Abstract
Ivabradine is a new bradycardic agent acting on the If channels of sinoatrial nodal cells to decrease the rate of diastolic depolarization and thus heart rate. The benefit of ivabradine over other negatively chronotropic agents is its absence of negative inotropy. Effective management of coronary artery disease, in terms of reducing morbidity and mortality, is reliant on controlling heart rate. Ivabradine has been shown to safely and effectively reduce heart rate without compromising cardiac function in patients with coronary artery disease and more recently in patients with heart failure and raised heart rate. Furthermore, ivabradine has been shown to have a favourable side-effect profile compared with alternative bradycardic agents. This article reviews the evidence for ivabradine in coronary artery disease and heart failure and compares its safety with alternative bradycardic agents for these conditions.
Keywords: coronary artery disease, drug safety, If channel, If channel inhibitor, ivabradine
Introduction
Ivabradine is a new therapeutic agent designed to reduce heart rate at rest and during exercise by selective inhibition of a novel receptor (If channel) located on the pacemaker-cell membrane within the sinoatrial node. As such, ivabradine joins a list of rate-limiting medications already available to prescribers for the control of heart rate in coronary artery disease (CAD). This review gives a brief overview of the physiological benefits of heart rate reduction in CAD and heart failure. The pharmacology of ivabradine and the physiological and clinical impact of inhibition of the If channel are reviewed. The results of recent clinical trials of ivabradine are also discussed giving context to the current location of ivabradine in treatment schedules for CAD and heart failure. In addition, ivabradine is reviewed in terms of its safety, and in relation to other rate-limiting medications.
Physiological principles of heart rate reduction in coronary artery disease and heart failure
The risk of cardiovascular mortality due to increased heart rate has been investigated in a large observational study of 24,913 patients with CAD [Diaz et al. 2005]. Patients with a baseline heart rate of at least 83 beats/min (bpm) were found to have a significantly higher risk of cardiovascular mortality [hazard ratio (HR) = 1.31; 95% confidence interval (CI) = 1.15–1.48; p < 0.001]. Patients with CAD and controlled heart rate were found to have a lower risk of cardiovascular mortality.
The principal symptom of CAD is chest pain due to myocardial demand for oxygen being in excess of physiological supply. In patients with CAD, this is characteristically because of the presence of atheroma causing stenosis of the coronary arteries. Decreasing heart rate allows the heart to function more effectively by increasing diastole, increasing coronary perfusion and allowing complete ventricular filling and therefore an increase in cardiac efficiency. Plaque rupture is a potentially fatal consequence of CAD and the risk is increased at higher heart rate because of haemodynamic stress. By controlling heart rate the risk of plaque rupture, and subsequent ischaemia, may be reduced. In patients with heart failure, lowering the heart rate with β-blockers is standard treatment resulting in significant mortality benefits in this patient group.
Until recent years there were two main types of heart rate limiting medication classes prescribed for patients with CAD – β-blockers and nondihydropyridine rate-limiting calcium channel blockers. The emergence of ivabradine offers an exciting alternative bradycardic agent in terms of efficacy and safety.
Physiology of action potentials and the role of the If channel
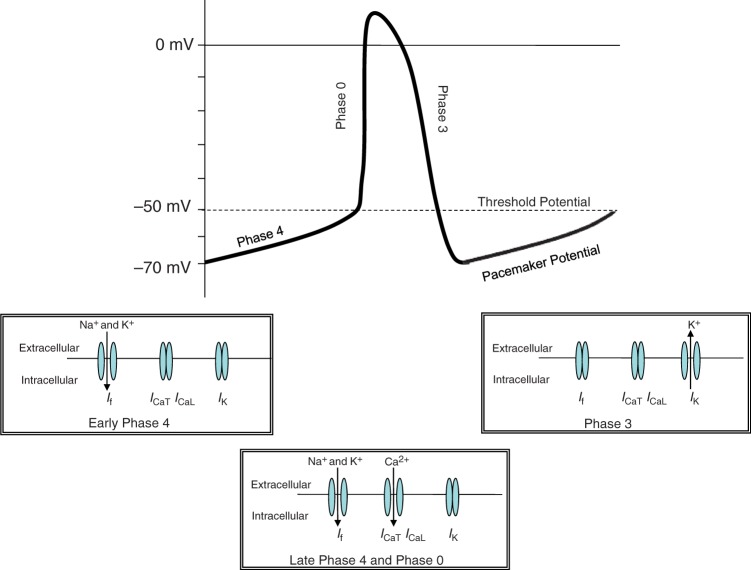
The sinoatrial node is composed of autonomous pacemaker cells. Because of sequential ionic movements across the cell membrane, via four separate channels, these cells cause an action potential to be generated which is then conducted across the heart, ultimately resulting in coordinated muscle contraction [Bois et al. 1996]. The If channel is made up of hyperpolarisation-activated, cyclic nucleotide gated channel subunits. The morphology of the cardiac If channel is similar to Ih channels found in neuronal cells [Bucchi et al. 2002]. Ivabradine blocks the If channel when the channel gate is open [Bucchi et al. 2007]. The If channel is activated at between −40 and −50 mV, which relates to an influx of both Na+ and K+ ions [Bucchi et al. 2002]. The opening of the If channel is dependent on both voltage and cyclic adenosine monophosphate (cAMP) intracellular concentration. When cAMP is bound to the If channel there is a higher likelihood of it being open, therefore allowing ivabradine binding. Adrenergic stimulation (e.g. sympathetic nervous system) increases cAMP concentration and hence binding to the If channel; the opposite is true in the presence of cholinergic stimulation (e.g. parasympathetic nervous system) [Sulfi and Timmis, 2006]. Pacemaker activity is spontaneously generated by the If current in the pacemaker cells of the sinoatrial node [Borer, 2004]. However, the rate of this activity can be influenced by external factors, including medication, hormones and sympathetic nerve activity [Scott et al. 2009].
