ADME is the four-letter acronym for absorption, distribution, metabolism and excretion that has described pharmacokinetics for 50 years. These terms were first presented together in English by Nelson in 1961, rephrasing resorption, distribution, consumption and elimination used by Teorell in 1937 [Nelson, 1961; Teorell, 1937]. Other relevant seminal works include Widmark’s description of first-order elimination in 1919 and Dost’s 1953 treatise defining the term pharmacokinetics [Widmark, 1919; Dost, 1953]. ADME(T) has become a standard term, widely used in the literature, in teaching, in drug regulation and in clinical practice.
ADME, as originally used, stood for descriptors quantifying drug: entering the body (A), moving about the body (D), changing within the body (M) and leaving the body (E). Over time, the use of ADME has diversified according to the needs of the user. In particular, it is used to describe mechanisms: crossing the gut wall (A); movement between compartments (D); mechanisms of metabolism (M); excretion or elimination (E); and transport (T) is sometimes added. Variable use of ADME often causes confusion.
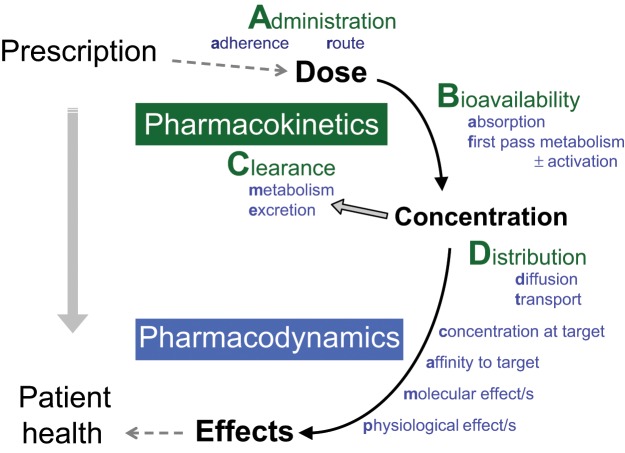
In teaching and applying pharmacokinetic principles we follow the active drug moiety through the body in space and time. The schematic shown in Figure 1 illustrates the pathway from prescription to patient health. For pharmacokinetics we use the acronym ABCD, standing for administration, bioavailability, clearance and distribution. Administration is factors relating to dosing and adherence. Bioavailability is the active drug moiety arriving in the systemic circulation (Nelson’s A). Clearance is the active drug leaving the systemic circulation (ME). Distribution is to the site/s of action (D). For each step the time course and mechanisms are considered with different factors dominating in different cases. For example, extent of bioavailability is usually more important than rate, but not always (e.g. anaesthesia and analgesia).
Figure 1.
From Prescription to Patient Health - mapping the medicine to the patient.
Administration of the drug by the chosen route requires a treatment plan, formulated in a prescription, adherence to that plan and description of the conditions of administration, that is, what actually happened. It should be noted at this point that adherence, not usually considered a pharmacokinetic variable, is a major clinical issue that can be readily quantified using pharmacokinetic data. An accurate description of drug administration is also essential to the interpretation of pharmacokinetic data, that is, how much, by what route, and at what time was the drug administered to the patient.
Bioavailability is the fraction of administered drug entering the systemic circulation [Rowland and Tozer, 2011]. For orally administered drugs, bioavailability is composed of absorption and first pass metabolism. Some authors include rate, as well as extent, in the definition of bioavailability [Atkinson, 2007]. The rate of drug entering the systemic circulation determines the maximum concentration and the time at which this occurs, but it does not affect steady-state concentration or determine maintenance dose.
Clearance is composed of metabolism and excretion of the active drug. Clearance, together with extent of bioavailability, determines average steady-state concentration and hence maintenance dose. The clearance of the pharmacologically active drug moieties is the clinically relevant quantifier of drug leaving the systemic circulation. The fate of inactive drug moieties is largely irrelevant.
Distribution of the drug to its site of action is the description of the differential distribution of the drug within the body. Distribution is easier to consider after bioavailability and clearance have defined steady-state concentration in the systemic circulation. Importantly, the concentration gradient between the systemic circulation and the site of action is often determined by transport processes. Although drug transport is important to bioavailability and clearance, it sits more comfortably under the heading of distribution. The rate of distribution is particularly relevant to bolus dosing, but in continuous dosing, rate is best considered after extent.
