Abstract
The thalidomide tragedy in the late 1950s and early 1960s served as a wakeup call and raised questions about the safety of medicinal products. The developed countries rose to the challenge putting in place systems to ensure the safety of medicines. However, this was not the case for low-resource settings because of prevailing factors inherent in them. This paper reviews some of these features and the current status of pharmacovigilance in Africa. The health systems in most of the 54 countries of Africa are essentially weak, lacking in basic infrastructure, personnel, equipment and facilities. The recent mass deployment of medicines to address diseases of public health significance in Africa poses additional challenges to the health system with notable safety concerns. Other safety issues of note include substandard and counterfeit medicines, medication errors and quality of medicinal products. The first national pharmacovigilance centres established in Africa with membership of the World Health Organization (WHO) international drug monitoring programme were in Morocco and South Africa in 1992. Of the 104 full member countries in the programme, there are now 24 African countries with a further nine countries as associate members. The pharmacovigilance systems operational in African countries are based essentially on spontaneous reporting facilitated by the introduction of the new tool Vigiflow. The individual case safety reports committed to the WHO global database (Vigibase) attest to the growth of pharmacovigilance in Africa with the number of reports rising from 2695 in 2000 to over 25,000 in 2010. There is need to engage the various identified challenges of the weak pharmacovigilance systems in the African setting and to focus efforts on how to provide resources, infrastructure and expertise. Raising the level of awareness among healthcare providers, developing training curricula for healthcare professionals, provisions for paediatric and geriatric pharmacovigilance, engaging the pharmaceutical industries as well as those for herbal remedies are of primary concern.
Keywords: adverse drug reactions, Africa, medicines safety, pharmacovigilance
Introduction
The intense pharmaceutical activities of the 1940s and 1950s resulted in the introduction of many medicinal products into the therapeutic setting, creating a sense of euphoria because of the availability of a panacea for all ailments. However, this euphoria was cut short by the thalidomide tragedy [McBride, 1961], which brought to the fore potential issues of medicines safety. This event was alien to the developing countries of Africa who were spared the thalidomide experience not by the presence of national systems and structures for regulating medicines but rather by the mere fact that there were simply very few medicines available because of financial and other considerations. These countries were not at all considered to be lucrative markets by the then emerging pharmaceutical industry. The developed countries rose to the challenge, putting in place systems to ensure the safety of medicines, but this was not the case for low-resource settings because of prevailing factors inherent in them. This paper reviews some of these features and the current status of pharmacovigilance in Africa
Features of the healthcare systems in Africa
Africa consists of 54 countries with about 1 billion people and an average gross national per capita income of US$1096 with a diverse proportion of the population living below the poverty line. The healthcare systems are essentially weak, lacking basic infrastructure, equipment and facilities. The number of healthcare personnel is grossly inadequate and there is a lack of the required expertise to manage prevalent health problems. For instance, a study in 12 African countries revealed that there were 0.09 physicians per 1000 population (range 0.03–0.14) and 0.55 nurses/midwives per 1000 population (range 0.03–2.01) [Kinfu et al. 2009]. The per capita expenditure on health in most sub-Saharan African countries is below US$100 compared with US$2560 in the UK and US$6719 in the USA [United Nations, 2007].
The morbidity profile is distinct from that of the developed countries of Europe and the USA, bearing a significant proportion of global infectious diseases accompanied recently by an alarming increase in noncommunicable diseases [WHO, 2008a]. This notable difference in the case mix accounts for the differential utilization of medicines which in turn influences the adverse event profiles.
Characteristics of pharmaceutical systems
The pharmaceutical system in African countries has its peculiarities and is characterized by a lack of quality medicines because of financial and physical factors. Compared with developed countries, there are few functional and reputable local pharmaceutical companies, procurement practices are inefficient, storage facilities are inadequate, and distribution networks are complex, ill defined and inefficient [Rovira, 2002, Kremer, 2002]. The pharmaceutical market is further compounded by lax border controls and customs entry points, which allow an inflow of substandard and counterfeit medicines. This is again propelled within the system by large informal marketing activities, and in the formal sector, there is misleading, inappropriate and aggressive promotion of pharmaceuticals. Interestingly, Africa’s share of the world’s pharmaceutical market as presented by the Pharmaceutical Research and Manufacturers of America is miniscule, approximating 1% [Kremer, 2002]. Between 50% and 80% of the populations of many African countries have poor access to essential medicines.