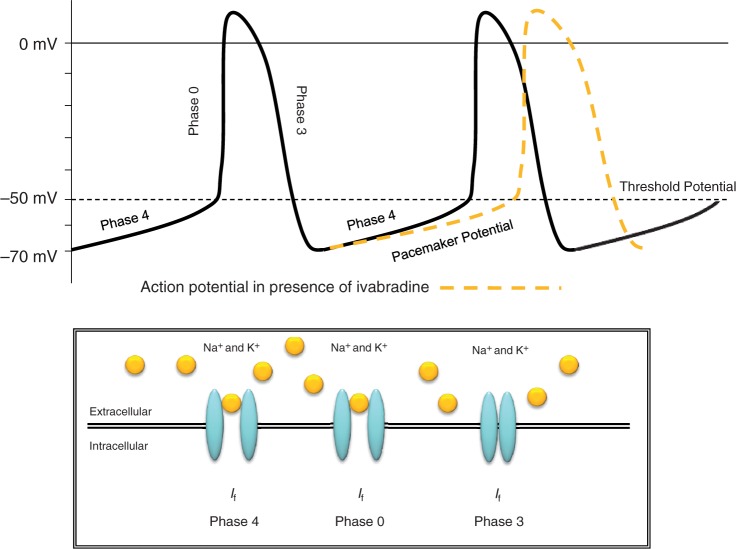
Figure 1 shows an action potential generated by a sinoatrial nodal cell. Each separate phase of the action potential relates to the opening, closing or both of different ion channels in the sinoatrial node. Figure 2 shows the effect ivabradine has on the action potential.
Figure 1.
Sinoatrial ion channel activity and the generation of an action potential.
Figure 2.
Pharmacodynamic If channel interaction and the effect on the sinoatrial action potential.
Pharmacology of ivabradine
Ivabradine is absorbed quickly and almost completely via the oral route. Peak plasma concentrations are seen after 2 h if taken with food [Sweetman, 2009]. Ivabradine is known to be plasma protein bound (∼70%) and undergoes extensive hepatic metabolism [Sweetman, 2009]. This produces at least four metabolites when administered in vivo, including two metabolites with only minor variations in structure compared with the parent drug: O- and N-demethylated metabolites [Francois-Bouchard et al. 2000; Klippert et al. 1998]. The N-demethylated metabolite is known to contribute to the pharmacological effect of the parent drug [Ragueneau et al. 1997] and is thought to be the main active metabolite [Portoles et al. 2006]. Metabolism occurs via the cytochrome P450 3A4 (CYP3A4) pathway; however, interaction between ivabradine and other inhibitors of the CYP3A4 enzyme does not appear to significantly affect the efficacy of ivabradine [Portoles et al. 2006]. Combination with strong CYP3A4 inhibitors such as azole antifungals (ketoconazole, itraconazole), macrolide antibiotics (clarithromycin, erythromycin), and HIV protease inhibitors is contraindicated. The combination of ivabradine with diltiazem or verapamil (moderate CYP3A4 inhibitors) results in an increase in ivabradine exposure (two to threefold increase in the area under the curve) and an additional heart rate reduction of 5 bpm. The concomitant use of ivabradine with these medications, although unlikely to cause significant clinical harm, is not recommended. Ivabradine has an elimination half life of 2 h [Sweetman, 2009]. In a dose-ranging study, ivabradine was shown to have a dose-dependent rate-limiting activity [Ragueneau et al. 1997]. In another study investigating potential electrophysiological alterations after ivabradine intravenous administration, ivabradine was shown to slightly increase the QT interval [Camm and Lau, 2003]. Table 1 provides a comparison of some of the key differences between ivabradine and β-blockers.
Table 1.
Comparison of cardiovascular effects and side effects of ivabradine and β-blockers.
| Medication | Mortality | Exercise-induced ST shift | Cardiac contractility | Peripheral vasoconstriction | Respiratory effects |
|---|---|---|---|---|---|
| β-blockers | Decrease | Time to shift prolonged | Decrease | Yes | Yes – causing wheeze |
| Ivabradine | Decrease | Time to shift prolonged | No effect | No effect | No effect |
Clinical efficacy data
Dose ranging and efficacy
Ivabradine is the first If current inhibitor to be clinically useful at reducing heart rate. This clinical benefit was first tested for efficacy in a cohort of 360 patients with stable angina [Borer et al. 2003]. This dose-ranging study tested doses of 2.5 mg, 5 mg and 10 mg of ivabradine or placebo twice daily over 2 weeks. A 3-month extension period then investigated the effect of titration to 10 mg of ivabradine twice daily. After 2 weeks, mean time to ST-segment depression was significantly increased in the group receiving 5 mg of ivabradine twice daily (44.1 s versus 9 s; p = 0.016), signifying an improvement in exercise tolerance and anti-ischaemic benefit in a dose-dependent fashion. Key clinical trials are reported in Table 2 and discussed below.
