Abstract
Anticoagulant drugs maintain a high potential for adverse events due to their inherent risk of haemorrhage and/or complex pharmacology. In addition, compromising the safety of these agents is the context in which they are principally used; that is, in the long-term prevention of thromboembolic diseases in an older patient population. These challenges are especially pronounced in the prevention of stroke in older persons with atrial fibrillation (AF), where the need for thromboprophylaxis is paramount and in whom the arrhythmia is most prevalent, but where the target population is simultaneously at high risk of adverse drug events. Essentially, this translates to the use of high-risk therapies on an indefinite basis, in persons who have multiple comorbidities, use polypharmacy, and who may have age-related functional and cognitive decline, culminating in a higher potential for medication misadventure. For this reason, anticoagulants mandate extra pharmacovigilance, and therefore the aim of this review is to address some of the key safety considerations in the use of anticoagulant drugs (warfarin, dabigatran, rivaroxaban), spanning the initiation of therapy to its ongoing management. Using the Medication Management Pathway (MMP) as a framework, in this review we canvas and highlight specific developments in practical strategies to facilitate the safe use of anticoagulants (particularly warfarin) in ‘at-risk' elderly patients including: comprehensive risk/benefit assessment using novel risk stratification tools; focused medicines review services; therapeutic drug monitoring services delivered in the primary care setting; and practical education strategies and resources targeting the older patient population. Until newer alternative anticoagulants become viable options for widespread use, clinicians will necessarily need to rely on specific resources and interventions to facilitate the safe use of currently available anticoagulants (i.e. warfarin) in ‘at-risk’ older people.
Keywords: anticoagulant, atrial fibrillation, medicines management pathway, medication safety, risk assessment, risk–benefit assessment, stroke prevention, warfarin
Introduction
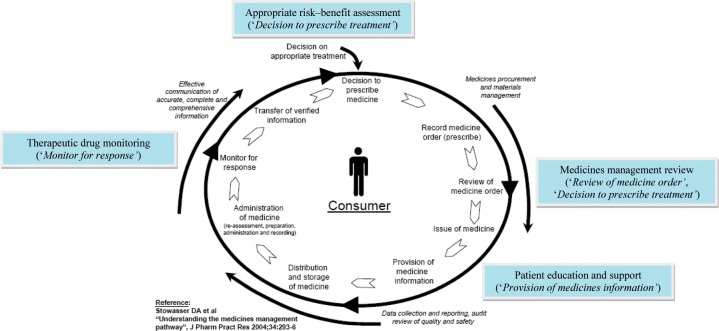
Assuring medication safety is an overarching principle of the Medicines Management Pathway (MMP) [Stowasser et al. 2004], and is most pertinent to ‘high-risk' pharmacotherapies which require significant intervention at each step. This is especially true for anticoagulants (e.g. warfarin) which maintain a higher potential for adverse events due to their inherent risk of haemorrhage and/or complex pharmacology and which mandate extra pharmacovigilance in the form of: appropriate risk–benefit assessment (‘Decision to prescribe treatment’); medicines management review (‘Review of medicine order’, ‘Decision to prescribe treatment’); patient education and support (‘Provision of medicines information’), and therapeutic drug monitoring (‘Monitor for response’) along this pathway.
In addition, compromising the safety of anticoagulants is the context in which they are principally used; that is, in the long-term prevention (primary and secondary) of thromboembolic diseases in an older patient population. Essentially, this translates to the use of high-risk therapies on an indefinite basis (i.e. lifelong), in persons who have multiple comorbidities, use polypharmacy, and who may have age-related functional and cognitive decline, culminating in a higher potential for medication misadventure. These challenges are especially pronounced in the prevention of stroke in older persons with atrial fibrillation (AF), where the need for thromboprophylaxis is paramount and in whom the arrhythmia is most prevalent [Benjamin et al. 1994; Wolf et al. 1991], but where the target population is simultaneously at high risk of adverse drug events. In short, the therapeutic dilemma for clinicians lies in that the safety of using anticoagulants is potentially undermined by the ever-increasing burden of cardiovascular disease on the background of a rapidly ageing population.
Unfortunately, the utilization of anticoagulant therapy remains suboptimal due to these underpinning safety considerations, despite it being a core evidence-based therapy. Furthermore, this contrasts with guidelines which emphasize this pharmacotherapy for both disease prevention and treatment in alliance with global health priorities in ‘healthy aging' and ‘cardiovascular disease'. Importantly, this underuse is most pronounced in the target elderly population which stands to benefit most from the therapy [Gladstone et al. 2009; Gallagher et al. 2008; Glazer et al. 2007; Ren and Bajorek, 2007; Bajorek et al. 2002]. Studies have shown that even after accounting for contraindications, older persons (compared with younger counterparts) are approximately five times less likely to receive anticoagulants (warfarin) for primary indications such as AF [Bajorek et al. 2002], and that this underutilization persists despite increasing awareness in recent years [Marcucci et al. 2010; Po and Lin, 2010]. Understandably, a fear of side effects underpins this [Gattellari et al. 2008; Bajorek et al. 2007], however, ‘old age’ per se has been cited as a contraindication to anticoagulation without objective risk–benefit assessment. In older people, both the risk of stroke and the risk of medication misadventure are high [Peterson, 2001], leaving clinicians to question whether initiating life-saving anticlotting therapy is done at the expense of causing potentially life-threatening side effects. Given that current evidence presents a favourable risk–benefit profile for use in the elderly [Copland et al. 2001], it is apparent that for too long clinicians have ‘overstated the inconvenience of warfarin and exaggerated its risks’ [Gattellari et al. 2009], and hence strategies designed to optimize therapy must necessarily target perceived age-related barriers to its safe use.
Therefore, the aim of this review is to address some of the key safety considerations in the use of antithrombotic drugs (specifically, anticoagulants), including specific and practical strategies to overcome these using the MMP [Stowasser et al. 2004] as an alternative framework (Figure 1). A particular emphasis will be placed on the use of anticoagulants for stroke prevention in AF, given the burden of disease and the high prevalence of this cardiac arrhythmia in the ‘at-risk' older population. A particular focus will be placed on the use of warfarin, but will include safety and practical issues regarding the widespread use of newer anticoagulants, such as dabigatran and rivaroxaban.
Figure 1.
The Medicines Management Pathway: a framework for assuring the safety of anticoagulants in older people.
Safety profiles of key anticoagulants
Antithrombotic agents comprise two main classes of drugs, antiplatelets and anticoagulants, and historically aspirin and warfarin, respectively, have been the mainstay agents used. To date, numerous clinical trials have provided convincing evidence that anticoagulants can prevent stroke in patients with AF, demonstrating that warfarin reduces this risk by approximately two thirds, whilst aspirin is less effective, reducing the risk by about 20% [Hart et al. 2007]. Concerted efforts over recent years to develop new agents (specifically anticoagulants) that are relatively devoid of major safety considerations have led to alternative drugs (e.g. rivaroxaban, dabigatran) expanding the treatment armamentarium. Whilst there has been much enthusiasm about the advent of these new agents, it must be tempered by considerations regarding their widespread use in clinical practice, including their alternate safety profiles and relevant pharmacoeconomic implications.
