Abstract
Orally taken tablets in different formulations continue to have a central role in the treatment of various psychiatric and medical conditions. In order to improve compliance, reduce the frequency of taking medications and minimize the peaks and troughs associated with certain immediate-release formulations, pharmaceutical companies have developed a number of novel methods of delivering oral solid dosage medications in the form of controlled-release (CR) formulations. Some CR formulations have been associated with pharmacobezoars and false-positive findings on certain physical investigations. Though CR drugs are commonly used in psychiatry, clinicians appear to have a limited understanding of how they are released for absorption once ingested. Some have insoluble parts that are excreted in faeces as ‘ghost pills’. Due to lack of awareness of this phenomenon to both patients and the physicians, anxiety has ensued in some patients. Some clinicians have been puzzled or have been dismissive when faced with curious patients wanting to know more after they had observed tablet-like looking structures in faeces. We present two cases from our clinical setting and a few drawn from the World Wide Web to highlight the role of CR medications and their association with the ghost pill phenomenon. The mechanisms involved in drug release relevant to psychiatry medications are also briefly reviewed. The ghost pill phenomenon occurs with certain CR medications. This is a normal and expected outcome related to drug-release mechanisms of some of these products. It is inevitable that some patients will see what looks like tablets or capsules in faeces. Raising awareness of this phenomenon among clinicians would facilitate discussions and information sharing at the initial process of medication prescribing. Awareness among patients and carers would also help to allay anxiety.
Keywords: Controlled release, drug release, ghost pill, venlafaxine, Oxycontine, diffusion, osmotic release
Introduction
Just over 100 years ago, a reputed British Pharmaceutical Journal article predicted that tablets will be a thing of the past and will be replaced by something different [Patel and Patel, 2010]. Today, however, tablets in various shapes and forms remain part of our clinical practice. It is estimated that drugs taken orally constitute around 90% of all medications and the market for these drugs continues to grow [Gabor et al. 2010]. It is acknowledged that taking medications orally remains the most preferred, safest, acceptable and most economical method of drug delivery [Gabor et al. 2010; Buxton and Benet, 2011].
Taking drugs orally several times a day and over a long period of time, however, has its own challenges, especially for patients with chronic physical health and mental health problems. Medications with a short half life need to be taken frequently each day, adding a further burden, and may actually complicate medication regimes with increased risk of poor compliance in the long run [Fleischhacker et al. 2003]. Studies indicate, however, that reduced frequency of taking medication to twice a day or less has been associated with improved compliance [Claxton et al. 2001; Kardas, 2007].
Immediate-release (IR) drugs are wholly available immediately for absorption following ingestion. To maintain therapeutic plasma levels, drugs with a short half life need to be taken several times each day. Due to the nature of the pharmacokinetic profile, some medications have been associated with side effects related to high peak serum concentration or local gastrointestinal (GI) tract irritation [Tang et al. 2005]. IR formulations taken several times during the day may have several corresponding troughs of lower plasma levels, with no therapeutic benefits [Verma and Garg, 2001; Tang et al. 2005].
To address some of these problems, drug companies have developed novel methods of controlling oral solid dosage release formulations that can be taken less frequently, once or twice daily while maintaining steady therapeutic levels. Controlled release, extended release, prolonged release, slow release, sustained release, long acting and modified release and their associated abbreviations (CR, XR, PR, SR, LA and MR) are some of the terms applied to medications that have been designed such that the active drug is released slowly and steadily in a predetermined manner over a predetermined period of time [Sansom, 1999; Jayanth et al.2011]. For the purpose of this article the term controlled release and its abbreviation CR will be used throughout to encompass all the above terms. Unlike the IR formulations where the whole lot is released and is available for absorption immediately following ingestion, CR formulations are released for absorption slowly and steadily over an extended period of time. While the structure of IR tablets disintegrates soon after being ingested or once it reaches the target site, CR formulations are made such that the structure of the tablet or capsule either disintegrates slowly over a predetermined period of time or remains intact, but the active drug is available for absorption in a novel way [Gabor et al. 2010]. When the shell housing the active drug does not disintegrate or does not get digested, it is passed out in the stool intact as a ‘ghost pill’. This can be a source of anxiety provoking experience and paranoia to patients, carers and professionals alike if the phenomenon is not known and the issue not handled sensitively as the following two cases illustrate.