Table 2.
Common side effects and their reported incidence from clinical trials.
| Trial | Drug regimen | Bradycardia | Eye disorders including phosphenes |
|---|---|---|---|
| INITIATIVE | Ivabradine versus atenolol | 2.2% (7.5 mg twice daily) and 5.4% (10 mg twice daily) versus 4.3% atenolol (p = NA) | 5 withdrawals in the ivabradine group versus none in the atenolol group (p = NA) |
| ASSOCIATE | Ivabradine versus placebo | 19 (4.2%) versus 2 (0.5%) (p = NA) | 9 (2%) versus 4 (0.9%) (p = NA) |
| BEAUTIFUL | Ivabradine versus placebo | 149 (6%) versus 21 (1%)* (p = NA) | 21 (0.4%) versus 12 (0.2%)* (p = NA) |
| SHIFT | Ivabradine versus placebo | 150 (5%) versus 32 (1%)$, 184 (6%) versus 48(1%)‡ (both p < 0.0001) | 89 (3%) versus 17 (1%) (p < 0.0001) |
Dropout rates – no reports of total incidence.
Symptomatic bradycardia.
Asymptomatic bradycardia.
NA, not available/reported.
Comparison against other antianginal agents
On the premise of the results found by Borer and colleagues [Borer et al. 2003] a trial was designed to test the 5 mg twice daily dose of ivabradine versus atenolol using a noninferiority design [Tardif et al. 2005]. The INITIATIVE trial investigated 939 patients with stable angina who were randomised to receive ivabradine 5 mg twice daily for 4 weeks then either 7.5 mg or 10 mg twice daily for a further 12 weeks, or atenolol 50 mg daily for 4 weeks before titration to 100 mg for a further 12 weeks. The primary endpoint of the study was exercise tolerance at month 1 and 4. Ivabradine was shown, at all doses, to be noninferior (86.8 ± 129.0 s, 91.7 ± 118.8 s, 78.8 ± 133.4 s for ivabradine 7.5 mg twice daily, 10 mg twice daily and atenolol 100 mg daily respectively; p < 0.001) to atenolol for increasing exercise tolerance. Episodes of angina were decreased by two-thirds in all groups.
The antianginal efficacy of ivabradine has also been tested against another antianginal drug, amlodipine [Ruzyllo et al. 2007]. Again, a double-blind parallel group design was employed to randomise 1195 patients with chronic stable angina to treatment with ivabradine 7.5 mg twice daily, ivabradine 10 mg twice daily or amlodipine 10 mg daily. The primary endpoint was the change in exercise tolerance seen at monthly intervals. Ivabradine was shown to have comparable anti-ischaemic ability to amlodipine at 3 months when exercise tolerance was improved by 27.6 ± 91.7 s, 21.7 ± 94.5 s and 31.2 ± 92.0 s for ivabradine 7.5 mg twice daily, ivabradine 10 mg twice daily and amlodipine 10 mg daily respectively (noninferiority p < 0.001). Again, in line with the results of the INITATIVE trial, episodes of angina were decreased in all the groups. No significant difference was seen between the groups.
Combination with other antianginal agents
A meta-analysis has shown that the combination of a calcium channel antagonist and a β-blocker is more effective at increasing exercise tolerance than either medication as monotherapy [Klein et al. 2002]. This dual approach to CAD management was investigated recently in the ASSOCIATE study in which the efficacy and tolerability of a combination of ivabradine and atenolol were studied [Tardif et al. 2009]. The trial used a randomised, double-blind design in 889 patients with chronic stable angina who were on atenolol 50 mg daily. They were then randomised to receive ivabradine 5 mg twice daily for 2 months titrating to 7.5 mg twice daily for a further 2 months, or placebo. The primary endpoint was again the change in exercise tolerance from baseline until the end of the study. After 4 months exercise duration in the ivabradine group was found to be 24.3 ± 65.3 s compared with 7.7 ± 63.8 s in the placebo group (p < 0.001), signifying the compound effect of double antianginal therapy.