The disarray in the pharmaceutical field could easily be attributed to the weak regulatory systems, resulting in poor drug registration, products with altered manufacturing/expiry dates/batch numbers or none of these labelling indicators, and a lack of quality control facilities.
Furthermore, traditional medicines and herbal remedies are extensively used, with an estimated 80% of the population taking herbal remedies [Hussain et al. 2009]. In spite of this high patronage, it is of interest that the contents of the preparations are often not well known and in some instances consist of a cocktail of potentially harmful ingredients [Ojewole and Olusi, 1976]. The use of these herbal remedies raises questions about safety which are yet to be addressed.
Self-medication practices, with easy access to both over-the-counter (OTC) and prescription-only medicines, are highly prevalent. Potent medicines are procured from uneducated, uninformed persons who operate in the informal sector and cause untold and devastating harm, the magnitude of which is not really known. The use of ‘gift medicines’ from family relations and friends within the country or from overseas is a potential source of harmful medicines. There are anecdotal reports of large amounts of nonsteroidal anti-inflammatory medicines being given to older patients for the treatment of painful conditions. These medicines are usually self administered with no supervision until adverse events like gastrointestinal bleeding develops.
The pharmaceutical scenario is further complicated by the irrational use of medicines by both healthcare providers and consumers. Medicines are prescribed irrationally with a potential for causing harm: polypharmacy, inappropriate dosing and coprescription of combinations likely to interact are all prevalent, although their contribution to morbidity and mortality is yet to be quantified. The use of objective indicators from the World Health Organization (WHO) and the International Network for Rational Use of Drugs has highlighted the irrational use of medicines, with inappropriate prescription of antibiotics and use of injections [WHO, 2006] being among the top methods of irrational use. The lack of objective sources of information is responsible to a large extent for inappropriate prescribing because promotional literature and outdated materials are used as reference information sources [Isah and Isah,1998].
Knowledge of beneficial responses and adverse events to medicines which develop in high resource settings cannot be assumed for those in the resource poor settings of Africa. There are genetic differences in the populations and environmental and cultural dissimilarities which might impact on response to medicines [Eliasson 2006]. McDowell and colleagues systematically reviewed the literature and summarized consistent findings about ethnicity and adverse drug reactions (ADRs) to cardiovascular drugs [McDowell et al. 2006]. Among other interesting results, they found a threefold higher risk of angioedema in black people compared with non-black people who took angiotensin-converting enzyme inhibitors, as well as a doubled risk of intracranial bleeding from thrombolytic therapy. An analogous situation concerns a relatively frequent general hypersensitivity reaction to the HIV drug abacavir, for which the described risk allele, HLA-B*5701, represents a highly specific and more sensitive marker in white people than in black people [Hetherington et al. 2002]. The recent work by Parikh and colleagues highlighted the role of common polymorphisms in the cytochrome P450 CYP2C8 impairing amodiaquine metabolism and its implications for malaria treatment in Africa [Parikh et al. 2007]. This finding has been further confirmed by work from Ghana [Kudzi et al. 2009], which underscored the CYP2C8 polymorphism in the population.
Pharmacovigilance in Africa
Pharmacovigilance is defined by WHO as ‘the science and activities related to the detection, assessment, understanding and prevention of ADRs or any other drug-related problems’ [WHO, 2002].
The introduction of pharmacovigilance systems in developed countries was triggered by the thalidomide episode in the 1960s [Venulet and Helling-Borda 2010]; however, the first centre established in Africa and reporting adverse reactions to the WHO Programme for International Drug Monitoring was in 1992.
The increase in the volume of medicinal products in Africa in the past three decades has not been backed by measures to set up systems to monitor and ensure their safety. The establishment of monitoring systems (organized collection and collation of reports of adverse effects of medicines and taking necessary regulatory action) in the wake of the thalidomide tragedy led to the establishment of pharmacovigilance systems in developed countries. The formation of the WHO Programme for International Drug Monitoring, which was eventually domiciled in Uppsala (UMC), provided the much needed global database and coordination required for the protection of patients. Though most developed countries established their pharmacovigilance systems in the 1960s and 1970s, several countries in Africa are only just beginning to consider the setting up of such systems.