Case reports
Case 1
Mr X is a 65-year-old man who has been experiencing mental health problems for most of his adult life in the setting of an abusive and traumatic childhood. His main symptoms were depressive symptoms with no biological features. Other symptoms were anger problems, mood swings, dissociative episodes at times and brief psychotic-like features in the form of auditory and visual hallucinations and paranoid-like ideas. In addition, he had type II diabetes. He developed severe pain in his legs and was consulting a pain specialist. Mr X was taking a number of medications including venlafaxine XL 150 mg for at least 7 years and OxyContin (oxycodone time-release formulation; Purdue Pharma L.P., Stamford, CT, USA) 40 mg twice daily during the previous 3 years. Other medications were quetiapine XL 200 mg, pregabalin, bisoprolol, aspirin, simvastatin and metformin. His condition was reasonably managed in the community.
During his outpatient appointment he revealed that he has been passing small round tablet-like objects in his stool. He denied having diarrhoea and there were no recent changes to his medication. Mr X became more vigilant of this new phenomenon. He observed more frequently that he was passing a roundish-looking structure. He was convinced that it was the OxyContin tablet. Around the same time, he felt that the pain in his legs was increasing. He was convinced that it was because the OxyContin was not being absorbed. Mr X informed his pain specialist who also became puzzled by his experience. Care home staff were unable to help as this was something they had not encountered before.
Case 2
Mrs Y is a 69-year-old woman who presented with mixed anxiety and depressive symptoms of 8-month duration. Mrs Y was also undergoing physical investigations to establish among other things the cause of her weight loss. She was eventually diagnosed with diverticular disease; however, her anxiety and depressive symptoms persisted. At the time of referral to the secondary mental health service, Mrs Y was taking venlafaxine IR 75 mg a day. She reported some improvement in her symptoms since taking venlafaxine. Following her outpatient review, the dose was increased to 150 mg and the formulation was changed from IR, taken twice daily, to extended release (XL), taken once a day. The dose was later increased to 225 mg a day. Though Mrs Y was prescribed venlafaxine XL (Efexor XL; Pfizer, New York, NY, USA), she was being given Venlalic XL tablets (Dallas Burston Ashbourne, Market Harborough, UK) by the pharmacy. Soon after, Mrs Y started observing tablets in her faeces. She took a sample to the doctor (Figure 1). She also revealed that she had informed several other health professionals and no one seemed to believe her. She informed the reviewing doctor that she had observed tablets in her faeces for 4 days consecutively prior to her appointment. Understandably, she was anxious and concerned about her experience, and felt that she was not absorbing her antidepressant medication. The reviewing doctor was puzzled by her experience so her medication was changed to the IR formulation and the phenomenon stopped. However, her anxiety and depressive symptoms persisted, therefore she was hospitalized for further assessment for 3 weeks. During her stay, venlafaxine was changed again to the XL formulation and Mrs Y observed on one occasion a tablet-like structure in her stool. Because of this ongoing concern, the medication was changed back to the IR formulation before her discharge.
Figure 1.
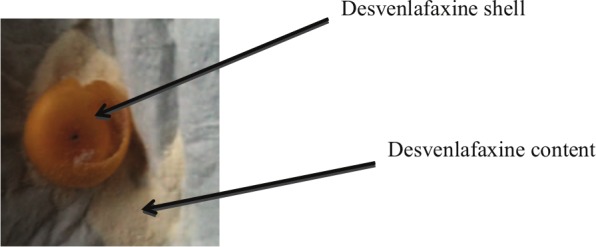
Mrs Y’s ‘ghost pill’ of Venlalic XL (venlafaxine).
Beyond our clinical settings
Concerns about passing visible parts of medications are not limited to clinical settings only; they are also discussed in Internet forums and chat rooms as the following cases demonstrate.
I have begun taking Pristiq® (desvenlafaxine tablet) for depression and have been finding the pill in my bag (Colostomy bag) next morning. When I called the doctor, she said not to worry it is just a ghost pill and that I am still getting the medicine I needed … I understand the meaning of ghost pill with a capsule but these are coated pills. I was just wondering if anyone else has had this issue (http://jpouch.org/eve/forums/a/tpc/f/9151071921/m/316103314, accessed 22 April 2012).
