Abstract
Objective:
To describe safety and efficacy laboratory monitoring of statin therapy at the University of Colorado Hospital Outpatient Clinics over a period of 3 years prior to the revised United States Food and Drug Administration statin labeling.
Methods:
This retrospective, observational study evaluated serum laboratory monitoring for safety and efficacy of statin therapy between July 2008 and June 2011. Adult patients prescribed chronic statin therapy were included. The primary objective of this study was to describe the frequency of outpatient liver function tests, lipid panels, and creatine kinase for patients on chronic statin therapy.
Results:
A total of 143 patients met study criteria. Over a 3-year period, the mean maximum frequency of measurements per patient of serum hepatic transaminases, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was higher than lipid panel measurements (5.2 ± 4.4 and 4.2 ± 2.0 respectively, p = 0.021). Only 22 of 143 patients (15.4%) had an elevation in ALT or AST. All elevations were less than three times the upper limit of normal and statin therapy was continued without changes in response to these elevations. Creatine kinase, though not a routine monitoring test, was infrequently measured (mean maximum frequency of measurements per patient 0.3 ± 0.8).
Conclusion:
Serum hepatic transaminases were routinely monitored in patients treated with chronic statin therapy. Given the absence of significant serum hepatic transaminase elevations, and clinician response to minor elevations, our data indicate that routine serum laboratory evaluations for statin toxicity are excessive.
Keywords: drug therapy monitoring, dyslipidemia, hepatic transaminases, hydroxyl-3-methyl-glutaryl coenzyme A reductase inhibitors, statin
Introduction
Hydroxyl-3-methyl-glutaryl coenzyme A reductase inhibitors, also known as statins, are commonly prescribed for the treatment of dyslipidemia. National Cholesterol Education Panel Adult Treatment Panel (ATP) III guidelines recommend statins as a first-line therapy for most patients with dyslipidemia based on proven efficacy in lowering low-density lipoprotein cholesterol (LDL-C) and overwhelming data demonstrating reduced cardiovascular morbidity and mortality across a broad range of patients [NCEP Adult Treatment Panel III, 2002; Baigent et al. 2005; Cholesterol Treatment Trialists’ Collaborative, 2012]. Although statins have shown beneficial effects on most lipid parameters and are generally well tolerated, the concern for statin-induced hepatotoxicity has been associated with statins ever since they were first approved for use by the US Food and Drug Administration (FDA) in 1987.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are the two serum hepatic transaminases that are commonly measured together in a hepatic panel, often referred to as liver function tests (LFTs). Asymptomatic elevations in either ALT or AST values more than three times the upper limit of normal (ULN) have been reported with all statins. Elevations in these hepatic transaminases are seen in less than 1% of patients treated with statins when used within the recommended starting dose range. However, this can increase to 2–3% of patients receiving atorvastatin 80 mg or when using a statin in combination with ezetimibe [Cohen et al. 2006]. Elevations in AST or ALT more than three times the ULN are most often transient and will resolve spontaneously in about 70% of cases even if statin therapy is continued unchanged [McKenney et al. 2006]. One estimate determined the incidence rate of idiopathic acute liver failure to be 0.5–1 case per million, and the incidence rate of possible statin-induced acute liver failure to be 0.2 cases per million [Bader, 2010]. Due to the low potential for hepatotoxicity issues and lack of data to support routine monitoring, there have been changes in the recommendations of hepatotoxicity monitoring since the ATP III guidelines were published.
The ATP III recommendations for monitoring of statin therapy are as follows: check lipid panel at baseline, 6–8 weeks after starting or adjusting the medication/dose, and then every 4–6 months; check LFTs at baseline, approximately 12 weeks after starting therapy, then annually or more frequently if indicated; and check creatine kinase (CK) at baseline and if the patient reports muscle soreness, tenderness, or pain [NCEP Adult Treatment Panel III, 2002]. Several years after the ATP III guidelines were published, a Liver Expert Panel composed primarily of hepatologists evaluated the liver-associated risks of statins. This Panel reported that routine monitoring could potentially identify patients with isolated increased transaminase levels, which might lead to unnecessary discontinuation of statin therapy [Cohen et al. 2006].