The descriptions of ADME by Teorell and Nelson are landmarks in clinical pharmacology [Nelson, 1961; Teorell, 1937]. While the usefulness of ADME has been shown many times, it also has the following limitations.
Absorption in ADME is discordant with bioavailability and consequently varyingly applied. For example, hepatic first-pass metabolism is inconsistently included or excluded under this heading. More recently, drug passage across the gut wall is being discussed in terms of mechanisms of absorption, metabolism and transport. The frequent inclusion of both the extent of drug absorption and the rate of drug absorption under one term also causes confusion.
Distribution, when considered following absorption, focuses attention on the rate of drug accumulation in peripheral compartments rather than the extent (the steady state concentration at the site of action relative to the concentration in the circulation).
Metabolism is the most important component of clearance but not the only component. Metabolism is also essential to the bioavailability of orally administered drugs. Furthermore, metabolism activates pro-drugs and sometimes produces active metabolites.
Excretion is frequently confused with elimination of the active drug, and these terms are frequently used interchangeably in the literature. Excretion is ‘the irreversible loss of chemically unchanged compound’ whereas elimination is ‘the irreversible loss of drug from the site of measurement’, that is, excretion plus metabolism [Rowland and Tozer, 2011]. Furthermore, drug excretion is often reported in terms of inactive metabolites leaving the body, while ignoring the fate of the active drug.
The majority of drug use is for the treatment of chronic disease; hence steady-state concentration of drug at the site of action is central to clinical pharmacokinetics. The ABCD acronym allows logical description of the active drug in space (extent) and time (rate) and provides a simple starting point for considering the variables that affect drug concentration at the site of action.
Knowledge of mechanisms is required to understand and predict changes in A, B, C or D, and the work of the last 50 years has revealed many layers of knowledge. For example, Clearance = metabolism + excretion and in turn renal (one route of) excretion = glomerular filtration + net tubular transport, which, for a small-molecule cationic drug, might be predominantly determined by organic cation transporter 2 in the basolateral membrane of the renal tubules coded for by the SLC22A2 gene and inhibited by cimetidine. One of the great joys of our discipline is that there are so many layers of knowledge to discover.
ADME is an old friend that has served pharmacokinetics well, but it has quirks that sometimes make teaching and applying pharmacokinetic principles difficult. Seventy-five years after Teorell, ABCD restates the descriptors of pharmacokinetics as they were originally described. Using both ADME and ABCD allows clear separation of the molecular mechanisms and the descriptors of the active drug moiety in the body in space and time.
Acknowledgments
We thank Professor John Miners, Professor Andrew McLachlan, Professor Andrew Somogyi and Dr Ben Snyder for their thoughtful comments.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no competing interests.
Contributor Information
Matthew P. Doogue, Department of Clinical Pharmacology, Flinders Medical Centre and Flinders University School of Medicine, Finders Drive, Bedford Park, Adelaide, South Australia, 5042, Australia
Thomas M. Polasek, Flinders University School of Medicine, Adelaide, Australia
References
- Atkinson A. (2007) Drug absorption and bioavailability. In: Atkinson A., Abernethy D., Daniels C., Dedrick R., Markey S. (eds), Principles of Clinical Pharmacology. Burlington, VT: Academic Press, pp. 37–49 [Google Scholar]
- Dost F. (1953) Der Blutspiegel: Kinetic der Konzentrationsablaufe in der Krieslaujflussigkeit. Leipzig: Thieme [Google Scholar]
- Nelson E. (1961) Kinetics of drug absorption, distribution, metabolism, and excretion. J Pharm Sci 50: 181–192 [DOI] [PubMed] [Google Scholar]
- Rowland M., Tozer T. (eds) (2011) Fundamental Concepts and Terminology, in Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. Baltimore, MD: Lippincott Williams & Wilkins, pp. 17–45 [Google Scholar]
- Teorell T. (1937) Kinetics of distribution of substances administered to the body. I. The extravascular modes of administration. Arch Int Pharmacodyn Thér 57: 205–225 [Google Scholar]
- Widmark E. (1919) Studies in the concentration of indifferent narcotics in blood and tissues. Acta Med Scand 52: 87–164 [Google Scholar]