Abstract
Aspirin or acetylsalicylic acid is an important therapy for many cardiology patients but hypersensitivity to this drug affects around 1% of the population and intolerance may affect up to 20%. While alternative medications to aspirin are available, in many cases there is a compelling need for aspirin therapy. In these patients, aspirin desensitization may be considered. However, this is a complex issue with a lack of international standardization. This article reviews the available evidence for aspirin desensitization and provides practical advice for the management of these patients.
Keywords: acetylsalicylic acid, allergy, aspirin, desensitization, hypersensitivity
Introduction
Aspirin is a cyclooxygenase-1 (COX-1) inhibitor that prevents platelet aggregation and is a cornerstone of treatment for patients with coronary artery disease [Davi and Patrono, 2007]. However, hypersensitivity or intolerance may restrict its use in some patients [Gollapudi et al. 2004]. Aspirin desensitization should be considered in such patients who require long-term therapy for cardiovascular indications. This is of particular importance in patients who require coronary artery stenting.
This paper will highlight the differences between aspirin intolerance and hypersensitivity before discussing the different types of hypersensitivity in detail. Subsequently, a discussion regarding the applicability and choice of a desensitization protocol will be explored for cardiac patients.
Aspirin intolerance
The National Institute for Clinical Excellence (NICE) in the UK have defined aspirin intolerance as either a proven hypersensitivity to aspirin, or a history of severe indigestion caused by low-dose aspirin [National Institute for Health and Clinical Excellence, 2005]. The prevalence of aspirin intolerance is between 6% and 20% with ‘true’ aspirin hypersensitivity occurring in 0.6–2.4% of the general population [Pfaar and Kilmek, 2006; Steg, 2005]. Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated in patients with a history of hypersensitivity including asthma, angioedema, urticaria, or rhinitis. However, in patients with a definitive need for aspirin, desensitization may offer a viable option for delivery of treatment.
Classification of aspirin hypersensitivity
Hypersensitivity reactions to aspirin have either a pharmacological or immunological basis, although patients may present with mixed reactions. Pharmacological reactions are dependent on inhibition of the COX-1 pathway while immunological/allergic reactions are mediated by drug-specific immunoglobulin E (IgE) production against aspirin [Castells, 2006]. This is the basis for the difference between an anaphylactoid and anaphylactic reaction. Anaphylactic reactions are IgE mediated whereas anaphylactoid reactions can resemble anaphylactic symptoms but are not IgE mediated. Furthermore, aspirin may induce a pharmacological reaction at one time but an immunological reaction at another time in the same patient [Silberman et al. 2005].
There are three basic clinical types of hypersensitivity reaction to aspirin: respiratory, cutaneous and systemic [Knowles et al. 2007; Gollapudi et al. 2004; Ramanuja et al. 2004]. While systemic reactions can be the most serious, respiratory and cutaneous reactions comprising urticaria and or angioedema are the most common.
Type 1: aspirin-exacerbated respiratory disease
Aspirin-exacerbated respiratory disease (AERD) consists of asthma and rhinitis/nasal polyps. AERD is also commonly referred to as aspirin-sensitive, aspirin-induced or aspirin-intolerant asthma (AIA). The prevalence of AIA is uncertain, but it has been estimated to affect about 1–20% of people with asthma [Jenkins et al. 2004; Ramanuja et al. 2004; Vally et al. 2002; Babu and Salvi, 2000]. Respiratory reactions to aspirin begin within minutes to hours after ingestion. Classic symptoms of asthma are often accompanied by rhinitis, conjunctival irritation and facial flushing. In addition, abdominal cramping may occur [Schaefer and Gore, 1999]. AERD most commonly occurs in patients between 30 and 40 years old, often after respiratory tract infection and is more common in women but is very uncommon in children [Ramanuja et al. 2004; Schaefer and Gore, 1999]. Most patients with AERD can successfully undergo aspirin desensitization therapy.