I am taking Wellbutrin XL® (bupropion). I started by taking 150 mg once daily for the past 2 weeks and the dose was increased to 300 mg once daily a few days ago. Here is the problem: The last 2 days I have eliminated at least one full pill twice when having a bowel movement. Does this mean I am not getting the medication into my system or what’s the deal here? (http://studenthealth.oregonstate.edu/answerspot/message.php?message=4709, accessed 16 September 2012).
I take OxyContin®, and I have some sort of stomach problem. Since last Friday … yes last Friday after only about 6 hours, I have to use the bathroom. I have loose stools and there is my pill! … I am in so much pain most of the time, due to my medication not being absorbed (http://www.healthboards.com/boards/pain-management/535207-might-well-flush-pills-they-go-there-anyway.html, accessed 30 August 2012).
Discussion
We have presented cases from a clinical setting for which the passage of the insoluble part of a tablet was a source of anxiety for two patients. The patients’ experiences were also baffling to the treating clinicians, at least initially. The cases presented highlighted that the passage of drug housing shells or the insoluble components in faeces can occur with certain CR drug formulations. The cases have also highlighted that there is limited awareness of the ‘ghost pill’ phenomenon among clinicians, patients and carers. Since the introduction of the first CR medication in the 1960s [Fyrh and Downie, 2003], more articles on CR formulations have been published; however, most are limited to drug manufacturing pharmaceutical areas and academic circles. Very little information has found its way to frontline clinicians.
Clinicians are familiar with the indications and common side effects of several CR formulations and have been prescribing some of these medications for a number of years. However, it appears that there is little awareness of how they are made and that some have insoluble components that are defecated intact and can be visible to the patient. The cases presented have demonstrated that a normal phenomenon can be a source of anxiety and mistrust if not handled well. Increased awareness, especially among prescribers, of how CR drugs differ from their IR counterparts and that some have intact shells that are passed in the faeces would help to reassure patients, allay anxieties and reduce mistrust when patients inform us of these events.
A number of technologies being used in the manufacturing of CR formulations are currently available and more novel ways of delivering oral formulations are being investigated [Gabor et al. 2010; Roger et al. 2010; Moodley et al. 2012]. Exactly how these various drugs are released in the GI system following ingestion is beyond our scope. Broadly, however, CR formulations released in the GI system use several drug-release mechanisms such as dissolution/erosion, diffusion and osmotic-controlled mechanisms, just to mention a few. In most cases, however, a combination of mechanisms is involved [Siegel and Rathbone, 2012]. Table 1 provides examples and the technologies involved in some of the commonly available CR formulations in psychiatry and a brief discussion on some of the mechanisms involved in CR medications is undertaken.
Table 1.
Some of the available controlled-release formulations and the mode of release.
| Drug | Release mode | Technology | Some indications | May have shells in stool | Manufacturing company |
|---|---|---|---|---|---|
| Desvenlafaxine Pristiq tablet |
Diffusion | Matrix tablet | Depression Social anxiety |
Yes | Wyeth Pharma (New Jersey, USA) |
| Dexmethylphenidate Focalin XR capsule |
Diffusion | SODAS (Elan Corporation - USA) | ADHD | Yes | Novartis |
| Methylphenidate Ritalin SR tablet |
Diffusion | SODAS (Elan Corporation - USA) | ADHD | Yes | Novartis (Switzerland) |
| Trazodone Oleptro |
Diffusion | Contramid (Laval, Canada) | Depression | No | Labopharm Inc. (Laval, Canada) |
| Venlafaxine capsule Effexor XR |
Diffusion | Encapsulated spheroid | Depression | Yes | Wyeth Pharma ( New Jersey, USA) |
| Bupropion tablets Wellbutrin XL |
Diffusion | Membrane-based release | Depression | Yes | GlaxoSmithKline (UK) |
| Oxycodone OxyContin |
Dissolution | Acro-Contin (Stamford, USA) | Pain relief | Yes | Purdue Pharma (Stamford, USA) |
| Oxybutynin CR Cystrin CR |
Erosion | TIMERx (USA) | Overactive bladder | No | Penwest Pharmaceuticals (Patterson, USA) |
| Quetiapine XL Seroquel |
Erosion | Matrix tablet | Schizophrenia Bipolar |
No | AstraZeneca (UK) |
| Carbamazepine Tergetol XR |
Osmotic | Zeros tablet technology (Switzerland) | Epilepsy | Yes | Novartis (Switzerland) |
| Venlafaxine extended release tablet |
Osmotic | Osmodex (North Carolina, USA) | Depression Social anxiety |
Yes | Osmotica Pharmaceutical (North Carolina, USA) |
| Methylphenidate Concerta | Osmotic | OROS (USA) | ADHD | Yes | Janssen (Belgium) |
| Paliperidone Invega tablet |
Osmotic | OROS (USA) | Schizophrenia | Yes | Janssen (Belgium) |
| Oxybutynin Ditropan |
Osmotic | OROS (USA) | Overactive bladder | Yes | UCB Pharma (Brussels, Belgium) |
ADHD, attention deficit hyperactivity disorder; CR, controlled release; SR, slow release; XL, extended release; XR, extended release.