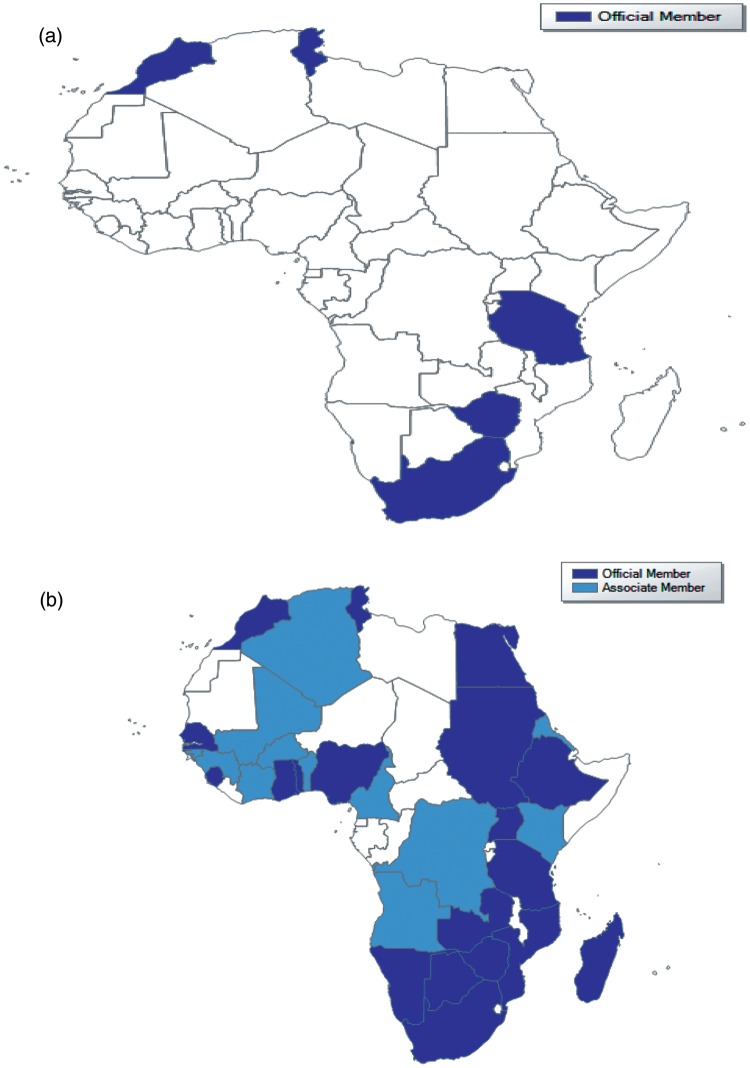
The establishment in African countries of pharmacovigilance systems (signal generation, confirmatory activities, communication and risk management) has lagged behind that of developed countries. In 2000, there were only five African countries in the WHO Programme, namely Morocco, South Africa, Tunisia, Tanzania and Zimbabwe. Since then, there has been an increase in the number of African countries joining the Programme. At the end of 2010, out of a total of 104 full member countries in the Programme, there were 24 African countries (Table 1). There are a further nine countries with associate membership (Table 2). This increase has largely been due to support from the WHO/WHO-UMC. The impact is best appreciated by the global map (Figure 1) illustrating the picture during the time periods 2000 and 2010. Financial and other logistic support from WHO and other donors [US Agency for International Development (USAID), Global Fund and other initiatives) has enabled capacity building and training of personnel in pharmacovigilance. This has been further strengthened by the setting up of Pharmacovigilance sans Frontiers (PVSF), a group of African consultants with interest in pharmacovigilance whose focus is on drug safety issues in the African setting. Again, the setting up of a WHO Collaborating Centre for Advocacy and Training in Accra, Ghana is another major step towards consolidating the initial gains made in the establishment of pharmacovigilance in Africa.
Table 1.