Type 2: cutaneous reactions
Aspirin-induced cutaneous disease consists of urticaria and angioedema. Cutaneous and systemic reactions to aspirin are less well characterized than AERD. Urticaria occurs either separately or simultaneously with angioedema. Patients with chronic idiopathic urticaria (CIU) are more sensitive to aspirin, urticaria being aggravated in 21–30% [Grattan, 2003; Schaefer and Gore, 1999]. When urticaria is active, patients are more likely to react to aspirin than if quiescent. Leukotriene-receptor antagonists can block NSAID-induced urticaria and angioedema reactions. Mixed reactions consisting of a combination of respiratory and cutaneous symptoms may also occur. Patients with CIU are not thought to be suitable for aspirin desensitization [Gollapudi et al. 2004].
Type 3: systemic reactions
Systemic reactions occur within minutes of ingesting aspirin and consist of hypotension, swelling, laryngeal oedema, generalized pruritis, tachypnoea and lapses in consciousness. Angioedema with hypotension is generally considered a ‘systemic’ rather than a cutaneous reaction to aspirin. Some authors report successful desensitization where systemic reactions have occurred [Castells. 2006; Silberman et al. 2005] while others do not[Gollapudi et al. 2004]. Because systemic reactions are potentially fatal, many authors recommend avoiding desensitization in these patients [Steg, 2005; Schaefer and Gore, 1999]. It should be noted that terminology regarding the type of hypersensitivity reactions is inconsistent in the published literature and standardization in this area is essential if aspirin desensitization is to be implemented safely.
Testing for hypersensitivity
There are no in vitro tests for aspirin hypersensitivity and cutaneous testing does not yield consistent results and therefore is not clinically useful [Ramanuja et al. 2004]. A clinical diagnosis, relying mostly on a careful clinical history and assessment, is key. The only definitive way to make a diagnosis is through a provocative aspirin challenge which can be done by oral, bronchial or nasal routes [Sweetman, 2009; Castells, 2006]. However, an aspirin challenge should generally not be performed purely for diagnostic purposes, especially if a systemic reaction has occurred, due to the possibility of a life-threatening reaction.
Clinical strategies for patients with aspirin hypersensitivity or intolerance
In all patients the clinical need for aspirin should be reassessed. In particular, in recent years the efficacy of aspirin in patients for the primary prevention of cardiovascular events has been questioned [Antithrombotic Trialists’ (ATT) Collaboration, 2009]. Indeed, even in patients with diabetes the use of aspirin in asymptomatic patients is no longer indicated [Scottish Intercollegiate Guidelines Network, 2010]. Furthermore, low-dose aspirin appears to be as efficacious as higher-dose aspirin [Antithrombotic Trialists’ (ATT) Collaboration, 2009; Scottish Intercollegiate Guidelines Network, 2007]; thus, if aspirin therapy is necessary then the dose should be reviewed and a low dose prescribed if appropriate, as hypersensitivity reactions may be dose dependent and patients who have not previously tolerated high doses of aspirin may be able to tolerate a low dose. This is also true of patients who have a history of aspirin-induced gastric bleeding. Those patients whose ulcers have healed and who are negative for Helicobacter pylori should be considered for treatment with a full-dose proton pump inhibitor and low-dose aspirin [National Institute for Health and Clinical Excellence, 2007]. If patients are unable to tolerate aspirin despite the above measures, then alternative antiplatelet therapies such as the thienopyridenes can be considered. However, due to the low cost of aspirin, desensitization may be a cost-effective therapeutic intervention in patients with aspirin intolerance [Shaker et al. 2008] compared with more expensive therapeutic alternatives.
While there is evidence of noninferiority with alternative drugs in many patients who require antiplatelet monotherapy [CAPRIE Steering Committee, 1996], some patients will require dual antiplatelet therapy, which includes aspirin, for example after coronary artery stenting. In these circumstances, aspirin desensitization should be considered on clinical grounds because there is limited evidence for nonaspirin-based dual antiplatelet combinations.