Warfarin
Since the conduct of pivotal trials in the 1980s and 1990s [Stroke Prevention in Atrial Fibrillation Investigators, 1991; Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators, 1990; Petersen et al. 1989], clinical guidelines have universally advocated the use of warfarin (vitamin K antagonist) as a first-line agent for stroke prevention in AF. Early trial findings highlighted that warfarin significantly reduced the incidence of stroke without a significant increase in the risk of major bleeding events, despite initial safety fears in view of its complex pharmacology and narrow therapeutic index [Jacobs, 2008]. However, translation of the evidence into clinical practice was hampered by the generalizability of the trial findings from highly selected trial participants to ‘real-world’ patients, and this was reflected in drug utilization patterns. Since then, numerous studies, including a meta-analysis of 29 trials involving 28,044 participants [Hart et al. 2007], have confirmed that the benefits of warfarin therapy (adjusted dose regimen, to a target international normalized ratio (INR) of between 2.0 and 2.9) outweigh the risks, affording a relative risk (RR) reduction in stroke of 64% with a minimal absolute increase in the rate of major extracranial haemorrhage (0.3% per year at INR below 3.5).
Furthermore, it has been demonstrated that the benefits of anticoagulation outweigh the risks even in those patients aged 85 years and older [Marinigh et al. 2010; Fang, 2009; Fang et al. 2006]. In particular, the findings from the BAFTA trial support the use of anticoagulant therapy for people over 75 years of age, where it is indicated for AF [Mant et al. 2007]. In this study, the only one to date to have examined the use of oral anticoagulation in patients over 75 years, the yearly risk of extracranial haemorrhage during warfarin therapy was a relatively low 1.4% (compared with 1.6% with aspirin), demonstrating outcomes comparable to previous clinical trials involving younger patients, and outcomes better than for aspirin. Importantly, the patients represented ‘real-life’ patients recruited from primary care.
Risk of intracranial haemorrhage
Specific safety concerns relate to the most feared bleeding complication of anticoagulation, that being intracranial haemorrhage (ICH). Studies to date have reported that the rate of ICH with warfarin is relatively low (0.3–0.6% per year), even among older patients (>75 years of age), and that the risk is not obviated through the use of aspirin as an alternative (risk of ICH of 0.2% per year) [Hart et al. 2005]. Factors which have been shown to increase this risk include uncontrolled hypertension [Wintzen et al. 1984] and INR intensity above 3.5 [Hart et al. 2005], both of which are modifiable risk factors; purposefully treating hypertension [Chapman et al. 2004] and maintaining INRs within the range 2.0–3.0 minimizes the risk of ICH to afford safer treatment. Other factors associated with a higher risk of ICH include prior stroke, cerebral amyloid angiopathy leukoariaosis, apolipoprotein E genotype, and falls risk, although the evidence for these remains inconclusive and no validated risk tools exist to predict the risk of ICH based on these factors [Fang, 2009].
Falls-related adverse events
As stated previously, despite currently available evidence, clinicians have remained reluctant in their prescription of warfarin, often citing old age per se as a contraindication to its use. More specifically, a risk of falls and frailty have been cited as characteristic elements of ageing that are perceived to be associated with a higher risk of adverse outcomes with warfarin therapy. In regard to falls risk, studies have shown that the risk of haemorrhagic complications is not increased in patients who fall whilst on warfarin therapy [Garwood and Corbett, 2008], nor is the rate of fall-related intracranial haemorrhagic injury [Bond et al. 2005], including among stroke patients undergoing rehabilitation [Stein et al. 1995]. Even those studies reporting a higher rate of ICH in persons with a higher falls risk, the authors have concluded that the benefits of therapy would outweigh these risks in all patients, except for those at low risk of stroke [Gage, 2005]. In a study using a Markov decision analytic model to determine optimal treatment in persons aged 65 years or older, which gave consideration to any potential for ICH, it was reported that persons taking warfarin would need to fall approximately 295 times within 1 year for warfarin to not be the optimal therapy [Man-Son-Hing et al. 1999].
Impact of frailty
In trying to unpack the specific dimensions of ageing that increase the risk of medication misadventure, the concept of frailty has been explored as a risk factor for adverse outcomes with anticoagulant use. In a study investigating the impact of frailty on the utilization of antithrombotics and related clinical outcomes, it was found that frail patients (as defined using validated tools) were less likely to receive warfarin therapy than non-frail patients, and that frail patients were significantly more likely to experience embolic stroke (RR 3.5, 95% confidence interval [CI] 1.0–12.0, p < 0.05), have a small nonsignificant increase in the risk of major haemorrhage (RR 1.5, 95% CI 0.7–3.0, p = 0.29), and had a greater mortality (RR 2.8, 95% CI 1.2–6.5, p = 0.01), concluding that frail older patients with AF are significantly less likely to receive warfarin than nonfrail and appear more vulnerable to adverse clinical outcomes, with and without antithrombotic therapy [Perera et al. 2009b].
Preference for aspirin
These safety concerns in the elderly have led some clinicians to prescribe aspirin in preference to warfarin, despite its reduced efficacy, believing it to be a safer option. Furthermore, very recent data suggests that aspirin may offer additional benefits in terms of reducing the long-term risk of death due to cancer, and this may further sway clinicians, as well as patients, toward its use [Rothwell et al. 2011]. However, studies have shown that antiplatelet agents are not without significant risk; the Warfarin Versus Aspirin for Stroke Prevention in Octogenerians with Atrial Fibrillation (WASPO) study showed that patients aged 80–90 years were significantly more likely to discontinue aspirin than warfarin therapy, principally due to the risk of gastrointestinal side effects [Rash et al. 2007]. Therefore, the current of mass of evidence overall supports the preferential use of warfarin in older persons at high risk of stroke, but in view of the challenges of using such complicated therapies, there remains a need for alternate anticoagulants that are more efficacious and safer than antiplatelet agents (aspirin), but which are more easily managed and administered than warfarin.