Dissolution/erosion controlled release
Dissolution refers to a process in which drugs dissolve in the given solvent such as the GI fluids. For oral solid drug release purposes, two types of dissolution are well known: the encapsulated or reservoir, and the matrix system [Wen and Park, 2010]. In the encapsulated system, the active drug is applied on inert beads, which are in turn coated with a slow soluble polymer. The thickness and the solubility of the polymer becomes the determining factor on the rate of drug release [Sansom, 1999; Wen and Park, 2010]. The beads can be filled in a capsule or compressed in a tablet form. A mixture of immediate and delayed release beads can be incorporated together in the same tablet or capsule. The active drug becomes available for absorption once the coating polymer on the beads dissolves.
Matrix dissolution, also called erosion controlled, release is the most commonly used system [Wen and Park, 2010]. The active drug is homogenously distributed in a matrix. The rate of drug release depends on the rate of matrix erosion following ingestion [Sansom, 1999; Wen and Park, 2010, Jayanthi et al. 2011].
Two types of erosion are known, bulky or homogenous erosion and surface erosion [Sansom, 1999; Siegel and Rathbone, 2012]. Bulky erosion occurs when water enters more rapidly than the rate at which the matrix erodes away, resulting in mass loss uniformly. In surface erosion, water inversion is slower than the rate at which the matrix erodes, resulting in the matrix being slowly eroded from the surface [Sansom, 1999; Siegel and Rathbone, 2012]. Matrix and reservoir dissolution can also employ the diffusion mechanism in addition to a dissolution process. Quetiapine XL is a typical example in this case.
Diffusion controlled release
The release of the active drug follows the principle of diffusion, with the flow of a solute (active drug) going from a higher to a lower concentration (GI tract). To achieve this; the active drug is either uniformly embedded in a matrix (monolithic matrix), or is contained in a reservoir (tablet or capsule) surrounded by insoluble polymer which acts as a semipermeable membrane [Wen and Park, 2010]. Different kinds of matrixes and polymers are commercially available for the purpose of CR drug manufacturing [Uhrich et al. 1999; Gabor et al. 2010; Tu et al. 2010]. Diffusion may use swellable hydrophilic or nonswellable polymers [Gabor et al. 2010]. Swellable polymers rapidly absorb fluids and swell on coming into contact with GI fluids, producing a protective gelatinous membrane around the active drug [Gabor et al. 2010; Siegel and Rathbone, 2012]. The surrounding gelatinous layer controls the rate at which water enters its core and the amount of drug being released. In this system, both dissolution and diffusion take place [Sansom, 1999]. Drugs embedded in a matrix may diffuse through the matric pores or matrix material [Siegel and Rathbone, 2012]. This mechanism is suitable for water-soluble drugs. Drugs using this mechanism may or may not have insoluble parts passing out in faeces.