African member countries of the World Health Organization international drug monitoring programme (2010).
| No. | Country | Year of entry | No. of reports |
|---|---|---|---|
| 01 | Morocco | 1992 | 4504 |
| 02 | South Africa | 1992 | 11,223 |
| 03 | Tunisia | 1993 | 4112 |
| 04 | Tanzania | 1993 | 546 |
| 05 | Zimbabwe | 1998 | 161 |
| 06 | Egypt | 2001 | 20 |
| 07 | Ghana | 2001 | 804 |
| 08 | Nigeria | 2004 | 30 |
| 09 | Mozambique | 2005 | 272 |
| 10 | Togo | 2007 | 45 |
| 11 | Uganda | 2007 | 86 |
| 12 | Ethiopia | 2008 | 111 |
| 13 | Namibia | 2008 | 215 |
| 14 | Sierra Leone | 2008 | 269 |
| 15 | Sudan | 2008 | 20 |
| 16 | Botswana | 2009 | 31 |
| 17 | Madagascar | 2009 | 271 |
| 18 | Senegal | 2009 | 90 |
| 19 | Kenya | 2010 | 21 |
| 20 | Zambia | 2010 | 37 |
| 21 | Cameroon | 2010 | 23 |
| 22 | Cote D’Ivoire | 2010 | 24 |
| 23 | Dem. Rep. Congo | 2010 | 192 |
| 24 | Burkina Faso | 2010 | 19 |
Table 2.
Associate African member countries of the World Health Organization international drug monitoring programme (2010).
| Serial no. | Countries |
|---|---|
| 1. | Algeria |
| 2. | Angola |
| 3. | Benin |
| 4. | Burundi |
| 5. | Guinea |
| 6. | Guinea-Bissau |
| 7. | Mali |
| 8. | Rwanda |
| 9. | Zanzibar |
Figure 1.
Map showing the disposition of African members of the World Health Organization international drug monitoring programme during the period 2000 (a) and 2010 (b).
The individual case safety reports (ICSRs) committed to the WHO global database (Vigibase) attest to the growth of pharmacovigilance in Africa. The number of reports from the five countries who had national pharmacovigilance systems in 2000 was 2695, increasing to about 25,000 in 2010 as the number of countries increased. The contribution is still extremely small within the global database, which has over 6 million reports. The data from Africa nonetheless represent a contribution from a population previously undocumented. The uniqueness of ADRs from the African population should be appreciated and put in context. As indicated above, the initial experiences during clinical trials are to a large extent limited to developed countries and cannot be assumed for the African population. The morbidity mix in Africa is distinct from developed countries and the population is relatively young. Again, ethno-racial differences exist [McDowell et al. 2006]. These factors and the personnel profile and therapeutic traditions to a large extent determine the choice of medicines, the responses and adverse effects.
The pharmacovigilance systems operational in African countries, like those in developed countries, are based essentially on spontaneous reporting. This has been facilitated by the introduction of the new tool Vigiflow, which enables online transmission of ICSRs to the Uppsala-based database (Vigibase), obviating the need for a local database. The robustness of this tool with its other supporting tools, notably Vigisearch and Vigimine, provides much needed technological expertise to countries that otherwise would not have been able to have the appropriate tools to manage their safety data and also to take advantage of the global data available to members of the WHO Programme for International Drug Monitoring. The advantages of the spontaneous reporting system – relative ease of operation and low cost, coverage of whole patient populations and lifecycle follow up of medicines, noninterference with prescribing habits and potential to allow for follow-up studies – is also applicable in the African setting. There are however hindrances to reporting, which include mainly inability to recognize ADRs, ignorance of the reporting requirements, lack of reporting forms, feeling of guilt following the occurrence of adverse effects and fear of litigation. In addition, complementary epidemiological methods (cohort and case control studies) required for signal validation are yet to be fully developed and applied in the African setting. However, in recognition of some of its known limitations (e.g. under reporting, limited information obtainable, lack of a denominator), another method of safety surveillance –cohort event monitoring (CEM) (WHO, 2008b) – has been introduced to address them and provide prompt answers to issues of safety. The CEM, championed by Dr David Coulter, is a modified form of prescription event monitoring with essential epidemiological features (inceptional, dynamic, longitudinal, descriptive), which enables the realization of its key objectives – characterization of known reactions, detection of signals of unrecognized reactions, identification of potential interactions with other medicines, complementary and alternative medicines and foods, and identification of risk factors and estimation of risk. Other objectives include the assessment of safety in pregnancy and lactation, providing evidence for effective risk management, hypothesis generation and identification of cohorts for further studies. CEM has been applied in further characterizing adverse events following the deployment of artemisinin-based combination therapy (ACT) (artemether–lumefantrine, artesunate–amodiaquine) in Ghana, Nigeria, Tanzania and Zimbabwe and the method is being scaled up or expanded in different countries. There is an ongoing CEM programme on antiretroviral therapy in Tanzania and Zimbabwe involving over 10,000 patients. The outcome of these studies will further ensure the safe use of these medicines in Africa. The development of the electronic reporting software CemFlow by WHO-UMC has promoted the use of this method. Of note also is the use of the PaniFlow software in a few African countries to monitor the safety of vaccines introduced following the influenza H1N1 pandemic.