Desensitization
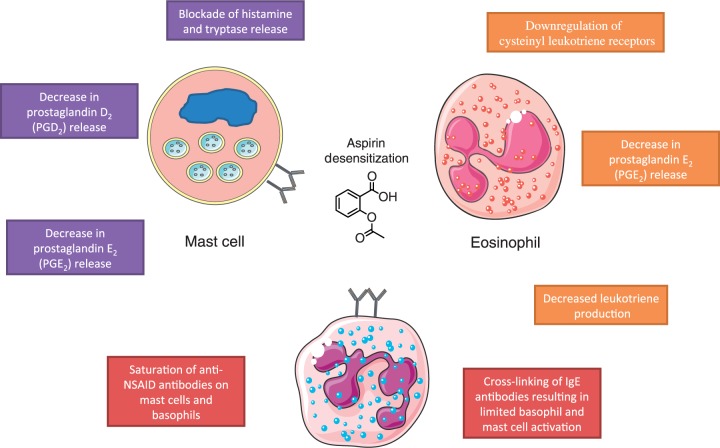
Desensitization for drug allergy has been defined as ‘the induction of temporary clinical unresponsiveness to drug antigens’ [Castells, 2006], or alternatively, ‘the elimination of pharmacological and immunological reactions by slowly increasing exposure to the drug’ [Gollapudi et al. 2004, pp.3020]. The mechanism for aspirin desensitization is not yet fully understood but it is thought that small incremental dosages decrease leukotriene production, downregulate cystienyl leukotriene receptors and decrease histamine and tryptase release from mast cells (Figure 1) [Castells, 2006; Stevenson and Szczeklik, 2006; Gollapudi et al. 2004; Ramanuja et al. 2004].
Figure 1.
Aspirin desensitization mechanism. NSAID, nonsteroidal anti-inflammatory drug.
Approach to aspirin desensitization
Aspirin desensitization has been successfully conducted in patients with aspirin-induced urticaria, angioedema, and asthma [Silberman et al. 2005] and can be effectively undertaken in the majority of patients with NSAID sensitivity, except those with CIU and systemic reactions [Gollapudi et al. 2004]. The different types of aspirin hypersensitivity reactions require different desensitization approaches. If reactions are of a mixed nature it would seem clinically prudent to use a protocol that initiated aspirin at the lower dose, due to the dose-dependent nature of the reaction.
An attempt should be made to ascertain if the mechanism of the reaction is pharmacological or immunological, before an appropriate desensitization regimen, if any, can be selected. This is often difficult as the patient’s condition may present as a mixed picture. Pharmacological reactions due to COX-1 inhibition tend to occur on first exposure to the drug and there is cross reactivity to other COX-1 inhibitory NSAIDs. In the UK, these include diclofenac, etodolac, fenoprofen, flurbiprofen, ibuprofen, indometacin, ketoprofen, mefanamic acid, nabumetone, naproxen, piroxicam, and sulindac. In these patients, aspirin desensitization should be strongly considered, even if the original causal drug was not aspirin, because the patient will cross react with aspirin due to the similar COX-1 inhibition mechanism.
Alternatively, IgE-mediated responses lack cross reactivity with other NSAIDs and there is a need for prior exposure to initiate an immune-mediated reaction. If this reaction is not systemic and the casual drug is a nonaspirin NSAID, then it is thought that treatment with aspirin can be started safely without desensitization therapy. This is possible because although antibodies against the nonaspirin NSAID may be present, specific antibodies against aspirin should not be present. Even if successful desensitization has been achieved, follow up by an allergist to perform a hypersensitivity check up and manage recurrent hypersensitivity symptoms after desensitization, which may have causes other than aspirin, is considered mandatory [Silberman et al. 2005]. However, easy access to trained allergists is not always possible in many countries, including the UK.
Desensitization protocols
Several examples of successful aspirin desensitization protocols have been published, but there does not appear to be an internationally agreed standard approach [Silberman et al. 2005; Ramanuja et al. 2004; Schaefer and Gore, 1999]. In general, patients are treated with increasing incremental doses of aspirin over set time intervals. After a positive response to aspirin and subsequent recovery, the dose at which the response occurred is repeated until no reaction occurs and the dose is increased until a maximum dose is reached. The desensitized state only exists as long as regular aspirin continues to be administered; an interruption of 1–5 days returns the patient to a desensitized state [Thomson Healthcare, 2010; Steg, 2005]. There are differences between the protocols used for patients with AERD and those with cutaneous sensitivity.