New oral anticoagulants
Targeted investment in research and development over the past decade has yielded viable and promising alternative agents to warfarin. In particular, dabigatran and rivaroxaban are two new oral anticoagulants which are relatively devoid of the some of the complexities of warfarin therapy (e.g. drug monitoring, dietary interactions), and which have demonstrated acceptable efficacy and safety profiles when compared with warfarin. In the RE-LY trial, dabigatran was compared with adjusted dose warfarin (INR 2–3), using an intention-to-treat analysis [Connolly et al. 2009]. In terms of the primary endpoint (stroke or systemic embolism), dabigatran was found to be noninferior to warfarin at the 110 mg dose (RR 0.91, 95% CI 0.74–1.11), with significantly less major bleeding (RR 0.80, 95% CI 0.69–0.93). At the 150 mg dose, dabigatran demonstrated superiority over warfarin (RR 0.66, 95% CI, 0.53–0.82), with no significant difference in major bleeding (RR 0.93, 95% CI 0.81–1.07). At the higher dose, dabigatran also reduced the risk of hemorrhagic stroke (RR 0.26, 95% CI 0.14–0.49), and demonstrated a strong trend to a reduction in overall mortality (RR 0.88, 95% CI 0.77–1.00). Whether the risks of bleeding are higher in ‘real-life’ patients is yet to be determined,
More recently, the findings from the pivotal ROCKET-AF trial, comparing rivaroxaban (20 mg dose) with adjusted dose warfarin (INR 2.5), have been reported [Mahaffey, 2010; ROCKET-AF Study Investigators, 2010]. With regard to reducing the risk of stroke and noncentral nervous system systemic embolism, rivaroxaban has been shown to be noninferior to warfarin based on an intention-to-treat analysis (RR 0.88, 95% CI 0.74–1.03), although demonstrating superiority to warfarin in the ‘on-treatment’ analysis (RR 0.79, 95% CI 0.65–0.95). Importantly, rates of major bleeding were similar to warfarin (RR 1.03, 95% CI 0.90–1.20), whilst those bleeding events that are most concerning to clinicians and patients were significantly lower in the rivaroxaban-treated group (i.e. ICH RR 0.59, 95% CI 0.37–0.93; critical organ bleed RR 0.69, 95% CI 0.53–0.91; bleeding-related death RR 0.50, 95% CI 0.31–0.79). A modest trend for a reduction in mortality was also reported (RR 0.92, 95% CI 0.82–1.03).
Overall, both agents appear to be at least noninferior to warfarin in terms of efficacy, with no increase in major bleeding overall. Other specific safety considerations for both agents are discussed in the following.
Dabigatran
Dabigatran etexilate, an oral prodrug, is a direct inhibitor of thrombin (a clotting factor within the coagulation cascade). Although, current findings highlight a favourable risk–benefit profile it should be noted that dabigatran is not without risk [Eisert et al. 2010; Escobar et al. 2010]. Given that the agent maintains a risk of bleeding, it should be avoided in patients who have bleeding disorders or active bleeding, and those who are using concurrent antithrombotic agents (including nonsteroidal anti-inflammatory drugs [NSAIDs]). It should be noted that dabigatran has no specific antidote in cases of serious bleeding.
Safety concerns have been raised regarding other adverse events such as an increased risk of myocardial infarction, and an increase in dyspepsia and gastrointestinal bleeding; regarding the latter, patients with pre-existing gastrointestinal conditions would need concomitant treatment with gastroprotectants (e.g. proton pump inhibitors) [Connolly et al. 2009]. Whilst it has more predictable pharmacokinetics, and does not need regular monitoring of clotting times, consideration might be given to checking liver function at baseline and during therapy as safety in patients with hepatic impairment has not yet been established (note that ximelagatran was withdrawn from the market due to concerns about hepatotoxicity). The agent is contraindicated in patients with severe liver disease and those with poor renal function (creatinine clearance <30 ml/min) due to its renal clearance. Significant drug interactions involve inhibitors of P-glycoprotein transport, including commonly used agents in older persons such as amiodarone verapamil, clarithromycin, and St John's wort. Quinidine is absolutely contraindicated.
Whilst this agent shows promise as a viable and relatively safe alternative to warfarin, pharmacoeconomic considerations may play an important role in determining its availability for widespread use. Although dabigatran is devoid of the cost of therapeutic drug monitoring that is required to ensure safe warfarin therapy, it is relatively more expensive to manufacture, and studies have shown that direct thrombin inhibitors might be cost-effective only in a small group of high-risk patients (i.e. those at high risk of bleeding with warfarin) [Valiya and Bajorek, 2005] and/or when supplier rebates are factored into costing [Braidy et al. 2010]. More recent Markov decision models using data from the RE-LY trial have confirmed that the cost-effectiveness of dabigatran is primarily dependent on its final market cost, being minimally affected by other factors [Freeman et al. 2011].
Rivaroxaban
Rivaroxaban is an oral direct factor Xa inhibitor (reversible antagonist) which was initially trialled and marketed for the short-term prevention of thromboembolism (e.g. deep venous thrombosis [DVT] and pulmonary embolism) postsurgically in patients undergoing total hip or knee replacement. Unlike warfarin, rivaroxaban has more predictable pharmacokinetics and hence does not require monitoring of clotting times (prothrombin time) or dosage titration, and has fewer drug interactions. However, there are specific safety considerations that need to be assessed in each individual patient, given that all anticoagulants increase the risk of bleeding and there is no antidote in the event of overdosage [Ufer, 2010]. The agent should not be used in patients with a significant risk of bleeding (including coagulopathies) or active bleeding.
The agent is partly metabolised in the liver and partly excreted in the urine, and therefore it is contraindicated in patients with significant hepatic impairment (particularly where INR is elevated) and/or renal impairment (creatinine clearance <l5 ml/min). Current trials will help evaluate whether the agent causes any significant hepatotoxicity when used long term. Rivaroxaban also interacts with certain drugs, particularly those which inhibit CYP3A4 or P-glycoprotein, such as the azole antifungals, HIV-protease inhibitor antivirals, cyclosporin, clarithromycin, diltiazem, dipyridamole, erythromycin, fluconazole, tamoxifen and verapamil, leading to an increased risk of bleeding. It should not be used in conjunction with other anticoagulants, and should be used cautiously during concurrent antiplatelet therapy and/or with other NSAIDs. As is the case for dabigatran, the relatively high cost of the drug (relative to warfarin) may render it cost effective only when supplier rebates are factored into costing [Braidy et al. 2010].
Rivaroxaban and dabigatran are recent additions to the anticoagulant options available in practice, and there are other potential candidates in the pharmaceutical pipeline, including apixaban (AVERROES [Eikelboom et al. 2010] and ARISTOTLE trials), edoxaban (ENGAGE AF-TIMI 48 trial [Ruff et al. 2010]), and betrixaban (EXPLORE-Xa trial). Whilst they offer some advantages, they are clearly not without risk [Ufer, 2010] and the cost of newer agents may be prohibitive, thus limiting widespread use especially in developing countries. For this reason, warfarin will likely remain a mainstay therapy for years to come, and in looking forward, the safety considerations regarding its use will need to focus less on comparisons with newer anticoagulants, and more on the risks of warfarin when used within combination regimens, including ‘triple therapy', in patients undergoing percutaneous coronary interventions (e.g. artery stenting). Studies have shown that combinations of warfarin, aspirin and clopidogrel are associated with a threefold increased risk of fatal and nonfatal haemorrhage when compared with warfarin monotherapy [Hansen et al. 2010].
Assuring medication safety along the Medicines Management Pathway
For current clinical practice, concerted efforts should continue to focus on facilitating the safe use of warfarin, and the key to assuring this lies in the application of practical and targeted interventions along key stages of the MMP [Stowasser et al. 2004].