Other initiatives to capture and characterize adverse events following the use of medicines include pregnancy registers, which are still in their infancy, and pharmacogenetic studies, which are useful in explaining some ethnic differences in response to medicines and adverse events, and in distinguishing people with an underlying genetic disposition from those who have adverse events caused by environmental or cultural factors.
Other safety issues
The scope of pharmacovigilance continues to broaden. Some of the other components include medication errors, counterfeit and substandard medicines, lack of efficacy, drug interactions, among others.
Medication errors
Medication errors remain a significant medicine-related problem. This has been highlighted by findings in Morocco [Ali et al. 2007; Bencheikh, 2009] and is yet to be fully evaluated in the African setting. If experiences in developed countries with advanced healthcare systems are considered, it is likely that African settings are yet to uncover the magnitude of this problem. Anecdotal reports suggest a differential disclosure pattern of staff and attitude of health establishments which discourage reporting of medication errors for fear of litigation and loss of clientele.
Counterfeit and substandard products
From the late 1980s efforts have been intensified to address the high rate of circulating substandard and counterfeit medicines. Reports from most African countries, including Kenya, Nigeria and Sierra Leone, underscore the extent of this problem [WHO, 2009, 2010]. The level of ingenuity and sophistication exhibited by the perpetrators is disturbing. The packaging of the counterfeit products is nearly identical to the genuine article and almost indistinguishable. The actual counterfeit ‘medicine’ may contain dummy capsules, formulations with very low to absent active ingredients, or products with altered manufacturing/expiry dates/batch numbers (or none of these labelling indicators).
Quality issues
There have been several reports of problems arising from defective quality of medicinal products in the African setting. An example is the recurrent contamination of products due to the inadvertent use of diethylene glycol (antifreeze) as diluent. In Nigeria, contamination of paracetamol by this substance resulted in the death of over 100 children in 1989. Again, in 2009 its use in the preparation of a teething mixture, ‘My Pikin’, containing paracetamol and chlorpheniramine, resulted in the death of over 100 children with over 100 cases of acute renal failure. There is urgent need to put in place more effective and efficient quality control measures to ensure the quality of medicinal products produced by local manufacturers in the African setting.
Public health programmes
There has been a concerted effort to improve supply and access to medicines in resource poor countries in recent decades. The essential medicines programme of WHO in the early 1980s was a great leap in this direction with the establishment of revolving drug funds to ensure sustainability. Several measures, including the removal of various legal and financial barriers, have been put in place to ensure the supply of medicines. This has required the active support of governmental and nongovernmental organizations, multinationals and donor agencies.