Aspirin-exacerbated respiratory disease
Aspirin challenge has been recommended for AERD that can only be controlled by unacceptably high doses of systemic corticosteroids, repeated polypectomies and/or sinus surgery, or patients requiring aspirin/NSAIDs for treatment of other diseases, for example, coronary disease, arthritis, or thromboembolism [Ramanuja et al. 2004]. A protocol has also been adapted for outpatients [Stevenson and Simon, 2006]. The starting dose to be used for the aspirin challenge should be based on the risk factors for each individual patient with AERD (Figure 2) [Hope et al. 2009].
Figure 2.
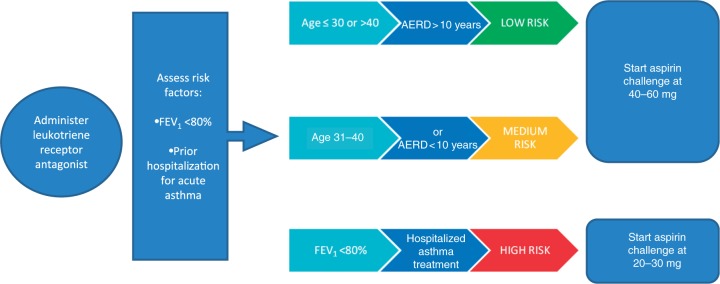
Risk stratification for aspirin challenge: for use in patients with a history of aspirin or nonsteroidal anti-inflammatory drug (NSAID) hypersensitivity-associated aspirin-exacerbated respiratory disease (AERD) [adapted from Hope et al. 2009]. FEV1, forced expiratory volume in 1 s.
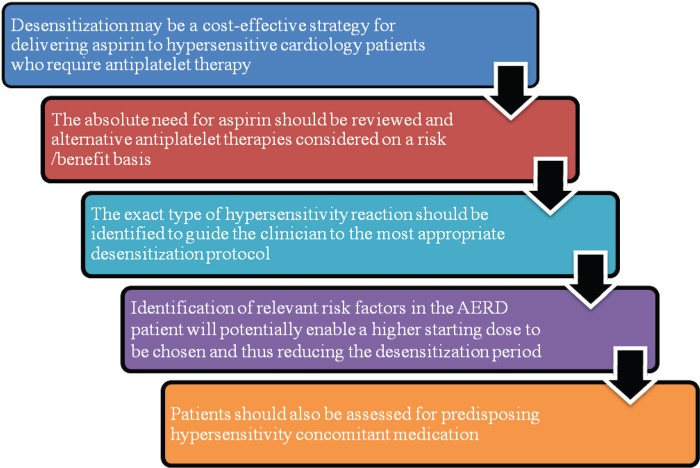
Figure 3.
Key points about aspirin desensitization. AERD, aspirin-exacerbated respiratory disease.
The use of leukotriene-modifying drugs, usually montelukast daily, for 1 week before aspirin challenge has been shown to reduce or eliminate bronchospastic reactions without blocking naso-ocular reactions. Corticosteroids do not exert a similar effect. It is currently recommended that patients continue asthma-controller medications (e.g. inhaled corticosteroids) before initiation of challenge and desensitization, but anticholinergics, antihistamines, sodium cromoglycate, and short-acting β-2 agonists be discontinued 24 h before challenge [Stevenson and Simon, 2006].
Aspirin- or NSAID-induced cutaneous reactions protocols
Wong and colleagues performed challenge-desensitization studies on 11 patients with a history of aspirin- or NSAID-induced urticaria or angioedema, nine of whom had coronary artery disease [Wong et al. 2000]. Ten patients were pretreated with antihistamines, and one was also pretreated with 60 mg prednisolone the night before and the morning of the desensitization because of a fragile clinical condition. Dosing was individualized for each patient and administered at intervals of 10–30 min, in the following increasing doses: 0.1 mg, 0.3 mg, 1 mg, 3 mg, 10 mg, 30 mg, 40 mg, 81 mg, 162 mg, and 325mg, although the latter two doses may be omitted depending on the final therapeutic dose required.