Coronary artery disease and left ventricular systolic impairment data
The largest trial investigating the efficacy of ivabradine is the BEAUTIFUL study [Fox et al. 2008a]. This trial investigates the potential of ivabradine to be of benefit to patients with CAD and left ventricular systolic dysfunction [Fox et al. 2006]. BEAUTIFUL was a randomised, double-blind, placebo-controlled, parallel-group trial in which patients randomised to the treatment arm were initialised on ivabradine 5 mg twice daily before being titrated to a target of ivabradine 7.5 mg twice daily if possible. The control group were given matching placebo in addition to appropriate cardiovascular medication. In total, 10,917 patients were randomised, the mean ejection fraction was 32% and the mean heart rate was 71.6 bpm [The BEAUTIFUL Study Group, 2008]. Eighty seven percent of patients were receiving β-blocker therapy as standard background treatment. The trial was outcome based and the primary outcome measure was a composite of cardiovascular death, acute myocardial infarction (MI) or hospitalisation for new or worsening heart failure. The trial had a median follow up of 19 months. Ivabradine did not affect the primary composite endpoint (HR = 1.00; 95% CI = 0.91–1.1; p = 0.94). In addition, the number of serious adverse effects in the ivabradine group was found to be lower than in the placebo group: 1233 (22.5%) versus 1239 (22.8%) (p = 0.7).
There were two main findings in the BEAUTIFUL trial. First, not all patients with CAD and left ventricular systolic impairment would benefit from the addition of ivabradine for the prevention of cardiovascular morbidity and mortality, but that a subgroup of patients with a heart rate greater than 70 bpm may benefit. Second, ivabradine at either 5 mg or 7.5 mg twice daily had a comparable side-effect profile to placebo.
The BEAUTIFUL investigators completed a subgroup analysis of the data captured from the BEAUTIFUL trial [Fox et al. 2008b]. In this analysis patients were grouped according to baseline heart rate as well as using heart rate as a continuous variable. The analysis found that patients with a heart rate of at least 70 bpm had a 34% (p = 0.0041) higher risk of cardiovascular death compared with patients with a heart rate less than 70 bpm. The investigators also found that there was a correlation between the degree to which the heart rate was higher than 70 bpm and the outcomes of mortality and heart failure related events; this correlation was found to be weaker for coronary outcomes. However, it should be emphasised that, ultimately, the primary endpoint of the BEAUTIFUL trial was negative.
The SHIFT trial was designed to compare the addition of ivabradine 7.5 mg twice daily or placebo to standard treatment, including a β-blocker, for patients with heart failure and a New York Heart Association (NYHA) classification of II–IV and ejection fractions less than 35% [Menown, 2007]. The SHIFT trial investigated the potential for ivabradine to improve cardiovascular outcomes, symptoms and quality of life in patients with heart failure and systolic dysfunction [Swedberg et al. 2010b]. A statistically significant reduction in the primary endpoint, a composite of cardiovascular mortality or hospital admission with worsening heart failure, was reported on the addition of ivabradine (HR = 0.82; 95% CI = 0.75–0.90; p < 0.0001) [Swedberg et al. 2010a]. However, this was mainly due to a reduced admission rate because the reduction in cardiovascular mortality alone was not statistically significant. On stratification of baseline heart rate, the greatest benefit was found in patients with an initial heart rate of at least 80 bpm [Böhm et al. 2010]. The major confounding factor with SHIFT, which will affect its ability to influence clinical practice, is the type of β-blocker used and the dose to which it had been titrated. Over 15% of patients received β-blockers that have no proven benefit to survival in heart failure [Teerlink, 2010]. Also, only 49% of patients reached 50% of the target β-blocker dose and only 23% of patients were at target dose [Swedberg et al. 2010a]. It is unclear if the benefits demonstrated in SHIFT would persist in a population of patients on an appropriate β-blocker at a target dose. What SHIFT did show was that for patients intolerant of β-blockers, ivabradine presents a potential alternative treatment option to control heart rate in heart failure.
When considering the results of both the SHIFT and BEAUTIFUL trials there would appear to be an association between the highest baseline heart rate and the greatest reduction in cardiovascular mortality. Until now the significance of this has yet to be explored fully in a trial specifically designed to investigate a cohort of patients with tachycardia. The SIGNIfY study will assess patients with CAD, normal left ventricular function and a resting heart rate of at least 70 bpm [Ferrari, 2009].
Safety and tolerability data
Dose comparison data
The safety of two standard doses of ivabradine was investigated as part of a long-term safety trial in 386 patients with chronic stable angina [López-Bescós et al. 2007]. A randomised, double-blind, parallel-group study was used to compare ivabradine 5 mg twice daily and 7.5 mg twice daily over a 12-month period. Patients were permitted to be on concomitant antianginal medication. Antianginal efficacy was also measured by monitoring the reduction in angina attacks between month 0 and 12. The number of angina attacks decreased by over 50% (p < 0.001) in both groups between month 0 and 12. The most commonly reported side effect was transient phosphene-like visual disturbances, which led to the withdrawal of four patients. These luminous phenomena are described as transient enhanced brightness in a limited area of the visual field and are generally mild or moderate in severity. Sinus bradycardia was reported in three patients warranting treatment withdrawal. Overall the results showed that ivabradine was tolerated well (side events 24 versus 32 for 5 mg and 7.5 mg doses respectively) at both doses, although there were more patients experiencing side effects at the higher dose.