In 2006, the National Lipid Association (NLA) Statin Safety Taskforce published recommendations stating that LFTs should be monitored before initiation of treatment and when clinically indicated [McKenney et al. 2006]. The NLA also provided further recommendations for statin management in response to LFT changes. The ATP III and the NLA consider a critical elevation to be an ALT or AST greater than three times the ULN and both recommend rechecking to confirm the elevation [NCEP Adult Treatment Panel III, 2002; McKenney et al. 2006]. Once confirmed, the NLA recommended clinical judgment to decide whether or not to continue, reduce the dose, or discontinue treatment altogether [McKenney et al. 2006]. Soon after, the FDA product labeling for lovastatin, simvastatin, and pravastatin was modified to recommend LFT monitoring before the initiation of treatment and when clinically indicated [Bader, 2010; Bristol-Myers Squibb, 2011; Merck & Co, 2010, 2011].
On 28 February 2012 the FDA released updated recommendations for the monitoring of LFTs [FDA, 2012], resulting in revisions of all statin labels. Revised product labeling now recommends monitoring LFTs prior to initiating statin therapy and then only when clinically indicated. The FDA concluded ‘serious liver injury with statins is rare and unpredictable in individual patients and that routine periodic monitoring of liver enzymes does not appear to be effective in detecting or preventing serious liver injury’.
The hypothesis of this study was that routine serum laboratory measurements were frequently undertaken in patients on chronic statin therapy at the University of Colorado Hospital Outpatient Clinics. The purpose of this study was to describe the frequency of safety and efficacy monitoring of statin therapy at the University of Colorado Hospital Outpatient Clinics over a period of 3 years prior to revised FDA statin labeling.
Methods
Study design
This was a retrospective, observational study evaluating safety and efficacy serum laboratory monitoring for statin therapy at the University of Colorado Hospital Outpatient Clinics between July 2008 and June 2011. Adult patients with a history of hyperlipidemia determined by International Classification of Diseases 9 (ICD-9) codes who were treated with statin therapy during this time period were identified through their electronic medical records (EPIC, Verona, WI, USA). An individual review of electronic medical records was conducted by one study investigator to assess whether patients met study inclusion or exclusion criteria. This protocol was approved by the Colorado Multiple Institutional Review Board.
Participants
Patients were included if they met study criteria. Inclusion criteria were age 18–89 years, a diagnosis of hyperlipidemia (confirmed by an ICD-9 code of 272.x), receiving primary care at a University of Colorado ambulatory care clinic, prescribed either atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, or simvastatin therapy between 1 July 2008 and 30 June 2011. Patients were excluded if they had documented active liver disease (determined by active hepatitis or hepatocellular carcinoma listed in the patient’s medical chart), physician documented nonadherence to their statin medication in the medical chart, greater than 15 months between primary care provider follow up, and the use of other medications which require routine outpatient LFT monitoring (pioglitazone, rosiglitazone, carbamazepine, felbamate, valproic acid, ethosuximide, oxcarbazepine, diclofenac, meloxicam, azathioprine, methotrexate, isoniazid, rifampin, pyrazinamide, dapsone, protease inhibitors, amiodarone, or dronedarone). These commonly used medications were determined using package inserts and a summary article listing medications requiring hepatic monitoring [Tice et al. 2001].
Objectives
The primary objective of this study was to describe the frequency of outpatient LFTs, lipid panels, and CK for patients on chronic statin therapy. The secondary objective was to describe the changes in statin therapy regimen in response to LFT elevations. LFT elevations were categorized as either less than three times the ULN or greater than three times the ULN.