Dilutions were prepared in water for oral administration using a dispersible aspirin tablet. The desensitization for patients with cutaneous reactions is more rapid than that for patients with AERD but starts with lower doses to provide an extra margin of safety. This protocol is particularly useful for patients with unstable coronary syndrome because it can be completed within a few hours.
Schaefer and Gore have successfully used a 3-day aspirin challenge protocol. Dosing occurs at 3 h intervals: on day 1, only placebo is given; on day 2, aspirin is given in increasing doses of 30 mg, 60 mg, and 120 mg; and on day 3, aspirin is given in increasing doses of 150 mg, 325 mg, and 650 mg.
This protocol was based on the results of a previous study in patients with asthma [Stevenson, 1988] on a patient with previous myocardial infarction and coronary artery bypass grafting in whom an urticarial facial rash developed 5 years previously. It has been suggested that this protocol only be used if there is a minimum risk of a respiratory or anaphylactoid reaction [Schaefer and Gore, 1999].
Silberman and colleagues used the following protocols to successfully rapidly desensitize patients hypersensitive to aspirin allowing prolonged safe treatment with aspirin and dual antiplatelet therapy with clopidogrel when percutaneous coronary intervention and stenting were required [Silberman et al. 2005]. Starting at 1 mg and doubling each dose every 30 min with a final dose of 100 mg (therefore lasting 3.5 h), or a simplified shorter version using five sequential doses (5, 10, 20, 40, and 75 mg), with the procedure lasting 2.5 h. None of the patients received pretreatment with antihistamines or corticosteroids, and β-blockers were withheld 24 h before desensitization. Patients were monitored in the coronary care unit: blood pressure, pulse, and peak expiratory flow were measured every 30 min, and cutaneous, naso-ocular, or pulmonary reactions were monitored closely until 3 h after the procedure. All patients had imperative indications for aspirin, including previous non-ST-elevated myocardial infarction, stable or unstable angina pectoris, or ST-elevated myocardial infarction: 14 because of indications for percutaneous coronary intervention, 11 of which were urgent. In one patient, multiple drug-coated stents had been deployed 4 days before aspirin hypersensitivity symptoms developed. Fourteen out of 16 were treatment successes. No late allergic reactions or adverse cardiac events were seen after a mean follow up of 14 months.
Furthermore, these protocols allow for immediate tolerance to be achieved in more than 90% of patients, who can then safely undergo interventional procedures, including stent placement, with dual antiplatelet therapy.
For patients with aspirin-induced cutaneous disease undergoing desensitization, pretreatment with a sedating antihistamine may be required and pretreatment with prednisolone may need to be considered depending on the patient’s clinical condition. The antihistamine may need to be continued after desensitization has been performed. Specifically, if the patient has CIU, the antihistamine should not be stopped but tapered to the lowest effective dose 1–2 days before oral challenge. This is because antihistamine withdrawal causes a flare up of the urticaria that may coincide with but be unrelated to the drug challenge, thus interfering with assessment.
Cardiovascular patients
These rapid regimens are not suitable for ongoing ST segment elevation acute coronary syndrome, in which reperfusion therapy and antiplatelet agents must be administered immediately. For most other patients with coronary artery disease who require aspirin, the procedure may be attempted with a high chance of successful long-term reintroduction of aspirin.
Due to a lack of evidence, it is recommended that aspirin desensitization is not performed in patients with anaphylaxis and acute coronary syndromes. However, if it is decided that a patient with coronary syndrome and previous anaphylaxis is to be challenged, then it should be performed in a monitored environment where resuscitation facilities are available. Patients with unstable coronary syndrome should undergo coronary intervention and medical management before aspirin desensitization is considered.