Bradycardia
As might be expected from a bradycardic agent, sinus bradycardia is one of the main side effects of the medication. Bradycardia occurs in 3.3% of patients, particularly within the first 2–3 months of treatment; only 0.5% of patients experience severe bradycardia below or equal to 40 bpm. In the BEAUTIFUL study, the rate of discontinuation in the ivabradine group compared with the placebo group was 149 (6%) versus 21 (1%) respectively [Fox et al. 2008a]. Of the 149 patients who withdrew because of bradycardia, only 34 (23%) were symptomatic. Bradycardia is dose dependent but it should be noted that the effect will plateau. This is because of the number of different ionic channels contributing to pacemaker potential (potentially up to 10) so that when 100% of the If channels are inhibited, heart rate is only reduced by a maximum of 25–30% [DiFrancesco and Camm, 2004].
Ivabradine is contraindicated when the patient’s resting heart rate is below 60 bpm prior to treatment. If, during treatment, the heart rate decreases persistently below 50 bpm at rest or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward, possibly to a dose of 2.5 mg twice daily (half a 5-mg tablet twice daily). Treatment should be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist.
Cross-reactivity with Ih channels
Hyperpolarization voltage-gated channels are not exclusive to pacemaker cells in the sinoatrial node but are also found in neuronal Ih channel retinal and CNS tissues [Cervetto et al. 2007]. Because of the lipophobic nature of the structure of ivabradine it does not cross the blood–brain barrier. However, there is cross-reactivity of ivabradine with Ih channels in ocular tissue. This creates the presence of phosphene-like transient adverse drug reactions in 15% of patients [Savelieva and Camm, 2006]. In the largest study of ivabradine so far, BEAUTIFUL, 37 patients (0.3%) dropped out because of a composite of eye disorders, including phosphenes, blurred vision and visual disturbance [Fox et al. 2008a]. All symptoms disappeared on withdrawal of the study medication. Phosphene effects are reported by 14.5% of patients and are generally experienced in the first 2 months of treatment [Cervetto et al. 2007]. The effects are increased with increasing ivabradine dose.
Electrophysiological safety
Ivabradine affects the rate of ventricular repolarisation, potentially due to a weak inhibitory effect on IKr channels [Savelieva and Camm, 2008]. This effect prolongs the repolarisation (QT interval) by no more than 2 ms, which is within the recommended guidelines for QT/QTc prolongation due to torsadogenic potential [Savelieva and Camm, 2006]. Perhaps more importantly, however, ivabradine should not be prescribed with other medications that can prolong the QT interval (e.g. sotalol or amiodarone). Ivabradine must also be avoided in patients with sick sinus syndrome because of the pharmacodynamic interactions within the sinoatrial node [Savelieva and Camm, 2006]. In contrast to β-blockers, ivabradine has no affect on conduction in the atrioventricular node and therefore is of no clinical benefit in the treatment of atrial fibrillation [Nixon et al. 2008].
Safety comparison with other bradycardic agents
Ivabradine is a negatively chronotropic agent without any significant negatively inotropic effect, unlike β-blockers [Klippert et al. 1998; Bois et al. 1996]. Another potential benefit of ivabradine is the lack of vasoconstriction which can cause a symptomatic decrease in distal perfusion in patients taking β-blockers [Savelieva and Camm, 2006]. In a small trial of patients with asthma, ivabradine was found to have no significant effect on respiratory function or wheeze [Babu et al. 2008]. Due to extensive experience of using β-blockers to treat CAD, and because they are relatively cheap in comparison to other bradycardic agents, it is unlikely that ivabradine will be used as a first-line agent unless patients are intolerant to β-blockers or there are contraindications [Begg, 2008]. One of the main adverse effects of β-blockers, even in patients who tolerate treatment well, is rebound tachycardia and hypertension. The potential for rebound tachycardia with ivabradine has been investigated and there appears to be no evidence of this effect [Borer and Le Heuzey, 2008], which is an obvious benefit for ivabradine compared with β-blockers.
Pooled safety data
A pooled subpopulation analysis using the data generated from five large randomised trials in a total of 2425 patients with angina pectoris has confirmed that ivabradine is well tolerated and that adverse drug reactions are rare [Tendera et al. 2009]. The dropout rate due to new-onset adverse drug reactions in all subgroups was less than 1.5%.
Other clinical considerations
The use of ivabradine is restricted to patients in sinus rhythm. One clinical concern is when patients who are prescribed ivabradine monotherapy as a rate-limiting strategy subsequently develop either supraventricular tachycardia or atrial fibrillation. Unlike β-blockers and nondihydropyridine rate-limiting calcium channel blockers, ivabradine provides no protective action at the level of the atrioventricular node and thus patients may be at risk of uncontrolled ventricular rates. The clinical implications of this are uncertain, but it is reassuring that ivabradine can be safely prescribed with β-blockers [Swedberg et al. 2010a; Fox et al. 2008a].
Future uses
Heart failure
The benefits of heart rate control in heart failure and in particular left ventricular systolic dysfunction are well understood and are multifaceted. When the heart rate is uncontrolled during left ventricular systolic impairment, increased strain is placed on the myocardium to meet physiological oxygen demand. This places the heart at risk of collagen accumulation and eventually left ventricular cardiac remodelling. In turn, this has a prohibitive effect on the heart by reducing the ejection fraction and the stroke volume as well as increasing the end diastolic volume, resulting in decreased functionality (see Figure 3). Other bradycardic agents, principally β-blockers, have a cardioprotective role in stable left ventricular systolic dysfunction. The following studies investigated the potential role and safety of ivabradine in treating heart failure.