Data collected
Data collected included age, sex, specific comorbidities, lipid-lowering therapy, lipid parameter monitoring, ALT monitoring, AST monitoring, and CK monitoring. The total number of medications was also collected and included chronic oral and injectable medications. If a patient was on different statins or different doses of a statin, the statin and dose the patient was on the majority of the time during the inclusion period were used. Only laboratory tests measured within the University of Colorado Hospital system were included. Lipid parameters were monitored as either individual components or as a lipid panel; data were collected from both. Because ALT and AST were measured using multiple order sets, it was identified whether they were measured within a comprehensive metabolic panel (CMP), hepatic panel, ALT alone, or AST alone. For the primary endpoint, if the numbers of measurements in a year were not equal for the components of a lipid panel or LFTs for a given patient, the maximum frequency for any single component was determined and used.
Data analyses
Descriptive statistics were used to describe data, and are presented in terms of mean (± standard deviation) for continuous variables and percentages for categorical values. χ2 statistics were used for comparisons between groups for categorical data and the t test was used for comparisons between groups for continuous data. A p value less than 0.05 was considered statistically significant.
Results
A total of 4079 patients with a diagnosis of hyperlipidemia between 1 July 2008 and 30 June 2011 were identified through electronic health records. From this sample, 500 patients were randomly selected and individually evaluated for inclusion. A total of 143 met study criteria. Baseline characteristics for the 143 patients are shown in Table 1. Simvastatin and atorvastatin were the most common statins prescribed (59.4% and 31.5% respectively). Thirty-one patients were prescribed a second lipid-lowering agent, with ezetimibe (10.5%) and fibric acid derivatives (7.7%) being the most common.
Table 1.
Baseline characteristics of the study population.
| Characteristic | Patients (n = 143) |
|---|---|
| Age, years: mean ± SD | 64 ± 10 |
| Gender: n (%) | |
| Men | 76 (52.8) |
| Women | 67 (46.9) |
| Comorbidities/past medical history: n (%) | |
| Coronary artery disease | 56 (39.2) |
| Diabetes mellitus | 56 (39.2) |
| Peripheral artery disease | 17 (11.9) |
| History of myocardial infarction | 16 (11.2) |
| History of myalgias | 10 (7.0) |
| Chronic liver disease | 2 (1.4) |
| History of rhabdomyolysis | 0 (0) |
| Statin: n (%) | |
| Simvastatin | 85 (59.4) |
| Atorvastatin | 45 (31.5) |
| Lovastatin | 6 (4.2) |
| Rosuvastatin | 4 (2.8) |
| Pravastatin | 2 (1.4) |
| Fluvastatin | 1 (0.7) |
| Additional lipid-lowering medication: n (%) | |
| Ezetimibe | 15 (10.5) |
| Gemfibrozil | 6 (4.2) |
| Fenofibrate | 5 (3.5) |
| Niacin | 3 (2.1) |
| Bile acid sequestrant | 2 (1.4) |
| Total number of medications a: mean ± SD | 7.9 ± 3.6 |
Total medications: determined at the last visit in the inclusion period. Oral and injectable chronic medications were included based on the patients’ medication list at that visit.
n, population size; SD, standard deviation.