In recent years, there has been a concerted effort to address the treatment of a number of diseases of public health significance in the African setting. These diseases include malaria, HIV/AIDs, tuberculosis, leprosy, lymphatic filariasis and helminthiasis. These diseases affect a large proportion of the population and recently have necessitated a donor-driven large-scale deployment of medicines to African countries. Some notable characteristics of the medicines used to manage these neglected tropical diseases include new (some fast-tracked registration) single entities or combinations, and old medicines which are known to be extremely toxic but for which no alternatives currently exist. Examples of the former include ACTs (artemether–lumefanthrine, artesunate– amodiaquine) and antiretroviral therapy (e.g. zidovudine, stavudine, nevirapine), whilst the latter class include medicines such as rifampicin, isoniazid and ethambutol, as well as triclabendazole, ivermectin, amphotericin B, suramin and para-aminosalicylic acid. These medicines are sometimes administered by community-level health workers in most public health programmes. A major limitation of the efforts to improve access to medicines and the rapid scale up to address the public health programmes is the lack of a monitoring programme of similar scale to address potential safety issues. This consideration is of utmost importance when factors determining response to medicines are considered.
Pharmacovigilance and the media in Africa
The media is an important organ for education of the population and dissemination of information and pharmacovigilance is one area in which the impact of media activities is most felt. There is however an acute need for an informed press so as to avoid undue sensationalism on issues relating to medicines safety, thus compromising patient care. A mass deworming exercise in Ghana was derailed by rumours, spread through the media, of deaths and serious adverse effects attributed to the medicines used for the programme [Dodoo et al. 2007]. Again, the handling of media reports in Ghana and Nigeria following the introduction of the ACTs led to apprehension and fear. On a positive note, the media kept in focus the saga of diethylene glycol contamination of paracetamol in Nigeria, educating the population and keeping them abreast of investigations.
In essence, the media should be viewed as an important stakeholder and its activities a veritable communication tool in pharmacovigilance. Therefore, media operatives should be educated in a structured manner on pharmacovigilance issues.
Challenges and the way forward
The tremendous growth in pharmacovigilance in the last decade has been associated with enormous challenges. This has been discussed in a detailed study by WHO [Olsson et al. 2010]. The pharmacovigilance systems are weak, lack resources, infrastructure and expertise. It is of utmost importance to engage governments to ensure political goodwill and commitment. Governments in the different African countries should provide the much required leadership to propel pharmacovigilance towards the desired goals of ensuring medicine safety. The provision of a policy and regulatory framework and funding will be a useful proactive step. This is necessary to ensure the sustainability of the young pharmacovigilance systems being developed. The regulatory systems whose activities should complement, synergize and relate with the pharmacovigilance systems are equally weak, lack resources and are inefficient.
The low index of suspicion, misconceptions and low level of awareness among healthcare providers is a major challenge because the identification of problems (e.g. ADRs) is an essential first step. Again, the population is naïve to ADRs and will mostly fail to relate the development of an ADR with intake of medicines, especially when they are prescribed. For most, only a beneficial outcome is expected from a medicine procured to treat an ailment. Consequently, the main thrust of activities in the nascent African setups is high-level advocacy to stakeholders – legislators, policy makers, government officials, health managers, officials in the education sector etc. – and a focused campaign to raise the level of awareness among healthcare providers and sensitization of the community. So far, efforts have been directed at the public sector. There is a need to engage private practitioners who offer services to a substantial proportion of patients. This activity should be carried out in such a manner as not to drive fear into the population with negative consequences. The development of a national policy and other legislative apparatus is an important measure to ensure sustainability of pharmacovigilance structures. Nigeria has taken the lead in preparing a policy document and the draft is awaiting adoption and ratification.
The low level of awareness of pharmacovigilance among healthcare professionals calls for its integration into undergraduate, postgraduate and professional curricula for medical, dental, pharmacy, nursing and other allied health disciplines. This early training will enable healthcare professionals to imbibe the pharmacovigilance culture earlier on in their careers. The enormous support from WHO with the establishment of the African pharmacovigilance network (Pharmacovigilance sans Frontier) will enable a realization of the awareness drive. Furthermore, the recent establishment in Accra, Ghana of the WHO Collaborating Centre for Advocacy and Training in Pharmacovigilance for national centres in Africa is a giant leap towards providing a focal point for pharmacovigilance activities in the continent. When fully functional, the centre should reach out to all African countries, despite the language barriers, and help build capacity and address the other needs of the countries with the support from the UMC in Sweden, WHO Headquarters and the WHO Regional Office for Africa. The inauguration of the African Society of Pharmacovigilance in 2009 in Rabat, Morocco has provided another platform for intellectual discourse and other activities to promote pharmacovigilance. The growing support of the Management Sciences for Health – USAID for pharmacovigilance, especially in the southern part of Africa, is also commendable.