MMP: ‘Decision to prescribe treatment’
The first consideration in using any treatment lies in the initial decision to prescribe treatment. For anticoagulants this decision is particularly one of safety, because the decision to not initiate treatment can be just as unsafe as the decision to initiate treatment (i.e. the risk of stroke from not taking treatment versus the risk of bleeding from treatment). Unfortunately, this decision-making has tended to focus more on the risks of treatment, rather than the benefits gained; indeed, studies have shown that clinicians are directed more by the ‘error of commission' rather than the ‘error of omission' in regard to the use of anticoagulants [Bajorek et al. 2007]. To some extent, this approach is understandable, given that an actual hemorrhagic event is easily identified whereas the prevention of a thromboembolic event is not (there is no event to detect), leading clinicians to adhere to the principle of ‘first do no harm’ (derived from the Hippocratic Oath [Edelstein, 1996]).
Studies evaluating the risk versus benefit of anticoagulants inherently conclude with statements that ‘the choice of antithrombotic prophylaxis is best individualized and should consider the patient's inherent stroke risk and the best estimate of the absolute benefits as well as bleeding risk, access to high-quality anticoagulation monitoring, and patient preferences’ [Hart et al. 2007]. Historically, the decision to prescribe treatment has been based on clinicians' interpretation of guidelines, and their perception of how applicable consensus recommendations are to their patients. Unfortunately, numerous studies have highlighted that adherence to guideline recommendations in this context is poor, with most focusing principally on the indications for treatment and with relatively sparse practical advice regarding the risks of treatment and contraindications. Careful patient selection via risk–benefit assessment is integral to decision-making here, but guidelines per se are ineffective as they fail to demonstrate how evidence can be actioned in individual patients.
Stroke risk assessment tools
To address this, in recent years more effort has been placed on developing algorithms and risk assessment tools to help clinicians objectively determine the appropriateness of treatment. At the first level, these tools have focused on quantifying the annual adjusted risk of stroke in an individual patient. The CHADS2 (Congestive heart failure, Hypertension, Age, Diabetes, Stroke) risk stratification scheme, derived from key clinical trials, has been validated and is probably the most widely used currently having demonstrated greater predictive value for stroke than other risk stratification schemes [Gage et al. 2004].
The CHA2DS2-VASc scheme, a recent derivative of CHADS2 which places more emphasis on age 75 years and previous stroke elements, also includes vascular disease, age 65–74 years and female gender as specific risk factors for stroke [Lip et al. 2010]. In validation studies, CHA2DS2-VASc has been able to correctly classify a higher proportion of patients as being at high risk of stroke. However, many elderly patients with AF will (by default) have a moderate or high score (i.e. moderate or high risk of stroke) using these tools, recognizing that advancing age is a key nonmodifiable risk factor for stroke, and therefore will necessarily require prophylaxis. As such, these tools have limited practical application in the older patient population where the need for treatment is obvious, but where the dilemma lies in determining which specific treatment to use in the presence of a potential for medication misadventure. Nevertheless, the assessment of a patient’s stroke risk is integral to guiding appropriate treatment, and recognition of this is reflected in more recent guidelines. For example, the European Society of Cardiology guidelines incorporate specific schema (i.e. CHA2DS2-VASc) into treatment algorithms, placing emphasis on the use of anticoagulants in patients with CHA2DS2-VASc scores of 1 or more, and recommending no antithrombotic treatment/aspirin for those with scores of 0 [Camm et al. 2010].
Bleeding risk assessment tools
For this reason, tools assessing the potential risk of bleeding have also been developed, such as the Outpatient Bleeding Risk Index (OBRI; comprising age >65 years, prior stroke, prior gastrointestinal bleed, and any of four comorbidities: recent myocardial infarction, anemia, diabetes, or renal insufficiency) [Beyth et al. 1998] and Kuijer’s model (comprising age >60 years, gender, and malignancy) [Kuijer et al. 1999]. Both tools have been validated for use, with the OBRI predicting major bleeding better than clinicians' clinical judgment; however, they been criticized for their lack of inclusion of other factors that may be predictive of bleeding in an older population (e.g. other comorbidities concomitant pharmacotherapy) and because their risk stratifications have used relatively lower age groups (60–65 years) compared with the ‘real-world' target population (mean age 75 years) [Kakar et al. 2006].
More contemporary bleeding risk assessment models have been developed and evaluated to account for a more comprehensive range of risk factors (i.e. age ≥70 years, gender, remote bleed, recent bleed, alcohol/drug abuse, diabetes, anemia, and antiplatelet therapy use), which are more targeted to an elderly population (i.e. patients >80 years of age), and which differentiate between recent and remote bleeds [Shireman et al. 2006]. Another commonly used, and validated tool, is HEMORR2HAGES, which includes hepatic or renal disease, ethanol abuse, malignancy, older (age >75 years), reduced platelet count or function, hypertension (uncontrolled), anemia, genetic factors, excessive fall risk, and stroke, but which places extra weight on prior bleeds [Gage et al. 2006].
A recent addition to bleeding risk tools is HAS-BLED, which takes into risk factors such as: Hypertension, Abnormal kidney and/or liver function; Stroke; Bleeding (previous history of bleeding, anemia or having predisposition to bleeding); Labile INR, Elderly and Drugs and/or alcohol (antiplatelet drugs, alcohol consumption of ≥8 drinks per week) [Pisters et al. 2010]. Using HAS-BLED scores has enabled the identification of those at higher bleeding risk who could be prescribed lower doses of dabigatran (110 mg twice a day) as an alternative to warfarin. More recently, HAS-BLED has been validated using data from the SPORTIF trial cohort of patients, demonstrating its useful predictive capacity for bleeding over previously published schemas, with greater simplicity, and identifying diabetes and heart failure (left ventricular dysfunction) as potential risk factors for bleeding in AF beyond those previously recognized [Lip et al. 2011].
Although such tools more completely address the difficulties and complexities involved in anticoagulation administration, there is further scope to incorporate frailty and polypharmacy into these risk assessments. It has been shown that frailty may be an important alternative way of characterizing patients within any risk versus benefit assessment, given that frailty appears to be a differentiating factor in determining treatment and in potentially identifying those likely to have adverse clinical outcomes [Perera et al. 2009a].
Stroke versus bleeding risk assessment tools
Whilst separate tools are available to assess stroke risk and bleeding risk independently, they have limited practical application because as separate tools they do not estimate the relative risk versus benefit of available treatment options in an individual patient, i.e. they do not bring the two elements of the risk versus benefit together. Studies have shown that assessing stroke risk alone is insufficient to guide treatment selection, highlighting that the older patient population has a higher prevalence of risk factors for both stroke and bleeding, reporting that 21.0% of older patients have diseases with a risk of haemorrhage, 26.7% have diseases with a risk of thrombosis, and 8.6% have diseases with a risk of both haemorrhage and thrombosis [Guo et al. 2010].