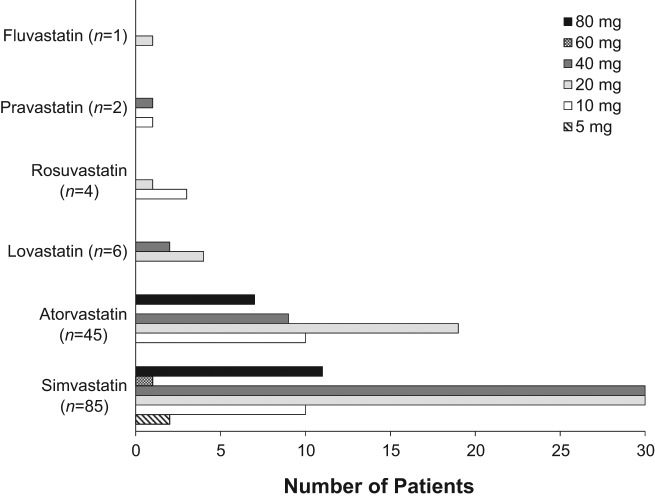
Doses of statin therapy ranged from 5 to 80 mg daily. Figure 1 displays the frequency of individual doses for each of the prescribed statins. The mean doses (± standard deviation) of simvastatin and atorvastatin were 33.6 mg (± 21.5) and 31.1 mg (± 23.5) respectively. The mean doses (± standard deviation) of the less commonly prescribed statins were rosuvastatin 12.5 mg (± 5.0), pravastatin 25 mg (± 21.2), lovastatin 26.7 mg (± 10.3), and fluvastatin 20 mg (± 0).
Figure 1.
Graph depicting the dosage range of simvastatin, atorvastatin, lovastatin, rosuvastatin, pravastatin, and fluvastatin prescribed.
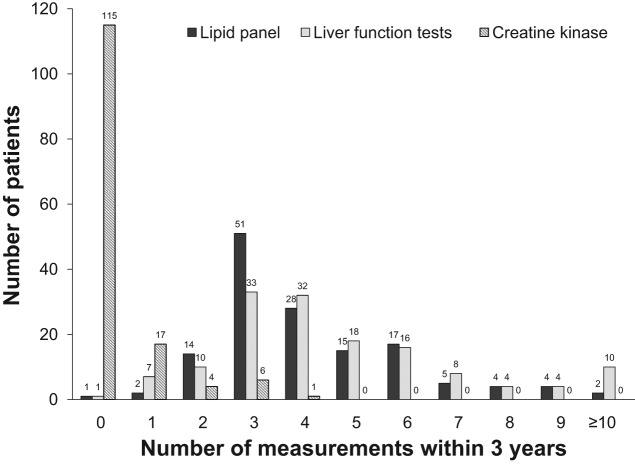
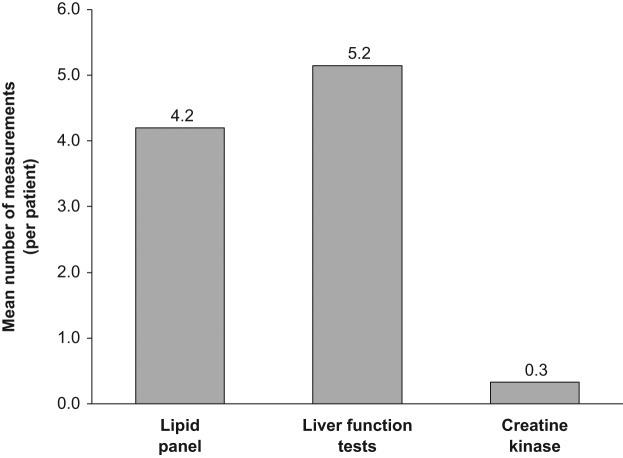
The maximum frequency of laboratory monitoring per patient was assessed for the 3-year inclusion period (see Figures 2 and 3). Using the maximum frequency, the total number of lipid tests was 601, LFT tests 736, and CK 47. Over this 3-year period, the mean per patient measurements of ALT and AST was higher than the mean per patient measurement of lipid panels (5.2 ± 4.4 and 4.2 ± 2.0 respectively, p = 0.021). LFTs were monitored through multiple order sets and the exact number of times monitored for a CMP was 592, hepatic panel 126, ALT 29, and AST 4. CK, not typically considered a routine monitoring test, was measured less frequently (mean number of measurements per patient 0.3 ± 0.8) than either of the other laboratory tests.
Figure 2.
Graph depicting the per patient maximum frequency of serum lipid panel measurements, liver function test measurements, and creatine kinase measurements over the 3-year study period.