Osmotic controlled release
The osmotic drug release mechanism uses the principle of osmosis, when water movement is from a low concentration (GI tract) to a higher concentration (active drug) through a semipermeable membrane [Sanson, 1999; Conley et al. 2006; Gabor et al. 2010; Gupta et al. 2010]. The release of a drug relies on creating osmotic pressure in an enclosed rigid compartment. The rigid case is surrounded by an insoluble semipermeable membrane. Typically, the membrane allows GI fluid to enter the chambers but will not allow the drug to diffuse out. Several separate chambers can be created to house the osmotic agent and the active drug. Once the tablet is ingested, the GI fluid gradually ingresses in the chambers. Hydration and swelling of the osmotic agent creates the needed pressure to push the drug out, usually through a leachable or laser drilled hole in the membrane [Conley et al. 2006; Gabor et al. 2010]. There can be one or several orifices depending on the design.
Different types of osmotic release mechanisms exist today since the first commercial osmotic drug in 1952 [Verma and Garg, 2001; Conley et al. 2006; Gabor et al. 2010; Gupta et al. 2010]. Osmotic delivery can be simple or elementary with one chamber; however, most are complex with two or more chambers. It is estimated that 60–80% of active drug is released through this type of system [Gupta et al. 2010]. Invega® (Janssen-Cilag International NV, Belgium) (paliperidone) is a typical example in this group. Paliperidone tablets are trilayered, with the two chambers closest to the surface housing the drug and the middle-pushing layer housing the osmotic agent [Gahr et al. 2011]. However, Concerta® (Janssen-Cilag, Belgium) (methylphenidate) has both IR and CR all in one capsule, releasing its cargo in two phases. Phase one comprises IR from its outer coat. Phase two, a delayed and extended release phase, depends on an osmotic release mechanism composed of two chambers. The first chamber contains the active drug to be released while the second one houses the osmotic agent. Medications using osmotic release mechanisms have intact drug-housing components that are expelled in faeces.
The role of oxycodone (OxyContinin) and venlafaxine (Efexor XL) in passing insoluble parts in stool
OxyContin
OxyContin is the CR formulation of oxycodone hydrochloride, a semisynthetic opioid analgesic structurally similar to codeine [Anderson et al. 2002]. It uses the Acro-Contin drug-delivery system consisting of a dual-control matrix of two hydrophobic macromolecules and an acrylic polymer [Anderson et al. 2002; Nersesyan and Slavin, 2007; Purdue Pharma, 2010]. Oxycodone is released from the tablets in two phases. Phase one comprises IR from the outer layer, followed by phase two, a slow release from an insoluble wax matrix process over the next 12 hours by dissolution, leaving behind an intact, empty drug-free wax matrix referred to as a ghost pill [Anderson et al. 2002; Nersesyan and Slavin 2007; Purdue Pharma, 2010].
A postmortem study by Anderson and colleagues on 36 patients who died following drug overdoses that included OxyContin noted that 15 out of 36 had what looked like intact OxyContin tablets in the stomach [Anderson et al. 2002]. However, tests on some of them revealed that they were either empty or had very little content.
OxyContin prescribing information to clinicians states: ‘OxyContin® must be swallowed whole and must not be cut, broken, chewed, crushed or dissolved. Taking cut, broken, chewed, crushed or dissolved OxyContin® tablets leads to rapid release and absorption of a potentially fatal dose of oxycodone.’ It adds: ‘Patients should be advised that they may pass empty matrix “ghosts” (tablets) via colostomy or in the stool, and that this is of no concern since the active medication has already been absorbed.’ [Purdue Pharma, 2010]. The patient information leaflet under the heading ‘how should I take the tablet?’ states: ‘You may see tablets in your stools (bowel movements). Do not be concerned. Your body has already absorbed the medicine.’ [Purdue Pharma, 2010].
Venlafaxine hydrochloride (Efexor XL)
Originally, venlafaxine was brought to the market as a sustained-release formulation, Efexor XL by Wyeth, now a subsidiary company of Pfizer. Efexor XL uses a diffusion mechanism. The active drug is on film-coated spheroids, which are packed into capsules. On ingestion, the outer cover of the capsule completely dissolves in the gastric fluids releasing the spheroids. The spheroids are made of inert insoluble ball-like figures on which the active drug is applied. The spheroids are additionally covered with a porous insoluble polymer (ethylcellulose) that acts as a semipermeable membrane through which the drug diffuses once in contact with GI fluids (Data on file 59, Pfizer). Spheroids may be visible in the stool after the medication has been used.