In addition, few algorithms specifically address those issues which characterize the challenges in an elderly population (function, cognition, social support, frailty) factors which are often not addressed in major clinical trials due to specific patient selection criteria. However, it is equally important to recognize here, that whilst age per se is not a reason to withhold anticoagulant therapy, not all older people are frail and/or cognitively impaired. For this reason, practical tools that facilitate a systematic, individualized risk–benefit assessment of each patient, and that identify potential barriers which can be overcome using available support services and resources, are necessary. Such individualized assessments should then be used as a basis for discussion between the patient and clinician regarding treatment options, and be supported by appropriate education to prevent warfarin-associated adverse events.
Studies have shown that targeted interventions that facilitate concurrent systematic and comprehensive review of a patient's risk factors for stroke and bleeding are effective. In a previous study it has been shown that an applied comprehensive risk-assessment process facilitated by a pharmacist, can significantly improve the utilization of therapy [Bajorek et al. 2005]. Using evidence-based algorithms to facilitate the review of individual stroke risk and bleeding risk (taking into account medical, functional, cognitive, medication-related, and social factors) the use of antithrombotics significantly increased from baseline (59.6% versus 81.2%, p < 0.00l). Overall, 36% of patients required changes to their baseline therapy, with 76.9% of these being ‘upgrades' to more effective treatment. Importantly, the process demonstrated that the goal of such tools should not be purely to increase the proportion of patients prescribed antithrombotics per se, but rather to improve the proportion of patients receiving appropriate therapy according to their risk versus benefit assessment; the process identified patients who had been prescribed warfarin for years, but in whom the risk–benefit profile had changed over time, rendering warfarin as no longer the most appropriate agent.
This highlights that risk stratification schemes and risk assessment tools are valuable aids to guide decision-making regarding the use of antithrombotics (particularly anticoagulants), but only when these tools are used simultaneously to complete the risk–benefit profile, and when they are use as part of routine care to re-evaluate the appropriateness of therapy over the long term when risks (in terms of both benefit and safety) may change over time.
Whilst such tools and processes have merit, they can only enhance the safety of drug use if they can be feasibly applied to practice and integrated into routine care, and therefore must necessarily be functional. Strategies include integrating elements of risk–benefit assessment within continuous quality audit cycles in hospitals, to identify treatment gaps and facilitate medication safety; Australia's Therapeutic Advisory Group's (TAG) Manual of Indicators for Quality Use of Medicines in Australian Hospitals includes a section devoted to optimizing the use of antithrombotic agents, including Indicator 1.6 ‘Percentage of patients with atrial fibrillation that are discharged on warfarin’ [NSW Therapeutic Advisory Group, 2007].
In addition, the use of information technology has become increasingly important in providing an accessible interface for the use of such tools as part of computerized decision support systems in an increasingly electronic and online health system. An example of this is the CARAT, an electronic (Web-based) Computerised Antithrombotic Risk Assessment Tool (www.caratcalculator.com), to aid clinicians in selecting appropriate antithrombotic therapy in older persons with AF [Bajorek et al. 2010]. Unlike paper-based guidelines, it facilitates a systematic review of risk factors and quantifies the estimated risk versus benefit of therapy in an individual patient, and is modelled on inputs from a previous study [Bajorek et al. 2005] and current evidence [Fang et al. 2008]. In a pilot study, clinicians reported being satisfied with CARAT's format (94%) and agreed with its estimates of risk (stroke and bleeding) and treatment recommendations (72%), indicating that the tool could be ‘very useful’ for practice, particularly for general practitioners in primary care [Masood et al. 2008].
MMP: ‘Review of medicine order’
An important finding from risk–benefit tool studies is that the use of antithrombotics must be re-reviewed periodically, as the risk–benefit profile changes in individual patients over time [Bajorek et al. 2005]. Interventions and services dedicated to the review of pharmacotherapeutic regimens may serve as critical platforms for such review. For example, Medication Management Review (MMR) including Home Medicines Review (HMR), are interventions which generally refer to the systematic evaluation of the patient's pharmacotherapeutic needs and drug regimen, a consumer interview and documentation of medication review findings followed by recommendations to optimize the drug regimen [Krska et al. 2001; Van der Meer et al. 1993]. Studies have shown that the treatment recommendations embedded in these types of interventions are useful in identifying drug-related problems, and in providing treatment recommendations that are founded in the principles of applying evidence-based medicine to clinical practice [Castelino et al. 2011, 2009]. In regard to cardiovascular medicines, pharmacist-led HMR services have been shown to significantly improve prescribing of evidence-based antithrombotic therapy, for patients at high-risk of cardiovascular events including patients with AF [Castelino et al. 2010].
MMP: ‘Provision of medicines information’
An often undervalued, yet critical, component of drug safety is patient education and knowledge. It has been stated that the ‘cornerstone of safe and efficacious warfarin use is patient understanding and knowledge of the risks/benefits’, to enable patients to effectively manage their treatment in conjunction with clinicians and other health professionals [Kakar et al. 2006]. This is fundamental to patients' ability to make informed decisions about the therapy [Howitt and Armstrong, 1999], facilitate adherence to treatment [Lane and Lip, 2005], and to support their day-to-day medication management [Bajorek et al. 2007]. Although older persons appear to be more accepting of warfarin therapy than clinicians perceive them to be, they report feeling somewhat abandoned in their management of warfarin due to the lack of ongoing support services in the community, specifically inadequate information provision; initial fears are founded by a relative lack of knowledge about the therapy. Indeed, older person's knowledge about warfarin and access to warfarin education programmes seems to be inadequate, highlighting the need to develop targeted educational strategies and resources to further improve knowledge about the therapy and thereby make it safer [Nasser et al. 2010].
There is scope to use information technology, including the Internet, more broadly as a readily accessible portal of information, but the practical utility of this information needs to be carefully considered in view of the functional challenges faced by elderly people (e.g. visual impairment, reduced comprehension with declining cognition). It has been shown that whilst the quality and suitability of information provided on warfarin-related Websites is overall adequate, the readability of information on most Internet sites is inadequate, requiring skills beyond that recommended for the general population [Nasser et al. 2009]. Well-designed Web-based resources (e.g. www.anticoagulation.com.au) have demonstrated good quality, suitability, and readability in terms of information content [Nasser et al. 2009], and may additionally serve as useful platforms to support patient self-management [Heneghan et al. 2006].
MMP: ‘Monitor for response’
The high risk of bleeding with anticoagulants necessitates that therapeutic drug monitoring is an essential component of long-term treatment, and dedicated anticoagulation clinics have been shown to keep the risks of bleeding ‘acceptably low', even in older persons (80 years of older) and those deemed to be frail [Tincani et al. 2009; Poli et al. 2007; Torn et al. 2005]. Given that the proportion of time that a patient spends in the therapeutic INR range (TTR) is associated with clinical outcomes, the importance of routine and reliable monitoring is obvious [Wan et al. 2008]. Studies have shown that TTR and percentage of INRs in range effectively predict INR control, and therefore predict adverse outcomes [Wan et al. 2008], and that the risk of stroke can be significantly reduced when the TTR is above about 70% [Morgan et al. 2009; Connolly et al. 2008]. This is also important to consider in the initial selection of therapy, given that any benefit from newer anticoagulants is inherently compared against the outcomes from warfarin, and it has been shown that local standards of care (i.e. INR control) affect the comparative benefits new treatments [Wallentin et al. 2010].