Figure 3.
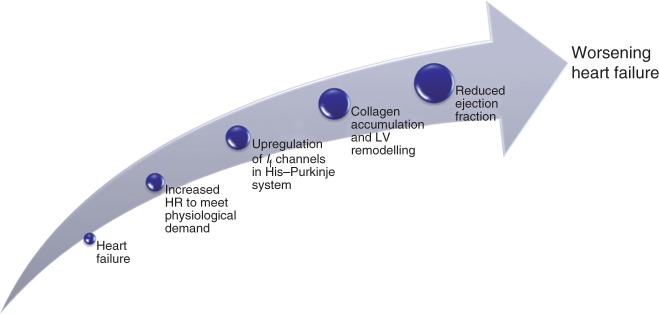
Effect of raised heart rate in heart failure. HR, heart rate; LV, left ventricular.
In a study in rats with congestive heart failure the use of ivabradine to produce a long-term heart rate reduction was shown to improve the left ventricular function, increase the stroke volume and maintain cardiac output during rate reduction [Mulder et al. 2007]. In a small study in humans, a single intravenous dose of ivabradine was administered to patients with regional or global left ventricular systolic impairment [Manz et al. 2002]. Heart rate at rest was reduced in the ivabradine group, however cardiac function including stroke volume and ejection fraction were preserved. Ivabradine has also been trialled in patients with NYHA classification III and ejection fractions of 21 ± 7%. Two infusions were found to decrease heart rate, increase stroke volume and left ventricular systolic work without increasing cardiac index [De Ferrari et al. 2008].
In heart failure, it is hypothesised that medication which decreases the heart rate will increase diastolic filling time and therefore stroke volume [DiFrancesco and Camm, 2004]. At the same time, myocardial oxygen demand is decreased and myocardial perfusion is increased as a result of the increased duration of diastole. However, the potential benefits may go beyond heart rate reduction. The physiological upregulation of If channels in the His–Purkinje system during advanced heart failure requires further elucidation and investigation for a potential future target [Savelieva and Camm, 2006]. There is also a need to further study the ability of ivabradine to influence the outcomes of patients with heart failure [Borer, 2006].
The use of ivabradine is currently contraindicated in patients with NYHA classification III–IV and should be used with caution in patients with NYHA classification I–II. No doubt, the results of the BEAUTIFUL and SHIFT studies will result in a review of these cautions.
Acute coronary syndrome
It is proposed that the rate-limiting effects of ivabradine may also be of benefit to patients with acute coronary syndrome (ACS). There are a number of reasons for this. First, ivabradine decreases heart rate, which increases diastole and improves myocardial perfusion while decreasing myocardial oxygen demand. Second, it has been hypothesised that haemodynamic shear forces experienced during systole as a result of contortion of the cardiac arteries may contribute to rupture of atherosclerotic plaques and lesions [Heidland and Strauer, 2001]. It is also known that increased heart rate in patients after a MI increases atherosclerosis [Perski et al. 1998]. By decreasing the heart rate without compromising inotropic functionality, ivabradine may provide benefit in these patients while, importantly, maintaining cardiac function. Third, it is theorized that to aid coronary artery perfusion in stenotic arteries, blood flow is rerouted to less stenosed coronary arteries, improving blood flow by using ‘subsidiary' vessels [Ferrari et al. 2006]. This steal phenomenon has a decreased effectiveness under tachycardic conditions, decreasing perfusion. Finally, the use of β-blockers in patients with ACS can increase the risk of atrioventricular blockade [Shattock and Camm, 2006]. Because ivabradine does not affect conduction via the atrioventricular node, the potential for atrioventricular block would be nullified.
Sinus tachycardia
Inappropriate sinus tachycardia is normally treated with β-blockers, although, β-blockade is not always effective or tolerated. However, there is published evidence, albeit from only one case report, of the effectiveness of ivabradine in the treatment of inappropriate sinus tachycardia [Schulze et al. 2008].
Conclusion
The emergence of ivabradine for rate control offers a new treatment option for patients with coronary artery disease and heart failure. A subgroup analysis has shown that ivabradine improves mortality in patients with an initial heart rate greater than 70 bpm and because it can limit heart rate at rest and during exercise, it is particularly useful for treating ambulatory angina pectoris. The main benefit of this bradycardic agent over existing therapies is its more favourable side-effect profile. The lack of negative inotropic action, vasodilation, hypotension and bronchospasm weigh heavily in the favour of ivabradine over β-blockers or rate-limiting calcium channel blockers. Ivabradine should currently be used as a second-line agent for managing angina, or as first-line treatment if the patient is intolerant to β-blockers or there are contraindications. The role of ivabradine in heart failure is still unclear, as well as unlicensed.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare that there is no conflict of interest.