Figure 3.
Graph depicting the mean number of serum lipid panel measurements, liver function test measurements, and creatine kinase measurements over the 3-year study period.
Only 22 of 143 patients (15.4%) had an elevation in ALT or AST (see Table 2). All of these elevations were less than three times the ULN. Statin therapy was continued without any change in response to the elevation. Seven of these patients were prescribed at least one additional lipid-lowering agent, and one patient was prescribed three lipid-lowering agents (statin with ezetimibe and fibric acid derivative). Of the 15 patients who were prescribed a statin with ezetimibe, four (26.7%) experienced an elevation in ALT or AST compared with only 18 of the 128 patients (14.1%) who received a statin but not with ezetimibe (p = 0.25). Of the 11 patients who were prescribed a statin with a fibric acid derivative, 4 (36.4%) experienced an elevation in ALT or AST compared with only 18 of the 132 patients (13.6%) who received a statin but not with a fibric acid derivative (p = 0.067).
Table 2.
The number of patients who experienced an elevation less than three times the upper limit of normal for a statin and corresponding dose.
| Lipid-lowering regimen | Statin dose (mg) |
||||
|---|---|---|---|---|---|
| 10a | 20a | 40a | 60a | 80a | |
| Atorvastatin | 1 (9) | 2 (17) | 1 (9) | – | 1 (4) |
| Atorvastatin + ezetimibe | – | – | – | – | 1 (3) |
| Lovastatin | – | 1 (4) | – | – | – |
| Rosuvastatin | 1 (3) | 1 (1) | – | – | – |
| Simvastatin | 1 (7) | 1 (22) | 3 (26) | 1(1) | 1 (6) |
| Simvastatin + ezetimibe | 1 (4) | – | – | – | 1 (3) |
| Simvastatin + fibric acid | – | 3 (6) | – | – | – |
| Simvastatin + ezetimibe + fibric acid | – | – | – | – | 1 (1) |
Number of patients with an elevation (total number of patients).
Discussion
This sample of patients on statin therapy from the University of Colorado Outpatient Clinics over the 3-year period should adequately reflect the larger patient population receiving chronic statin therapy. With respect to the new FDA recommendations released in 2012 regarding statin therapy and the changes with statin package inserts, this evaluation was warranted. Over our 3-year period of evaluation, routine serum monitoring for toxicity (i.e. ALT, AST, CK) was cumulatively more frequent than routine serum monitoring for efficacy (i.e. lipid panel monitoring). Consistent with NLA recommendations and revised product labeling, our findings further confirm that routine LFT monitoring is excessive.
Of the patients on statin therapy included in this study, only 22 of 143 patients experienced a minor elevation in LFTs over 3 years of evaluation. According to NLA recommendations, statin-treated patients with isolated asymptomatic transaminase levels between 1 and 3 times the ULN, there is no need to discontinue the statin. However, the NLA recommends repeating serum laboratory measurements to confirm the elevations along with ruling out other etiologies when an isolated asymptomatic transaminase level is found to be more than three times the ULN [McKenney et al. 2006]. The NLA also recommends that consideration should be given to continuing the statin, reducing the dose, or discontinuing the statin based on clinical judgment [McKenney et al. 2006]. This is different from the ATP III guideline which recommends stopping the statin if LFTs increase more than three times the ULN [NCEP Adult Treatment Panel III, 2002]. None of these elevations seen in our population were greater than three times the ULN, so it is not surprising and it is reassuring that none of our patients had therapy interrupted or modified in response to minor elevations.