Routine monitoring is therefore key to therapeutic safety, however, whilst dedicated monitoring is readily facilitated in an acute care and/or ambulatory setting via on-site pathology services and clinics, this is logistically and practically challenging in the primary care setting. There is a critical need for point-of-care services to provide ready access to therapeutic monitoring, as well as other resources for patients. Community Pharmacy-based Anticoagulation Monitoring services using point-of-care INR testing provide an alternative model of care, and can: (1) maintain the control of patients INR within the target range to help achieve optimal anticoagulation outcomes (i.e. maintain safe and efficacious control of patient INR without any major or minor warfarin-related adverse events); (2) improve patient knowledge which, in turn, is linked to fewer warfarin-related complications; (3) improve patients quality of life by reducing treatment-related distress; (4) foster timely and appropriate interprofessional collaboration between pharmacists and general practitioners; and (5) deliver care which is cost-comparable to existing pathology services [McLachlan et al. 2005].
Current research is exploring the opportunities to deliver point-of-care services in a more ‘mobile' format, such as within the primary care setting (i.e. in general practice surgeries), or aligned with home medicines review services in older persons discharged (on warfarin therapy) from hospital to the community setting [Peterson, 2010]. There has also been much interest in patient self-management, including self-adjustment of doses, with a systematic review highlighting that patient self-monitoring improves the quality of anticoagulation, and significantly reduces the rate of thromboembolism, all-cause mortality, and major haemorrhage [Heneghan et al. 2006]. However, in the ‘at-risk’ older population, self-management may not be feasible due to functional and cognitive impairments.
Summary
It has been stated that even after accounting for the many cited contraindications to warfarin, the evidence continues to support the use of warfarin, as both a safe and effective therapy in older persons. Importantly, any risk of haemorrhage is ‘dwarfed’ by the significant benefits to be gained in terms of strokes prevented [Kapoor, 2007]. Until newer anticoagulants become viable treatment options for widespread use, efforts must be directed at supporting clinicians in overcoming the perceived barriers to treatment. The MMP identifies key steps in the safe use of anticoagulant therapy, and strategies to facilitate the safe use of warfarin in ‘at-risk’ elderly patients include comprehensive and individualized risk–benefit assessment using novel risk stratification tools (which remove age per se as a contraindication), focused medicines review services, therapeutic drug monitoring services delivered in the primary care setting, and practical education strategies and resources targeting the older patient population.
Funding
The preparation of this manuscript did not receive funding. The papers cited in the article (as authored by various people and organisations) may have been funded for their original research studies.
Conflict of interest statement
None to declare.
References
- Bajorek B.V., Krass I., Ogle S.J., Duguid M.J., Shenfield G.M. (2002) The impact of age on antithrombotic use in elderly patients with non-valvular atrial fibrillation. Australasian J Ageing 21: 36–41 [Google Scholar]
- Bajorek B.V., Krass I., Ogle S.J., Duguid M.J., Shenfield G.M. (2005) Optimizing the use of antithrombotic therapy for atrial fibrillation in older people: A pharmacist-led multidisciplinary intervention. J Am Geriatrics Soc 53: 1912–1920 [DOI] [PubMed] [Google Scholar]
- Bajorek, B.V., Masood, N. and Krass, I. (2010) Computerised Antithrombotic Risk Assessment Tool (CARAT) for Stroke Prevention in Atrial Fibrillation. Available at: www.caratcalculator.com [DOI] [PubMed]
- Bajorek B.V., Ogle S.J., Duguid M.J., Shenfield G.M., Krass I., Bajorek B.V., et al. (2007) Management of warfarin in atrial fibrillation: views of health professionals, older patients and their carers. Med J Australia 186: 175–180 [DOI] [PubMed] [Google Scholar]
- Benjamin E., Levy D., Vaziri D., D'Agostino R., Belanger A., Wolf P. (1994) Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. J Am Med Assoc 271: 840–844 [PubMed] [Google Scholar]
- Beyth R.J., Quinn L.M., Landefeld C.S. (1998) Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 105: 91–99 [DOI] [PubMed] [Google Scholar]
- Bond A., Molnar F., Li M., Mackey M., Man-Son-Hing M. (2005) The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thrombosis J 3(1): 7 January 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. (1990) The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 323: 1505–1511 [DOI] [PubMed] [Google Scholar]
- Braidy N., Bui K., Bajorek B. (2010) Evaluating the impact of new anticoagulants in the hospital setting. Pharmacy Practice, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm A.J., Kirchhof P., Lip G.Y.H., Schotten U., Savelieva I., Ernst S., et al. (2010) Guidelines for the management of atrial fibrillation. Eur Heart J 31(19): 2369–2429 [DOI] [PubMed] [Google Scholar]
- Castelino R.L., Bajorek B.V., Chen T.F. (2009) Targeting Suboptimal Prescribing in the Elderly: A Review of the Impact of Pharmacy Services. Ann Pharmacother 43: 1096–1106 [DOI] [PubMed] [Google Scholar]
- Castelino R.L., Bajorek B.V., Chen T.F. (2011) Are interventions recommended by pharmacists during Home Medicines Review evidence-based? J Eval Clin Practice 17(1): 104–110 [DOI] [PubMed] [Google Scholar]
- Castelino R.L., Chen T.F., Guddattu V., Bajorek B.V. (2010) Use of evidence-based therapy for the prevention of cardiovascular events among older people. Eval Health Professions 33: 276–301 [DOI] [PubMed] [Google Scholar]
- Chapman N., Huxley R., Anderson C., Bousser M.G., Chalmers J., Colman S., et al. (2004) Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: The PROGRESS Trial. Stroke 35: 116–121 [DOI] [PubMed] [Google Scholar]
- Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151 [DOI] [PubMed] [Google Scholar]
- Connolly S.J., Pogue J., Eikelboom J., Flaker G., Commerford P., Franzosi M.G., et al. (2008) Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 118: 2029–2037 [DOI] [PubMed] [Google Scholar]
- Copland M., Walker I., Tait R. (2001) Oral anticoagulation and hemorrhagic complications in an elderly population with atrial fibrillation. Arch Intern Med 161: 2125–2128 [DOI] [PubMed] [Google Scholar]
- Edelstein L. (1996) The Hippocratic Oath: Text, Translation and Interpretation. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Eikelboom J.W., O'Donnell M., Yusuf S., Diaz R., Flaker G., Hart R., et al. (2010) Rationale and design of AVERROES: Apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 159: 348–353e341. [DOI] [PubMed] [Google Scholar]