The pharmaceutical industry also poses a major challenge to the growth of pharmacovigilance. As a major stakeholder, it should be proactive in efforts to ensure the safety of medicines and not merely opting to remain a marketing outfit as seen in African countries. It is hoped that appropriate legislation will address this aberration.
As alluded to above, the practice of traditional and complementary medicine remains ambiguous about the practitioners and the tools of the art. The toxic potential of such medicines raises significant safety concerns. There is enormous difficulty in characterizing their adverse events and engaging the practitioners who fear the undermining of their practice and possible litigation. The safe use of the medicines used widely in this sphere dictates that the practitioners be engaged in pharmacovigilance issues to enable the detection of adverse events induced by herbal remedies.
The clinical care component of pharmacovigilance is a very important consideration which is yet to be addressed. The clinical evaluation and management of patients suffering from adverse reactions in hospitals require more impetus and should be integrated into the health system and accorded the status of a clinical discipline. The establishment of pharmacovigilance (ADR) clinics would provide a forum to address the enormous neglected problem of case management.
The need to explore the areas of paediatric and geriatric pharmacovigilance cannot be overemphasized considering the fact that children and older people constitute high-risk groups. The African population is a young one and the ADRs or adverse events in this population most times are unrecognized as witnessed from the reports committed to the global database [Jasso-Gutiérrez et al. 2009]. The population of older people is growing and their medical needs are peculiar in Africa as in developed countries.
A major problem for the growth and development of pharmacovigilance is the funding arrangements – financial and logistic support [Pirmohammed et al 2007]. This should be appropriately addressed to enable the provision of the basic needs and running cost of pharmacovigilance systems. Recipient countries need support to seek assistance by way of proposals from funding agencies because studies have shown a failure to address pharmacovigilance and possible lack of this expertise to articulate the requests [Stergachis et al. 2010].
Conclusion
In all, pharmacovigilance has grown remarkably in Africa in the last decade and its importance in the healthcare system is being realized. However, notable deficiencies exist which need to be addressed to ensure sustainability of these initial efforts at promoting pharmacovigilance. The recognition of this discipline and its integration into the healthcare system is paramount
The peculiarities of pharmacovigilance problems must be appreciated and research efforts geared to identifying and solving these unique issues. Pharmacovigilance is not a luxury but a definite must.
Acknowledgments
The authors note with thanks the contribution from the staff of the WHO-UMC Uppsala and WHO Geneva as well as members of the African Pharmacovigilance network (Pharmacovigilance sans Frontier – PVSF). The support of WHO for pharmacovigilance programmes is gratefully acknowledged. In particular, the support and contributions of Lembit Rago, Mary Couper (retired), both from WHO Geneva, and the Directors of WHO-UMC Uppsala (Ralph Edwards – immediate past and Marie Lindquist – current) to the growth of pharmacovigilance in Africa are duly acknowledged. Some aspects of this paper was first presented at the World Conference of Basic and Clinical Pharmacology (WorldPharma) July, 2010 at Copenhagen, Denmark. The support of the organisers is also gratefully acknowledged.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts in preparing this article.