Our low rates of LFT elevations are similar to the low incidence of hepatic enzyme elevations seen in prospective placebo-controlled trials. In the Primary Prevention of Acute Coronary Events with Lovastatin in Men and Women with Average Cholesterol Levels (AFCAPS/TexCAPS) trial, the incidence of any elevation in AST or ALT above the ULN was 1.0% and 3.3% respectively in the lovastatin group and 1.0% and 2.1% respectively in the placebo group [Downs et al. 1998]. However, the incidence of consecutive elevations more than three times the ULN for either AST or ALT was 0.6% with lovastatin and 0.3% with placebo [Downs et al. 1998]. In the Heart Protection Study (HPS), the incidence of ALT elevations two to four times the ULN was 1.35% in the simvastatin group and 1.28% in the placebo group [Heart Protection Study Collaborative Group, 2002]. Significant elevations in the HPS, defined as more than four times the ULN, were seen in 0.42% in the simvastatin group compared with 0.31% in the placebo group [Heart Protection Study Collaborative Group, 2002]. The primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) also reported a low incidence of hepatic enzyme elevations [Colhoun et al. 2004]. The incidence of at least one elevation more than three times the ULN for ALT was 1% in both the atorvastatin and placebo group and this incidence was even lower for AST elevations (0.3% with placebo, 0.4% with atorvastatin) [Colhoun et al. 2004]. In the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), elevations of ALT more than three times the ULN were similar between the rosuvastatin (0.3%) and placebo (0.2%) groups [Ridker et al. 2008]. Although we only had seven patients on atorvastatin 80 mg, which as previously mentioned can increase the risk of elevated LFTs more than three times the ULN, the risk remains low. Of these seven patients, none experienced an elevation more than three times the ULN. Two experienced an elevation less than three times the ULN, while one was also on ezetimibe in addition to atorvastatin 80 mg.
Similar to other reports, statin combination therapy appeared to result in a higher propensity for elevated LFTs. Seven patients in our study who experienced LFT elevations were treated with ezetimibe or a fibric acid derivative in combination with a statin. Both of these medication classes are known to increase LFTs when used with statin therapy. Although 15 of 143 (10.5%) patients included in the study were on statin with ezetimibe, four of the 22 (18.2%) patients who experienced elevations were prescribed statin with ezetimibe combination therapy. Fibric acid derivatives were used in 11 of 143 (7.7%) patients overall, yet 4 of the 22 (18.2%) patients who experienced elevations were prescribed stain with fibric acid derivative combination therapy. Both of these medication classes have been shown to increase the risk of elevated LFTs when added to statin therapy, although the risk is low. Two studies evaluated the incidence of elevations in ALT and AST at least three times the ULN with the combination use of ezetimibe and simvastatin [Bays et al. 2004; Goldberg et al. 2004]. The incidence of elevations in these two studies with combination treatment ranged from 1.5% to 1.7% compared with a range of 0–1.1% when evaluating incidence with placebo, ezetimibe monotherapy, or simvastatin monotherapy [Bays et al. 2004; Goldberg et al. 2004]. In the SAFARI trial, all elevations in ALT and AST more than three times the ULN were seen in the combined drug therapy, simvastatin plus fenofibrate group, compared with no reports in the simvastatin group [Grundy et al. 2005]. None of the elevations seen in our study were greater than three times the ULN. Even with the elevation, statin therapy was appropriately continued in all 22 patients.
According to the Centers for Medicare and Medicaid Services [Center for Medicare and Medicaid Services, 2012], the cost of monitoring LFTs are: ALT $7.50, AST $7.33, hepatic function test $11.57, and CMP $14.97. Since ALT, AST, and the hepatic function test offer information specifically for LFTs, these costs were used directly. Since a CMP offers more information than just LFTs, the cost difference between a CMP and a basic metabolic panel (BMP; is $9.94) was used when determining overall cost spent, which was $5.03. Using these values, the estimated total cost spent for LFT monitoring for the 143 patients in this study was roughly $4700.00 over 3 years, which is about $10.91 per person annually. When extrapolated to the larger global statin-treated patient population, the costs to routinely monitor hepatic function in statin-treated patients is staggering. It has been estimated that roughly 29.7 million patients were on statin therapy in the United States in 2005, and at that time this was expected to continue to increase [Stagnitti, 2008]. If we were to apply the average amount spent per person annually to the 29.7 million patients on statins in 2005, this would result in a national annual saving of $324 million.