- Eisert W.G., Hauel N., Stangier J., Wienen W., Clemens A., van Ryn J. (2010) Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thrombosis Vascular Biol 30: 1885–1889 [DOI] [PubMed] [Google Scholar]
- Escobar C., Barrios V., Jimenez D. (2010) Atrial fibrillation and dabigatran: has the time come to use new anticoagulants? Cardiovasc Therapeut 28: 295–301 [DOI] [PubMed] [Google Scholar]
- Fang M. (2009) Antithrombotic therapy for the treatment of atrial fibrillation in the elderly. J Intervent Cardiol Electrophysiol 25: 19–23 [DOI] [PubMed] [Google Scholar]
- Fang M., Go A., Chang Y., Borowsky L., Pomernacki N., Singer D. (2008) Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am College Cardiol 51: 810–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M.C., Go A.S., Hylek E.M., Chang Y., Henault L.E., Jensvold N.G., et al. (2006) Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatrics Soc 54: 1231–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J.V., Zhu R.P., Owens D.K., Garber A.M., Hutton D.W., Go A.S., et al. (2011) Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 154(1): 1–11 [DOI] [PubMed] [Google Scholar]
- Gage B.F. (2005) Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 118: 612–617 [DOI] [PubMed] [Google Scholar]
- Gage B.F., van Walraven C., Pearce L., Hart R.G., Koudstaal P.J., Boode B.S.P., et al. (2004) Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 110: 2287–2292 [DOI] [PubMed] [Google Scholar]
- Gage B.F., Yan Y., Milligan P.E., Waterman A.D., Culverhouse R., Rich M.W., et al. (2006) Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 151: 713–719 [DOI] [PubMed] [Google Scholar]
- Gallagher A., Rietbrock S., Plumb J., van Staa T. (2008) Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thrombosis Haemostasis 6: 1500–1506 [DOI] [PubMed] [Google Scholar]
- Garwood C.L., Corbett T.L. (2008) Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann Pharmacother 42: 523–532 [DOI] [PubMed] [Google Scholar]
- Gattellari M., Worthington J., Zwar N. (2009) Warfarin: An inconvenient truth. Stroke 40: 5–7 [DOI] [PubMed] [Google Scholar]
- Gattellari M., Worthington J., Zwar N., Middleton S. (2008) Barriers to the use of anticoagulation for nonvalvular atrial fibrillation: a representative survey of Australian family physicians. Stroke 39: 227–230 [DOI] [PubMed] [Google Scholar]
- Gladstone D., Bui E., Fang B., Laupacis A., Lindsay M., Tu J. (2009) Potentially preventable strokes in high risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 40: 235–240 [DOI] [PubMed] [Google Scholar]
- Glazer N., Dublin S., Smith N., French B., Jackson L., Hrachovec J. (2007) Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med 167: 246–252 [DOI] [PubMed] [Google Scholar]
- Guo Y., Wu Q., Zhang L., Yang T., Zhu P., Gao W., et al. (2010) Antithrombotic therapy in very elderly patients with atrial fibrillation: Is it enough to assess thromboembolic risk? Clin Interventions Aging 5: 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Sorensen R., Clausen M., Fog-Petersen M., Raunso J., Gadsboll N., et al. (2010) Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 170: 1433–1441 [DOI] [PubMed] [Google Scholar]
- Hart R.G., Pearce L.A., Aguilar M.I. (2007) Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146: 857–867 [DOI] [PubMed] [Google Scholar]
- Hart R.G., Tonarelli S.B., Pearce L.A. (2005) Avoiding central nervous system bleeding during antithrombotic therapy: recent data and ideas. Stroke 36: 1588–1593 [DOI] [PubMed] [Google Scholar]
- Heneghan C., Alonso-Coello P., Garcia-Alamino J.M., Perera R., Meats E., Glasziou P. (2006) Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet 367: 404–411 [DOI] [PubMed] [Google Scholar]
- Howitt A., Armstrong D. (1999) Implementing evidence based medicine in general practice: audit and qualitative study of antithrombotic treatment for atrial fibrillation. Br Med J 318: 1324–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L. (2008) Warfarin pharmacology, clinical management, and evaluation of hemorrhagic risk for the elderly. Cardiol Clin 26: 157–167 [DOI] [PubMed] [Google Scholar]
- Kakar P., Lane D., Lip G.Y.H. (2006) Bleeding risk stratification models in deciding on anticoagulation in patients with atrial fibrillation. Chest 130: 1296–1299 [DOI] [PubMed] [Google Scholar]
- Kapoor J. (2007) Letter to the Editor, “The management of atrial fibrillation”. Lancet 370: 1608–1608 [DOI] [PubMed] [Google Scholar]
- Krska J., Cromarty J., Arris F., Jamieson D., Hanford D., Duffus P. (2001) Pharmacist-led medication review in patients over 65: a randomised, controlled trial in primary care. Age Ageing 30: 205–211 [DOI] [PubMed] [Google Scholar]
- Kuijer P.M.M., Hutten B.A., Prins M.H., Buller H.R. (1999) Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med 159: 457–460 [DOI] [PubMed] [Google Scholar]
- Lane D., Lip G.Y.H. (2005) Anti-thrombotic therapy for atrial fibrillation and patients' preferences for treatment. Age Ageing 34: 1–3 [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Frison L., Halperin J.L., Lane D.A. (2011) Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J Am College Cardiol 57: 173–180 [DOI] [PubMed] [Google Scholar]
- Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M. (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest 137: 263–272 [DOI] [PubMed] [Google Scholar]
- Mahaffey, K. (2010) Stroke prevention using the oral direct factor Xa inhibitor rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation (ROCKET AF). Presented at the American Heart Association Scientific Sessions, Chicago, IL, 15 November 2010.
- Man-Son-Hing M., Nichol G., Lau A., Laupacis A. (1999) Choosing antithrombotic therapy for elderly persons with atrial fibrillation who are at risk for falls. Arch Intern Med 159: 677–685 [DOI] [PubMed] [Google Scholar]
- Mant J., Hobbs F.D.R., Fletcher K., Roalfe A., Fitzmaurice D., Lip G.Y.H., et al. (2007) Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 370: 493–503 [DOI] [PubMed] [Google Scholar]
- Marcucci M., Iorio A., Nobili A., Tettamanti M., Pasina L., Marengoni A., et al. (2010) Factors affecting adherence to guidelines for antithrombotic therapy in elderly patients with atrial fibrillation admitted to internal medicine wards. Eur J Intern Med 21(6): 516–523 [DOI] [PubMed] [Google Scholar]
- Marinigh R., Lip G.Y.H., Fiotti N., Giansante C., Lane D.A. (2010) Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am College Cardiol 56: 827–837 [DOI] [PubMed] [Google Scholar]
- Masood M., Krass I., Bajorek B. (2008) Computerised Antithrombotic Risk Assessment Tool (CARAT) Project: Developing an algorithm for antithrombotic selection for stroke prevention in patients with Atrial Fibrillation. Canberra, Australia: Australasian Pharmaceutical Science Association (APSA) [Google Scholar]
- McLachlan, A., Spindler, M., Fois, R., Krass, I., Chen, T. and Bajorek, B. (2005) A community pharmacy based anticoagulant management service (Project ID 2002-027; Commonwealth Department of Health and Ageing, Third Community Pharmacy Agreement).
