamine (Gibco), 10 μg/ml insulin (Sigma), and 100 U/ml penicillin/ streptomycin (Gibco) in a humidified atmosphere of 2.5% CO2 at 25°C. Routine cell subculture was performed as previously described (Lo et al., 1993).
Pmi28 cells were a kind gift from A. Starzinski-Powitz (Goethe Universität, Germany). Mouse Pmi28 cells were grown on BIOCOAT I T-75 flasks (Becton-Dickinson) in F-10 (HAM) media supplemented with 20% FCS (Gibco) and 100 U/ml penicillin/ streptomycin (Gibco) in a humidified atmosphere of 6% CO2 at 37°C. Cells were passaged at a 1:10 ratio every 3 days.
Myotube Formation Assay
Myogenesis was induced in confluent A1 cells by lowering the fetal calf serum concentration from 10% to 0.25%. Cells were incubated in a humidified atmosphere of 2.5% CO2 at 25°C. After 5 days, >90% of cells fused into multinucleate myotubes as previously described (Lo et al., 1993). Reprogramming was induced by incubating the myotubes in standard growth medium. All inhibitors used in the present manuscript are specified in Table S1.
In Pmi28 cell cultures, myogenesis was induced as previously described (Duckmanton et al., 2005). Cells were plated at high density onto collagen-coated plastic dishes and maintained for 3 days in differentiation media, consisting of Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 6% horse serum (HS; Sigma) and 100 U/ml penicillin/ streptomycin (Gibco).
Myotube Purification
Myotubes were trypsinized for 1 min, neutralized with media, and sieved through a 100 μm mesh followed by a 35 μm mesh (Cell MicroSieve; BioDesign Inc. of New York). Myotubes were retained on the 35 μm mesh, washed with media to eliminate mononucleates, and collected from the sieve into dishes precoated with 0.75% gelatin.
Pmi28 myotubes were purified by addition of 50 μM Ara-C (Sigma), which specifically eliminates proliferating cells, to the differentiation media. The purity of the resulting cultures was assessed by fluorescence microscopy following incubation with Hoechst 33258 (2 μg/ml).
Statistical Analysis
Statistical analyses were performed with Prism 4.0, and unpaired two-tailed t tests were applied unless otherwise stated. Paired two-tailed t tests were carried out to analyze RT-PCR experiments.
Acknowledgments
We are grateful to the members of the Brockes lab and Dr. Yutaka Matsubayashi for fruitful discussions and to Dr. Emmanuel Boucrot for providing reagents. This research was supported by an MRC Programme grant to J.P.B.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Accession Numbers
The GenBank accession number for the CDS of Notophthalmus viridescens Sox6 reported in this paper is KJ801973.
Supplemental Information
References
- Albeck J.G., Mills G.B., Brugge J.S. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol. Cell. 2013;49:249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S.J., McCormick F. Kinetic and biochemical correlation between sustained p44ERK1 (44 kDa extracellular signal-regulated kinase 1) activation and lysophosphatidic acid-stimulated DNA synthesis in Rat-1 cells. Biochem. J. 1996;320:237–245. doi: 10.1042/bj3200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckmanton A., Kumar A., Chang Y.T., Brockes J.P. A single-cell analysis of myogenic dedifferentiation induced by small molecules. Chem. Biol. 2005;12:1117–1126. doi: 10.1016/j.chembiol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ferretti P., Brockes J.P. Culture of newt cells from different tissues and their expression of a regeneration-associated antigen. J. Exp. Zool. 1988;247:77–91. doi: 10.1002/jez.1402470111. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Yeh M., Liu A. Sox6 is required for normal fiber type differentiation of fetal skeletal muscle in mice. Dev. Dyn. 2007;236:2062–2076. doi: 10.1002/dvdy.21223. [DOI] [PubMed] [Google Scholar]
- Harrisingh M.C., Perez-Nadales E., Parkinson D.B., Malcolm D.S., Mudge A.W., Lloyd A.C. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa Y., Gates P.B., Chang Y.T., Simon H.G., Brockes J.P. Distinctive expression of Myf5 in relation to differentiation and plasticity of newt muscle cells. Int. J. Dev. Biol. 2004;48:285–291. doi: 10.1387/ijdb.031787yi. [DOI] [PubMed] [Google Scholar]
- Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kamakura S., Moriguchi T., Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- Kumar A., Velloso C.P., Imokawa Y., Brockes J.P. Plasticity of retrovirus-labelled myotubes in the newt limb regeneration blastema. Dev. Biol. 2000;218:125–136. doi: 10.1006/dbio.1999.9569. [DOI] [PubMed] [Google Scholar]
- Lo D.C., Allen F., Brockes J.P. Reversal of muscle differentiation during urodele limb regeneration. Proc. Natl. Acad. Sci. USA. 1993;90:7230–7234. doi: 10.1073/pnas.90.15.7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y.H., Zhang W., Chen X., George J., Ng H.H. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lööf S., Straube W.L., Drechsel D., Tanaka E.M., Simon A. Plasticity of mammalian myotubes upon stimulation with a thrombin-activated serum factor. Cell Cycle. 2007;6:1096–1101. doi: 10.4161/cc.6.9.4141. [DOI] [PubMed] [Google Scholar]
- Mansour S.J., Matten W.T., Hermann A.S., Candia J.M., Rong S., Fukasawa K., Vande Woude G.F., Ahn N.G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- Murphy L.O., Smith S., Chen R.H., Fingar D.C., Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Ishiguro K., Gaubatz S., Livingston D.M., Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- Pagès G., Lenormand P., L’Allemain G., Chambard J.C., Meloche S., Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajalunga D., Mazzola A., Franchitto A., Puggioni E., Crescenzi M. The logic and regulation of cell cycle exit and reentry. Cell. Mol. Life Sci. 2008;65:8–15. doi: 10.1007/s00018-007-7425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube W.L., Brockes J.P., Drechsel D.N., Tanaka E.M. Plasticity and reprogramming of differentiated cells in amphibian regeneration: partial purification of a serum factor that triggers cell cycle re-entry in differentiated muscle cells. Cloning Stem Cells. 2004;6:333–344. doi: 10.1089/clo.2004.6.333. [DOI] [PubMed] [Google Scholar]
- Tanaka E.M., Gann A.A., Gates P.B., Brockes J.P. Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J. Cell Biol. 1997;136:155–165. doi: 10.1083/jcb.136.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E.M., Drechsel D.N., Brockes J.P. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr. Biol. 1999;9:792–799. doi: 10.1016/s0960-9822(99)80362-5. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Pajalunga D., Ferrantelli F., Soddu S., Salvatori G., Sacchi A., Crescenzi M. Terminally differentiated skeletal myotubes are not confined to G0 but can enter G1 upon growth factor stimulation. Cell Growth Differ. 1996;7:1039–1050. [PubMed] [Google Scholar]
- Vassilev L.T., Vu B.T., Graves B., Carvajal D., Podlaski F., Filipovic Z., Kong N., Kammlott U., Lukacs C., Klein C. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Velloso C.P., Kumar A., Tanaka E.M., Brockes J.P. Generation of mononucleate cells from post-mitotic myotubes proceeds in the absence of cell cycle progression. Differentiation. 2000;66:239–246. doi: 10.1046/j.1432-0436.2000.660410.x. [DOI] [PubMed] [Google Scholar]
- Velloso C.P., Simon A., Brockes J.P. Mammalian postmitotic nuclei reenter the cell cycle after serum stimulation in newt/mouse hybrid myotubes. Curr. Biol. 2001;11:855–858. doi: 10.1016/s0960-9822(01)00234-2. [DOI] [PubMed] [Google Scholar]
- Walsh K., Perlman H. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Weber J.D., Cheng J., Raben D.M., Gardner A., Baldassare J.J. Ablation of Goalpha overrides G1 restriction point control through Ras/ERK/cyclin D1-CDK activities. J. Biol. Chem. 1997;272:17320–17326. doi: 10.1074/jbc.272.28.17320. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ebisuya M., Ashida F., Okamoto K., Yonehara S., Nishida E. Continuous ERK activation downregulates antiproliferative genes throughout G1 phase to allow cell-cycle progression. Curr. Biol. 2006;16:1171–1182. doi: 10.1016/j.cub.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Sharrocks A.D., Whitmarsh A.J. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Yun M.H., Gates P.B., Brockes J.P. Regulation of p53 is critical for vertebrate limb regeneration. Proc. Natl. Acad. Sci. USA. 2013;110:17392–17397. doi: 10.1073/pnas.1310519110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