Despite the routine monitoring of LFTs, the results of monitoring in our patient population did not warrant any changes in statin therapy. With statin therapy being continued there were unnecessary extra healthcare costs for patients associated with LFT monitoring. Since elevations were small and considered clinically irrelevant, the routine monitoring of LFTs in this patient population should be considered as preventable costs to the healthcare system. These data, along with the new FDA recommendations, should help minimize the concern patients or clinicians may have regarding statins and hepatotoxicity. Moreover, the costs needed to perform routine monitoring seem unjustified.
It has been estimated that it may take 5–10 years for an update to a guideline or recommendation to be implemented in physician practice [Phillips et al. 2001]. Moreover, some data show that there are delays in implementing FDA recommendations related to statin safety [Alford et al. 2012]. These delays in implementation lead to clinical inertia in healthcare for patients. As the Lipid Treatment Assessment Project (L-TAP) first showed several years ago, despite the NCEP guidelines being published prior to the survey, only 38.4% of the patients achieved their LDL-C target level [Pearson et al. 2000]. Although goal attainment rates have improved, as demonstrated in the L-TAP 2 evaluation [Waters et al. 2009], there is continued observation that doses of statins are typically not titrated up or optimized in clinical practice [Pearson et al. 2000]. Given this delay in practice, clinicians need to be reminded of new recommendations, especially shortly after a new update. Since LFT monitoring had previously been recommended for years, this new recommendation to no longer routinely monitor LFTs may take time to be implemented. Routinely checking LFTs adds no additional benefit and is no longer recommended, therefore clinicians should be reminded early on about the update to help put this change into practice. If not, LFTs may continue to be monitored and money will be spent on a practice that is not supported by clinical evidence which leads to a form of clinical inertia.
This was a retrospective study with a relatively small sample size. Since this was a retrospective medical record review, past medical history and medications prescribed were used based on the accuracy of documentation in the electronic health records (EHR). The association between LFT monitoring and statin therapy was assessed by excluding patients on other medications that have recommendations for routine LFT monitoring. However, other less commonly identified medications or clinical conditions for which LFT monitoring is needed may not have been covered in our exclusion criteria. Even with these exclusion criteria, it is possible that not all LFT monitoring parameters were directly associated with the statin therapy. It is also possible that some laboratory monitoring was not included in the EHR. Lastly, this study did not include patients who may have previously had an elevation greater than three times the ULN and statin therapy was discontinued prior to the start of our inclusion period.
Conclusion
Routine measurement of serum LFTs was more common than measurement of serum lipid panels over a 3-year period in patients on chronic statin therapy. Elevated serum LFTs were infrequent, and although not statistically significant, were more common in patients also treated with ezetimibe or a fibric acid derivative, but increases were less than three times the ULN. Statin therapy was not changed in this small subset of patients. Given the results of this descriptive analysis and the lack of evidence to support routine monitoring of LFTs in patients on statin therapy, clinicians should abandon routine LFT monitoring in statin-treated patients, which is consistent with new FDA recommendations. Repeat LFT monitoring should only be done when clinically indicated.
Acknowledgments
Dr Lowe (principle investigator) was responsible for the study design, data collection, analysis and interpretation, manuscript preparation and attaining Institutional Review Board (IRB) approval. Dr Marrs was responsible for data analysis and interpretation, and manuscript preparation. Dr Saseen was responsible for assisting with study design, data collection, analysis and interpretation, manuscript preparation, and maintaining IRB approval.
Footnotes
Funding: The authors received no financial support for the research, authorship, or publication of this article.