References
- Babu K.S., Gadzik F., Holgate S.T. (2008) Absence of respiratory effects with ivabradine in patients with asthma. Br J Clin Pharmacol 66: 96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg A. (2008) SIGN advice on angina care is reinforced by recent evidence. Guidelines in Practice 11: 1–6 [Google Scholar]
- Böhm M., Swedberg K., Komajda M., Borer J.S., Ford I., Dubost-Brama A., et al. (2010) Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 376: 886–894 [DOI] [PubMed] [Google Scholar]
- Bois P., Bescond J., Renaudon B., Lenfant J. (1996) Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol 118: 1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer J.S. (2004) Drug insight: If inhibitors as specific heart-rate-reducing agents. Nat Clin Pract 1: 103–109 [DOI] [PubMed] [Google Scholar]
- Borer J.S. (2006) Therapeutic effects of If blockade: Evidence and perspective. Pharmacol Res 53: 440–445 [DOI] [PubMed] [Google Scholar]
- Borer J.S., Le Heuzey J. (2008) Characterization of the heart rate-lowering action of ivabradine, a selective If current inhibitor. Am J Therapeut 15: 461–473 [DOI] [PubMed] [Google Scholar]
- Borer J.S., Fox K., Jaillon P., Lerebours G. (2003) Antianginal and antiischemic effects of ivabradine, an If inhibitor, in stable angina: A randomised, double-blind, multicentred, placebo-controlled trial. Circulation 107: 817–823 [DOI] [PubMed] [Google Scholar]
- Bucchi A., Barbuti A., Baruscotti M., DiFrancesco D. (2007) Heart rate reduction via selective ‘funny’ channel blockers. Curr Opin Pharmacol 7: 208–213 [DOI] [PubMed] [Google Scholar]
- Bucchi A., Baruscotti M., DiFrancesco D. (2002) Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J Gen Physiol 120: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm A.J., Lau C. (2003) Electrophysiological effects of a single intravenous administration of ivabradine (S 16257) in adult patients with normal baseline electrophysiology. Drugs R&D 4: 83–89 [DOI] [PubMed] [Google Scholar]
- Cervetto L., Demontis G.C., Gargini C. (2007) Cellular mechanisms underlying the pharmacological induction of phosphenes. Br J Pharmacol 150: 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari G.M., Mazzuero A., Agnesina L., Bertoletti A., Lettino M., Campana C., et al. (2008) Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Failure 10: 550–555 [DOI] [PubMed] [Google Scholar]
- Diaz A., Bourassa M.G., Guertin M., Tardif J. (2005) Long-term prognostic value of resting heat rate in patients with suspected or proven coronary artery disease. Eur Heart J 26: 967–974 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Camm A.J. (2004) Heart rate lowering by specific and selective If current inhibition with ivabradine: A new therapeutic perspective in cardiovascular disease. Drugs 64: 1757–1765 [DOI] [PubMed] [Google Scholar]
- Ferrari R. (2009) A step further with ivabradine: SIGNIfY (Study assessing the morbidity–mortality benefits of the If inhibitor ivabradine in patients with coronary artery disease). Eur Heart J Suppl 11(Suppl D): D19–D27 [Google Scholar]
- Ferrari R., Cargnoni A., Ceconi C. (2006) Anti-ischaemic effect of ivabradine. Pharmacol Res 53: 435–439 [DOI] [PubMed] [Google Scholar]
- Fox K., Ferrari R., Tendera M., Steg P.G., Ford I. (2006) Rationale and design of a randomised, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease and left ventricular systolic dysfunction: the morbidity-mortality evaluation of the If inhibitor ivabradine in patients with coronary disease and left ventricular dysfunction (BEAUTIFUL) study. Am Heart J 152: 860–866 [DOI] [PubMed] [Google Scholar]
- Fox K., Ford I., Steg P.G., Tendera M., Ferrari R. (2008a) Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 372: 807–816 [DOI] [PubMed] [Google Scholar]
- Fox K., Ford I., Steg P.G., Tendera M., Robertson M., Ferrari R., et al. (2008b) Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet 372: 817–821 [DOI] [PubMed] [Google Scholar]
- Francois-Bouchard M., Simonin G., Bossant M., Bousier-Neyret C. (2000) Simultaneous determination of ivabradine and its metabolites in human plasma by liquid chromatography – tandem mass spectrometry. J Chromatogr B 745: 261–269 [DOI] [PubMed] [Google Scholar]
- Heidland U.E., Strauer B.E. (2001) Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation 104: 1477–1482 [DOI] [PubMed] [Google Scholar]
- Klein W.W., Jackson G., Tavazzi L. (2002) Efficacy of monotherapy compared with combined antianginal drugs in the treatment of chronic stable angina pectoris: A meta-analysis. Coron Artery Dis 13: 427–436 [DOI] [PubMed] [Google Scholar]
- Klippert P., Jeanniot J., Polve S., Lefevre C., Merdjan H. (1998) Determination of ivabradine and its N-demethylated metabolite in human plasma and urine, and in rat and dog plasma by a validated high-performance liquid chromatographic method with fluorescence detection. J Chromatogr B 719: 125–133 [DOI] [PubMed] [Google Scholar]