References
- Ali L., Benkirane R., Soulaymani R. (2007) Detecting medication errors in pharmacovigilance database: capacities and limits. Int J Risk Saf Med 19: 1–18 [Google Scholar]
- Bencheikh R.S. (2009) Medication errors: pharmacovigilance centres in detection and prevention. Br J Clin Pharmacol 67: 687–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodoo A., Adjei S., Couper M., Hugman B., Edwards R. (2007) When rumours derail a mass deworming exercise. Lancet 370: 465–466 [DOI] [PubMed] [Google Scholar]
- Eliasson E. (2006) Ethnicity and adverse drug reactions. BMJ 332: 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington S., Hughes A.R., Mosteller M., Shortino D., Baker K.L., Spreen W., et al. (2002) Genetic variations in HLA-B region and hypersensitivity to abacavir. Lancet 359: 1121–1122 [DOI] [PubMed] [Google Scholar]
- Hussain K., Majeed M.T., Ismail Z., Sadikun A., Ibrahim P. (2009) Traditional and complementary medicines: quality assessment strategies and safe usage. Southern Med Review 2: 19–23 [PMC free article] [PubMed] [Google Scholar]
- Isah A.O., Isah E.C. (1998) Drug information sources utilised by junior hospital doctors in the absence of a formal service in a developing country setting. Nat Postgrad Med J 5: 23–27 [Google Scholar]
- Jasso-Gutiérrez L., Castellanos-Solis E.C., Santos-Preciado J.I. (2009) The importance of pharmacovigilance in the pediatric population. Bol Med Hosp Infant Mex 66: 3–15 [Google Scholar]
- Kinfu Y., Dal Poz M.R., Mercer H., Evans D.B. (2009) The health worker shortage in Africa: are enough physicians and nurses being trained?. Bull World Health Organ 87: 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer M. (2002) Pharmaceuticals and the developing world. J Econ Perspect 16: 67–90 [DOI] [PubMed] [Google Scholar]
- Kudzi W., Dodoo A.N., Mills J.J. (2009) Characterisation of CYP2C8, CYP2C9 and CYP2C19 polymorphisms in a Ghanaian population. BMC Med Genet 10: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W.G. (1961) Thalidomide and congenital abnormalities. Lancet 278: 1358 [Google Scholar]
- McDowell S.E., Coleman J.J., Ferner R.E. (2006) Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 332: 1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojewole J.A., Olusi S.O. (1976) Effects of cow’s urine concoction on plasma glucose concentration in fasted rats. Trans R Soc Trop Med Hyg 71: 241–245 [PubMed] [Google Scholar]
- Olsson S., Shanthi P., Stergachis A., Couper M. (2010) Pharmacovigilance activities in 55 low – and middle- income countries. A questionnaire-based analysis. Drug Saf 33: 689–703 [DOI] [PubMed] [Google Scholar]
- Parikh S., Ouedraogo J.B., Goldstein J.A., Rosenthal P.J., Kroetz D.L. (2007) Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther 82: 197–203 [DOI] [PubMed] [Google Scholar]
- Pirmohamed M., Atuah K.N., Dodoo A.N.O., Winstanley P. (2007) Pharmacovigilance in developing countries. BMJ 335: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira J. (2002) Pharmaceuticals, globalization and developing countries: recent developments and challenges. Pharmaceut Policy Law 5: 7–10 [Google Scholar]
- Stergachis A., Bartlein R.J.K., Dodoo A., Nwokike J., Kachur P.S. (2010) A situational analysis of pharmacovigilance plans in the Global Fund Malaria and U.S. President’s Malaria Initiative proposals. Malaria J 9: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (2007) Human Development Report – Per Capita Health Expenditures by Country. www.hdr.undp.org (accessed 18 September 2011).
- Venulet J., Helling-Borda M. (2010) WHO’s international drug monitoring – the formative years, 1968–1975. Preparatory, pilot and early operational phases. Drug Saf 33: e1–e23 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2002) The Importance of Pharmacovigilance Safety Monitoring of Medicinal Products, Geneva, p. 7 [Google Scholar]
- World Health Organization. (2006) Using indicators to measure country pharmaceutical situations. Fact Book on WHO level I and level II monitoring indicators, Geneva [Google Scholar]
- World Health Organization. (2008a) The Global Burden of Disease, Geneva [Google Scholar]
- World Health Organization. (2008b) Practical Handbook on Pharmacovigilance of Antimalarial Medicines, Geneva [Google Scholar]
- World Health Organization. (2009) Pharmacovigilance sans Frontiers (PVSF) Meeting Reports on Counterfeit and Substandard Medicines, Maputo, Mozambique [Google Scholar]
- World Health Organization. (2010) Pharmacovigilance sans Frontiers (PVSF) Meeting Reports on Counterfeit and Substandard Medicines, Lome, Togo [Google Scholar]
- Yadav S. (2008) Status of adverse drug reaction monitoring and pharmacovigilance in selected countries (2008). Indian J Pharmacol 40(Suppl. 1: S4–S9 [PMC free article] [PubMed] [Google Scholar]