Prescribing information on Efexor XL states: ‘Venlafaxine prolonged-release capsules contain spheroids, which release the active substance slowly into the digestive tract. The insoluble portion of these spheroids is eliminated and may be seen in faeces’ [Pfizer, 2011a].
The patient information leaflet states the following under the side effect section:
Do not be concerned if you see small white balls or granules in your stool after taking this medicine … as they travel through your stomach and intestines, venlafaxine is slowly released. The spheroid ‘shell’ does not dissolve and is passed out in your stools. So even though you may see spheroids in your stools, your dose of medicine has been absorbed [Pfizer, 2011b].
Several brands of venlafaxine extended-release formulations made with different technologies are available today. Wyeth also produced extended-release tablets of desvenlafaxine, an active metabolite of venlafaxine under the brand name Pristiq available in the USA and Canada. The patient information leaflet states the following under the heading ‘How should I take Pristiq®?’: ‘When you take Pristiq®, you may see something in your stool that looks like a tablet. This is the empty shell from the tablet after the medicine has been absorbed by your body’ [Pfizer, 2011c]. Mrs Y was taking Venlalic, a generic product that uses an osmotic release mechanism; hence it is expected to have an intact empty shell left behind.
Advantages and disadvantages of using controlled-release medications
Several benefits can be derived from using CR formulations. These include reduced dose frequency, and as a result, this can lead to improved compliance. Based on how they are made and released, CR formulations are less likely to be abused or misused. They have a reduced side-effect profile especially those related to rapid rise in peak serum concentration and local irritation due to a slow release or targeted nature of delivery, resulting in some cases in reduced local irritation and a steady rise in serum levels. The above could result in improved drug tolerance, reduced peak-to-trough variations and maintaining plasma levels within therapeutic ranges. CR formulations also provide increased duration of drug therapeutic effect [Wen and Park, 2010; Jayanthi et al. 2011; Moodley et al. 2012]. For pharmaceutical companies, CR formulations mean having exclusivity on the market, financial gains and in some cases drug patent life extension [Davar and Ghosh, 2010].
CR formulations, however, can be more expensive than their counterpart IR formulations. Their role in the day-to-day clinical setting remains a matter of debate given their cost. In general, CR formulations contain larger amounts of drugs than IR formulations as they are expected to last over a long period of time. If not taken correctly, they can result in serious untoward effects, including dose dumping and death [Jayanthi et al. 2011; Schier et al. 2003]. Schier and colleagues described a case of one critically ill patient with a nasogastric tube who died after two doses of crushed CR nifedipine were administered. Therefore, CR drugs should not be cut, chewed, crushed or dissolved unless the manufacturer advises [Sansom, 1999; Schier et al. 2003]. CR formulations have also been associated with the formation of pharmacobezoars; that is, obstruction in the GI tract by tablets or medication capsule components in the background of altered GI motility or anatomy. Several cases have been reported involving both CR and IR formulations. Though most cases involved physical medications, psychiatric medications such as clomipramine (Anafranil, Novartis, Switzerland), slow-release clomipramine, meprobamate and Effexor XR (Pfizer, USA) have also been reported [Simpson, 2011]. The estimated incidence of significant GI-related problems including pharmacobezoars is around 1 in 76 million tablets distributed, the majority (1 in 29 million) being in relation to taking Procardia XL (Pfizer, USA) (nifedipine slow release) [Bass et al. 2002].
Risk factors for developing pharmacobezoars include narrowing of the GI system secondary to surgical procedures, cancer, GI ulcers, or natural reduced motility related with old age and dehydration [Bass et al. 2002; Prisant and Spaulding, 2006; Simpson, 2011]. Some manufactures specifically advise not to prescribe certain CR formulations if patients have a history of GI narrowing [Prisant and Spaulding, 2006]. CR formulations can be a source of false positives on physical investigations, such as barium enema, and may appear as polyps on an endoscopy [Prisant and Spaulding, 2006]. Clinicians need to be aware and well informed of the pros and cons related to CR formulations to be able to inform patients and others and to be able to practice safely given that CR medications are frequently prescribed and newer ones will be appearing on the market.