Implications for patients taking β-blockers and angiotensin converting enzyme inhibitors
The use of β-blockers may increase sensitivity to allergens which may result in a more serious hypersensitivity response [Sweetman, 2009; Lang, 2008, 1995; TenBrook ret al. 2004; Toogood, 1987]. Furthermore, β-blockers may reduce the response to adrenaline, which forms part of the emergency treatment for severe hypersensitivity reactions [Sweetman, 2009; Working Group of the Resuscitation Council (UK), 2008; Lang, 1995]. Therefore, it is recommended that β-blockers should be discontinued for 24 h before a challenge or desensitization regimen is attempted [Working Group of the Resuscitation Council (UK), 2008]. However, in patients with symptomatic coronary disease this should be assessed on a case-by-case basis.
Patients with a history of angioedema unrelated to angiotensin converting enzyme (ACE) inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor and these should be used with caution or avoided in patients with a history of idiopathic or hereditary angioedema [AstraZeneca UK Ltd, 2010; Sweetman, 2009]. Patients receiving ACE inhibitors during desensitization treatment have been found to have sustained systemic reactions [AstraZeneca UK Limited, 2010; Sweetman, 2009]. In the same patients, these reactions have not been seen when ACE inhibitors were temporarily withheld but they have reappeared upon inadvertent readministration of the medicinal product [AstraZeneca UK Ltd, 2010]. It has been suggested that ACE inhibitors should be withheld prior to desensitization in order to prevent systemic reactions, although angiotensin receptor antagonists would be a reasonable alternative in most patients.
Conclusions
Desensitization should be carried out in a multidisciplinary setting where there is access to resuscitation facilities and close monitoring of the patient can be carried out. Current protocols for aspirin desensitization vary and there is currently no internationally agreed approach. The type of reaction and severity of the aspirin reaction must be fully established, as protocols for aspirin desensitization differ for patients experiencing respiratory and cutaneous reactions. Patients who have had previous systemic reactions to aspirin should not generally undergo aspirin desensitization because of the risk of a fatal reaction. Once desensitized, it is extremely important that the patients be maintained on regular aspirin therapy because a break in therapy of 1–5 days can result in the patient returning to a sensitized condition [Thomson Healthcare, 2010]. It has been suggested that aspirin desensitization is underused due to difficulty diagnosing AERD [Stevenson and Simon, 2006; Castells, 2006]. In addition, the process of diagnostic challenge and desensitization is potentially hazardous, requires specialist knowledge, and is time, staff, and space intensive. Leukotriene receptor antagonists significantly reduce the incidence of aspirin-induced bronchospastic responses and modify those that occur, which has resulted in more aspirin desensitization taking place in outpatient centres.
More studies are required to establish the risks and benefits to patients with unstable cardiovascular disease, to identify predictive risk factors for reaction severity to allow for higher starting doses, and finally for a desensitization protocol to be developed for anaphylactic patients. Furthermore, there is a need for greater international standardization of terminology and desensitization protocols.
Acknowledgments
The authors would like to thank Dr Alan Webb for assistance in the preparation of the manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest statement: The authors declare that there are no conflicts of interest
Contributor Information
Phil Lambrakis, NHS Highland, Raigmore Hospital, Inverness, UK.
Gordon F. Rushworth, Highland Clinical Research Facility, Centre for Health Science, Inverness, UK
Jane Adamson, NHS Highland, Raigmore Hospital, Inverness, UK.
Stephen J. Leslie, Consultant Cardiologist, Cardiac Unit, Raigmore Hospital, Inverness IV2 3UJ, and University of Stirling, Highland Campus, Old Perth Road, Inverness IV2 3JH, UK.
References
- Antithrombotic Trialists’ (ATT) Collaboration. (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AstraZeneca UK Ltd (2010) Zestril tablets: summary of product characteristics. Last updated 16 March 2010. Available at: www.medicines.org.uk (accessed 18 August 2011).