- López-Bescós L., Filipova S., Martos R. (2007) Long-term safety and efficacy of ivabradine in patients with chronic stable angina. Cardiology 108: 387–396 [DOI] [PubMed] [Google Scholar]
- Manz M., Reuter M., Lauck G., Omran H., Jung W. (2002) A single intravenous dose of ivabradine, a novel If inhibitor, lowers heart rate but does not depress left ventricular function in patients with left ventricular dysfunction. Cardiology 100: 149–155 [DOI] [PubMed] [Google Scholar]
- Menown I.B.A. (2007) Ivabradine: A new strategy for management of stable angina. Br J Hosp Med 68: 321–325 [DOI] [PubMed] [Google Scholar]
- Mulder P., Barbier S., Chagraoui A., Richard V., Henry J.P., Lallemand F., et al. (2007) Long-term heart rate reduction induced by the selective If current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation 109: 1674–1679 [DOI] [PubMed] [Google Scholar]
- Nixon R., Kruszewski K., Bloe C., Leslie S.J. (2008) Ivabradine: A new treatment for chronic stable angina. Br J Cardiac Nurs 3: 170–175 [Google Scholar]
- Perski A., Hamsten A., Lindvall K., Theorell T. (1998) Heart rate correlates with severity of coronary atherosclerosis in young post-infarction patients. Am Heart J 116: 1369–1373 [DOI] [PubMed] [Google Scholar]
- Portoles A., Calvo A., Terleira A., Laredo L., Resplandy G., Gorostiaga C., et al. (2006) Lack of pharmacokinetic interaction between omeprazole or lansoprazole and ivabradine in healthy volunteers: An open-label, randomised, crossover, pharmacokinetic interaction clinical trial. J Clin Pharmacol 46: 1195–1203 [DOI] [PubMed] [Google Scholar]
- Ragueneau I., Laveille C., Jochemsen R., Resplandy G., Funck-Brentano C., Jaillon P. (1997) Pharmacokinetic–pharmacodynamic modelling of the effects of ivabradine, a direct sinus node inhibitor, on heart rate in healthy volunteers. Clin Pharmacol Therapeut 64: 192–203 [DOI] [PubMed] [Google Scholar]
- Ruzyllo W., Tendera M., Ford I., Fox K. (2007) Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: A 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs 67: 393–405 [DOI] [PubMed] [Google Scholar]
- Savelieva I., Camm A.J. (2006) Novel If current inhibitor ivabradine: Safety considerations. Adv Cardiol 43: 79–96 [DOI] [PubMed] [Google Scholar]
- Savelieva I., Camm A.J. (2008) If inhibition with ivabradine: Electrophysiological effects and safety. Drugs Saf 31: 95–107 [DOI] [PubMed] [Google Scholar]
- Schulze V., Steiner S., Hennersdorf M., Strauer B. (2008) Ivabradine as an alternative therapeutic trial in the therapy of inappropriate sinus tachycardia. Cardiology 110: 206–206 [DOI] [PubMed] [Google Scholar]
- Scott A.E., Kruszewski K., Leslie S.J. (2009) Sinus node If channel inhibition – a new therapeutic approach to heart rate lowering. Curr Drug Ther 4: 1–6 [Google Scholar]
- Shattock M., Camm A.J. (2006) Pure heart rate reduction: The If channels from discovery to therapeutic target. Br J Cardiol 13: 27–35 [Google Scholar]
- Sulfi S., Timmis A.D. (2006) Ivabradine – the first selective sinus node If channel inhibitor in the treatment of stable angina. Int J Clin Pract 60: 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedberg K., Komajda M., Böhm M., Borer J.S., Ford I., Dubost-Brama A., et al. (2010a) Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 376: 875–885 [DOI] [PubMed] [Google Scholar]
- Swedberg K., Komajda M., Böhm M., Borer J.S., Ford I., Tavazzi L. (2010b) Rationale and design of a randomised, double-blind, placebo-controlled outcome trial of ivabradine in chronic heart failure: The Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT). Eur J Heart Fail 12: 75–81 [DOI] [PubMed] [Google Scholar]
- Sweetman S. (2009) Martindale: The Complete Drug Reference, 36th Pharmaceutical Press: London [Google Scholar]
- Tardif J., Ford I., Tendera M., Bourassa M.G., Fox K. (2005) Efficacy of ivabradine, a new selective If inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 26: 2529–2536 [DOI] [PubMed] [Google Scholar]
- Tardif J., Ponikowski P., Kahan T. for the ASSOCIATE Study Investigators (2009) Efficacy of the If current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: A 4-month, randomized, placebo-controlled trial. Eur Heart J 30: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerlink J.R. (2010) Ivabradine in heart failure – no paradigm SHIFT… yet. Lancet 376: 847–849 [DOI] [PubMed] [Google Scholar]
- Tendera M., Borer J.S., Tardif J. (2009) Efficacy of If inhibition with ivabradine in different subpopulations with stable angina pectoris. Cardiology 114: 116–125 [DOI] [PubMed] [Google Scholar]
- The BEAUTIFUL Study Group (2008) The BEAUTIFUL study: Randomised trial of ivabradine in patients with stable coronary artery disease and left ventricular systolic dysfunction – baseline characteristics of the study population. Cardiology 110: 271–282 [DOI] [PubMed] [Google Scholar]