What the British National Formulary says
In the UK, most clinicians refer to the British National Formulary (BNF) as a quick reference on all sorts of issues related to medication prescribing. It is not apparent how many clinicians read the summary of product characteristics of the medications they prescribe or the patient leaflet insert found in packages where ghost pill-like phenomenon information is sometimes mentioned. The BNF, however, remains silent on matters relating to the ghost pill, except for Concerta® (Janssen-Cilag, Belgium), for which it says under counselling: ‘the tablet membrane may pass through gastrointestinal tract unchanged’. It does not mention other drugs that may exhibit the same phenomenon. Adding this would help to inform clinicians who can then quickly consult the BNF if asked by curious patients.
Summary and conclusion
The phenomenon of passing insoluble parts that look like tablets in stools is well known in drug-manufacturing circles. However, little seems to be known among patients and clinicians and this can be a source of anxiety and mistrust. Clinicians are familiar with several areas relating to drug prescribing, such as indications and contraindications, significant drug interactions and side effects. However, clinicians need to be aware of the ghost pill phenomenon too. Highlighting this issue in the clinical setting may go a long way to allay fears and anxieties. We believe a brief mention in the BNF would help increase awareness and knowledge among clinicians.
Footnotes
Funding: No funding from any sources was obtained for the purpose of this study.
Conflict of interest statement: The authors declare no conflicts of interest.
References
- Anderson D., Fritz K., Muto J. (2002) Oxycontin: the concept of a “ghost pill” and the postmortem tissue distribution of oxycodone in 36 cases. Anal Toxicol 26:448–459 [DOI] [PubMed] [Google Scholar]
- Bass D., Prevo M., Waxman D. (2002) Gastrointestinal safety of an extended release, nondeformable, oral dosage form (OROS): a retrospective study. Drug Saf 25: 1021–1033 [DOI] [PubMed] [Google Scholar]
- Buxton I., Benet L. (2011) Pharmacokinetics: the dynamics of drug absorption, distribution, metabolism and elimination. In: Brunton L., Chabner B., Knollmann B. (eds), Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill, pp. 17–40 [Google Scholar]
- Chen X., Wen H., Park K. (2010) Challenges and new technologies of oral controlled release. In: Wen H., Park K. (eds), Oral Controlled Release Formulation Design and Drug Delivery. Theory to Practice. New York: John Wiley, p. 257–277 [Google Scholar]
- Claxton A., Cramer J., Pierce C. (2001) Systematic review of the association of dose regime and medication compliance. Clin Ther 23: 1296–1310 [DOI] [PubMed] [Google Scholar]
- Conley R., Gupta S., Sathyan G. (2006) Clinical spectrum of the osmoticcontrolled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin 22: 1879–1892 [DOI] [PubMed] [Google Scholar]
- Data on file 59, Pfizer.
- Davar N., Ghosh S. (2010) Oral controlled release-based products for life cycle management. In Wen H., Park K. (eds), Oral Controlled Release Formulation Design and Drug Delivery. Theory to Practice. New York: John Wiley, pp. 305–320 [Google Scholar]
- Fleischhacker W., Oehl M., Hummer M. (2003) Factors influencing compliance in schizophrenia patients. J Clin Psychiatry 64(Suppl. 16): 10–13 [PubMed] [Google Scholar]
- Fyhr P., Downie K. (2003) Extended release drug delivery technology. Amarin Development AB. Available at: http://www.iptonline.com/pdf_viewarticle.asp?cat=6&article=181 (accessed 16 September 2012).