- Morgan C.L., McEwan P., Tukiendorf A., Robinson P.A., Clemens A., Plumb J.M. (2009) Warfarin treatment in patients with atrial fibrillation: Observing outcomes associated with varying levels of INR control. Thrombosis Res 124: 37–41 [DOI] [PubMed] [Google Scholar]
- Nasser, S., Mullan, J. and Bajorek, B. (2009) Assessing the quality, suitability and readability of web-based patient information on warfarin. Presented at the Australasian Pharmaceutical Sciences Association Annual Scientific Meeting, Hobart, Tasmania, Australia, 9–11 December 2009.
- Nasser S., Mullan J., Bajorek B. (2010) Patient education about warfarin therapy: a review of the knowledge, information needs and education of older patients. Family Practice, in press. [Google Scholar]
- NSW Therapeutic Advisory Group (2007) Indicators for Quality Use of Medicines in Australian Hospitals. http://wwwciaphealthnswgovau/nswtag/QUMIndicatorshtml
- Perera V., Bajorek B.V., Matthews S., Hilmer S.N. (2009a) The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing 38: 156–162 [DOI] [PubMed] [Google Scholar]
- Perera V., Bajorek B.V., Matthews S., Hilmer S.N., Perera V., Bajorek B.V., et al. (2009b) The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing 38: 156–162 [DOI] [PubMed] [Google Scholar]
- Petersen P., Boysen G., Godtfredsen J., Andersen E., Andersen B. (1989) Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK study. Lancet 1: 175–179 [DOI] [PubMed] [Google Scholar]
- Peterson G. (2001) Rational drug usage in the elderly: walking the fine line between polypharmacy and undertreatment. Australian Pharmacist 20: 248–254 [Google Scholar]
- Peterson, G. (2010) The Role of Community Pharmacy in Post Hospital Management of Patients Initiated on Warfarin (Project ID 2007/08-04; Commonwealth Department of Health and Ageing, Fourth Community Pharmacy Agreement); Final Report.
- Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J.G.M., Lip G.Y.H. (2010) A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 138(5): 1032–1033 [DOI] [PubMed] [Google Scholar]
- Po H., Lin Y.-J. (2010) Antithrombotic treatment before stroke onset and stroke severity in patients with atrial fibrillation and first-ever ischemic stroke: an observational study. Neurol Asia 15: 11–17 [Google Scholar]
- Poli D., Antonucci E., Marcucci R., Fatini C., Alterini B., Mannini L., et al. (2007) Risk of bleeding in very old atrial fibrillation patients on warfarin: relationship with ageing and CHADS2 score. Thrombosis Res 121: 347–352 [DOI] [PubMed] [Google Scholar]
- Rash A., Downes T., Portner R., Yeo W., Morgan N., Channer K. (2007) A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenerians with atrial fibrillation (WASPO). Age Ageing 36: 151–156 [DOI] [PubMed] [Google Scholar]
- Ren S., Bajorek B. (2007) Utilisation of Antithrombotic Therapy for Stroke Prevention in Atrial Fibrillation: Then and Now. Manly, Australia: Australasian Pharmaceutical Science Association (APSA) [DOI] [PubMed] [Google Scholar]
- ROCKET-AF Study Investigators. (2010) Rivaroxaban—once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: Rationale and Design of the ROCKET AF study. Am Heart J 159: 340–347.e341 [DOI] [PubMed] [Google Scholar]
- Rothwell P., Fowkes F., Belch J., Ogawa H., Warlow C., Meade T. (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31–41 [DOI] [PubMed] [Google Scholar]
- Ruff C., Giugliano R., Antman E., Crugnale S., Bocanegra T., Mercuri M., et al. (2010) Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: Design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation - Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J 160: 635–641.e632 [DOI] [PubMed] [Google Scholar]
- Shireman T.I., Mahnken J.D., Howard P.A., Kresowik T.F., Hou Q., Ellerbeck E.F. (2006) Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest 130: 1390–1396 [DOI] [PubMed] [Google Scholar]
- Stein J., Viramontes B., Kerrigan D. (1995) Fall-related injuries in anticoagulated stroke patients during inpatient rehabilitation. Arch Phys Med Rehabilitation 76: 840–843 [DOI] [PubMed] [Google Scholar]
- Stowasser D., Allinson Y., O’Leary K. (2004) Understanding the medicines management pathway. J Pharm Practice Res 34: 293–296 [Google Scholar]
- Stroke Prevention in Atrial Fibrillation Investigators. (1991) The Stroke Prevention in Atrial Fibrillation Study: final results. Circulation 84: 527–539 [DOI] [PubMed] [Google Scholar]
- Tincani E., Baldini P., Crowther M., Zanasi A., Ferrari P., Cenci A., et al. (2009) Bleeding rates in patients older than 90 years of age on vitamin K antagonist therapy for nonvalvular atrial fibrillation. Blood Coagulation Fibrinolysis 20: 47–51 [DOI] [PubMed] [Google Scholar]
- Torn M., Ward L., Bollen E., van der Meer F., van der Wall E., Rosendaal F. (2005) Risks of oral anticoagulant therapy with increasing age. Blood Coagulation Fibrinolysis 165: 1527–1532 [DOI] [PubMed] [Google Scholar]
- Ufer M. (2010) Comparative efficacy and safety of the novel oral anticoagulants dabigatran, rivaroxaban and apixaban in preclinical and clinical development. Thrombosis Haemostasis 103: 572–585 [DOI] [PubMed] [Google Scholar]
- Valiya S.N., Bajorek B.V. (2005) Ximelagatran cost effectiveness for stroke prevention in atrial fibrillation. J Pharm Practice Res 35: 279–283 [Google Scholar]
- Van der Meer F., Rosendaal F., Vandenbroucke J., Briet E. (1993) Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med 153: 1557–1562 [DOI] [PubMed] [Google Scholar]
- Wallentin L., Yusuf S., Ezekowitz M., Alings M., Flather F., Franzosi M., et al. (2010) Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 376(9745): 975–983 [DOI] [PubMed] [Google Scholar]
- Wan Y., Heneghan C., Perera R., Roberts N., Hollowell J., Glasziou P., et al. (2008) Anticoagulation control and prediction of adverse events in patients with atrial fibrillation/CLINICAL PERSPECTIVE. Circulation: Cardiovasc Qual Outcomes 1: 84–91 [DOI] [PubMed] [Google Scholar]
- Wintzen A.R., de Jonge H., Loeliger E.A., Bots G.T.A.M. (1984) The risk of intracerebral hemorrhage during oral anticoagulant treatment: A population study. Ann Neurol 16: 553–558 [DOI] [PubMed] [Google Scholar]
- Wolf P.A., Abbott R.D., Kannel W.B. (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983–988 [DOI] [PubMed] [Google Scholar]