Conflict of interest statement: The authors have no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Contributor Information
Rachel N. Lowe, Department of Clinical and Administrative Sciences, California Northstate University College of Pharmacy, Rancho Cordova, CA, USA
Joel C. Marrs, Department of Clinical Pharmacy, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Anschutz Medical Campus, Aurora, CO, USA
Joseph J. Saseen, University of Colorado Anschutz Medical Campus (C238), Skaggs School of Pharmacy and Pharmaceutical Sciences, 12850 E. Montview Blvd, V20-2126, Aurora, CO 80045, USA
References
- Alford J., Saseen J., Allen R., Nair K. (2012) Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States food and drug administration dose restrictions. Pharmacotherapy 32: 623–630 [DOI] [PubMed] [Google Scholar]
- Bader T. (2010) The myth of statin-induced hepatotoxicity. Am J Gastroenterol 105: 978–980 [DOI] [PubMed] [Google Scholar]
- Baigent C., Keech A., Kearney P., Blackwell L., Buck G., Pollicino C., et al. (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366: 1267–1278 [DOI] [PubMed] [Google Scholar]
- Bays H., Ose L., Fraser N., Tribble D., Quinto K., Reyes R., et al. (2004) A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther 26: 1758–1773 [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb (2011) Pravachol (package insert). Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda (accessed 24 December 2012).
- Centers for Medicare and Medicaid Services (2012). Clinical Laboratory Fee Schedule. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html (accessed 26 June 2012).
- Cholesterol Treatment Trialists’ Collaborative (2012) The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380: 581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Anania F., Chalasani N. (2006) An assessment of statin safety by hepatologists. Am J Cardiol 97: 77C–81C [DOI] [PubMed] [Google Scholar]
- Colhoun H., Betteridge D., Durrington P., Hitman G., Neil H., Livingstone S., et al. (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364: 685–696 [DOI] [PubMed] [Google Scholar]
- Downs J., Clearfield M., Weis S., Whitney E., Shapiro D., Beere P., et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279: 1615–1622 [DOI] [PubMed] [Google Scholar]
- FDA (2012) FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol-lowering Statin Drugs. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm (accessed 24 December 2012).
- Goldberg A., Sapre A., Liu J., Capece R., Mitchel Y. (2004) Efficacy and safety of ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 79: 620–629 [DOI] [PubMed] [Google Scholar]
- Grundy S., Vega G., Yuan Z., Battisti W., Brady W., Palmisano J. (2005) Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am J Cardiol 95: 462–468 [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360: 7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney J., Davidson M., Jacobson T., Guyton J. (2006) Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 97: 89C–94C [DOI] [PubMed] [Google Scholar]
- Merck & Co (2010) Mevacor (package insert). Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda (accessed 24 December 2012).
- Merck & Co (2011) Zocor (package insert). Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda (accessed 24 December 2012).
- NCEP Adult Treatment Panel III (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421 [PubMed] [Google Scholar]
- Pearson T., Laurora I., Chu H., Kafonek S. (2000) The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med 160: 459–467 [DOI] [PubMed] [Google Scholar]
- Phillips L., Branch W., Cook C., Doyle J., El-Kebbi I., Gallina D., et al. (2001) Clinical inertia. Ann Intern Med 135: 825–834 [DOI] [PubMed] [Google Scholar]
- Ridker P., Danielson E., Fonseca F., Genest J., Gotto A., Kastelein J., et al. (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359: 2195–2207 [DOI] [PubMed] [Google Scholar]
- Stagnitti M. (2008) Trends in statins utilization and expenditures for the U.S. civilian noninstitutionalized population, 2000 and 2005. Statistical Brief #205. May 2008. Agency for Healthcare Research and Quality, Rockville, MD: [PubMed] [Google Scholar]
- Tice S., Parry D. (2001) Medications that require hepatic monitoring. Hosp Pharm 36: 456–464 [Google Scholar]
- Waters D., Brotons C., Chiang C., Ferrieres J., Foody J., Jukema J., et al. (2009) Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation 120: 28–34 [DOI] [PubMed] [Google Scholar]