- Gabor F., Fillafer C., Neutsch L., Ratzinger G., Wirth M. (2010) Improving oral delivery. Handb Exp Pharmacol 197: 345–398 [DOI] [PubMed] [Google Scholar]
- Gahr M., Kölle M., Schönfeldt-Lecuona C., Lepping P., Freudenmann R. (2011) Paliperidone extended-release: does it have a lace in antipsychotic therapy? Drug Des Devel Ther 5: 125–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B., Thakur N., Jain N., Banweer J., Jain S. (2010) Osmotically controlled drug delivery system with associated drugs. J Pharmaceut Sci 13: 571–588 [DOI] [PubMed] [Google Scholar]
- Jayanthi B., Manna P., Madhudhan S., Mohanta G., Manavalan R. (2011) Per oral extended products – an overview. J App Pharm Sci 1: 50–55 [Google Scholar]
- Kardas P. (2007) Comparison of patient compliance with once-daily and twice-daily antibiotic regimens in respiratory tract infections: results of a randomized trial. J Antimicrob Chemother 59: 531–536 [DOI] [PubMed] [Google Scholar]
- Moodley K., Pillay V., Choonara Y., du Toit L., Ndesendo V., Kumar P., et al. (2012) Oral drug delivery systems comprising altered geometric configurations for controlled drug delivery. Int J Mol Sci 13: 18–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan H., Slavin K. (2007) Current approach to cancer pain management: Availability and implications of different treatment options. Ther Clin Risk Manag 3: 381–400 [PMC free article] [PubMed] [Google Scholar]
- Patel R., Patel J. (2010) Novel technologies of oral controlled release drug delivery system. Syst Rev Pharm 1: 128–132 [Google Scholar]
- Pfizer (2011a) Venlafaxine hydrochloride capsule. Summary of the product characteristics. New York: Pfizer [Google Scholar]
- Pfizer (2011b) Efexor XL package leaflet. New York: Pfizer [Google Scholar]
- Pfizer (2011c) Pristiq extended release tablets (desvenlafaxine) medication guide. New York: Pfizer [Google Scholar]
- Prisant L., Spaulding V. (2006) Antihypertensive pharmacobezoar. J Clin Hypertens 8: 296–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue Pharma (2010) OxyContin (oxycodone hydrochloride controlled release tablets). Stamford, CT: Purdue Pharma L.P. Available at: http://www.purduepharma.com/pressroom/news/oxycontinpi.pdf (accessed 5 January 2013). [Google Scholar]
- Roger E., Lagarce F., Garcion E., Benoit J. (2010) Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine (Lond) 5: 287–306 [DOI] [PubMed] [Google Scholar]
- Sansom L. (1999) Oral extended-release products. Aust Prescr 22: 88–90 [Google Scholar]
- Schier J., Howland M., Hoffman R., Nelson L. (2003) Fatality from administration of labetalol and crushed extended-release nifedipine. Ann Pharmacother 37: 1420–1423 [DOI] [PubMed] [Google Scholar]
- Siegel R., Rathbone M. (2012) Overview of controlled release mechanisms. In: Siepmann J., Siegel R., Rathbone M. (eds), Fundamentals and Applications of Controlled Release Drug Delivery, Advances in Delivery Science and Technology. Berlin: Springer, pp. 19–46 [Google Scholar]
- Simpson S. (2011) Pharmacobezoars described and demystified. Clin Toxicol (Phila) 49: 72–89 [DOI] [PubMed] [Google Scholar]
- Tang E., S., K., Chan L., W., Heng P., W., S. (2005) Coating of Multiparticulates for Sustained Release. Amer J. Drug Delivery 3: 17–28 [Google Scholar]
- Tu J., Shen Y., Mahalingam R., Jasti B., Li X. (2010) Polymers in oral modified release system. In: Wen H., Park K. (eds), Oral Controlled Release Formulation Design and Drug Delivery. Theory to Practice. New York: John Wiley, pp. 71–88 [Google Scholar]
- Uhrich K., Cannizzaro S., Langer R., Shakesheff K. (1999) Polymeric systems for controlled drug release. Chem Rev 99: 3181–3198 [DOI] [PubMed] [Google Scholar]
- Verma R., Garg S. (2001) Current status of drug delivery technologies and future directions. Pharma Technol On-Line 25: 1–14. Available at: http://www.pharmanet.com.br/pdf/drugdelivery.pdf (accessed 20 July 2012). [Google Scholar]
- Wen H., Park K. (2010) Introduction and an overview of oral controlled release formulation design. In: Wen H., Park K. (eds), Oral Controlled Release Formulation Design and Drug Delivery. Theory to Practice, New York: John Wiley, pp. 1–19 [Google Scholar]