- Babu K.S., Salvi S.S. (2000) Aspirin and asthma. Chest 118: 1470–1476 [DOI] [PubMed] [Google Scholar]
- Castells M. (2006) Desensitisation for drug allergy. Curr Opin Allergy Clin Immunol 6: 476–481 [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee. (1996) A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348: 1329–1339 [DOI] [PubMed] [Google Scholar]
- Davi G., Patrono C. (2007) Platelet activation and atherothrombosis. N Eng J Med 357: 2482–2494 [DOI] [PubMed] [Google Scholar]
- Gollapudi R.R., Terstein P.S., Stevenson D.D., Simon R.A. (2004) ASA sensitivity implications for patients with coronary artery disease. J Am Med Assoc 292: 3017–3023 [DOI] [PubMed] [Google Scholar]
- Grattan C.E.H. (2003) Aspirin sensitivity and urticaria. Clin Exp Dermatol 28: 123–127 [DOI] [PubMed] [Google Scholar]
- Hope A.P., Woessner K.A., Simon R.A., Stevenson D.D. (2009) Rational approach to ASA dosing during oral challenges and desensitisation of patients with ASA-exacerbated respiratory disease. J Allergy Clin Immunol 123: 406–410 [DOI] [PubMed] [Google Scholar]
- Jenkins C., Costello J., Hodge L. (2004) Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ 328: 434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S.R., Drucker A.M., Weber E.A., Shear N.H. (2007) Management options for patients with aspirin and nonsteroidal anti- inflammatory drug sensitivity. Ann Pharmacother 41: 1191–1200 [DOI] [PubMed] [Google Scholar]
- Lang D.M. (1995) Anaphylactoid and anaphylactic reactions. Hazards of beta-blockers. Drug Saf 12: 299–304 [DOI] [PubMed] [Google Scholar]
- Lang D.M. (2008) Do beta-blockers really enhance the risk of anaphylaxis during immunotherapy?. Curr Allergy Asthma Rep 8: 37–44 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2005) Clopidogrel and modified release dipyridamole in the prevention of occlusive vascular events. NICE technology appraisal guidance 90. London: National Institute for Health and Clinical Excellence; Available at: www.nice.org.uk/guidance/TA90 (accessed 18 August 2011). [Google Scholar]
- National Institute for Health and Clinical Excellence (2007) MI: secondary prevention. NICE clinical guideline 48. London: National Institute for Health and Clinical Excellence; Available at: www.nice.org.uk/guidance/CG48 (accessed 18 August 2011). [Google Scholar]
- Pfaar O., Klimek L. (2006) Aspirin desensitization in aspirin intolerance: update on current standards and recent improvements. Curr Opin Allergy Clin Immunol 6: 161–166 [DOI] [PubMed] [Google Scholar]
- Ramanuja S., Breall J.A., Kalaria V.G. (2004) Approach to ‘ASA allergy’ in cardiovascular patients. Circulation 110: e1–e4 [DOI] [PubMed] [Google Scholar]
- Schaefer O.P., Gore J.M. (1999) ASA sensitivity: the role for ASA challenge and desensitisation in postmyocardial infarction patients. Cardiology 91: 8–13 [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network. (2007) Risk estimation and the prevention of cardiovascular disease, SIGN No. 97. Scottish Intercollegiate Guidelines Network: Edinburgh [Google Scholar]
- Scottish Intercollegiate Guidelines Network. (2010) Management of diabetes, SIGN No. 116. Scottish Intercollegiate Guidelines Network: Edinburgh [Google Scholar]
- Shaker M., Lobb A., Jenkins P., O’Rourke D., Takemoto S.K., Sheth S., et al. (2008) An economic analysis of ASA desensitisation in ASA-exacerbated respiratory disease. J Allergy Clin Immunol 121: 81–87 [DOI] [PubMed] [Google Scholar]
- Silberman S., Neukirch-Stoop C., Steg P.G. (2005) Rapid desensitisation procedure for patients with ASA hypersensitivity undergoing coronary stenting. Am J Cardiol 95: 509–510 [DOI] [PubMed] [Google Scholar]
- Steg P.H.G. (2005) Hypersensitivity to ASA in patients with coronary artery disease: rapid desensitisation is feasible. European Society of Cardiology Council. E-journal of Cardiology Practice 3. Available at: http://www.escardio.org/communities/councils/ccp/e-journal/volume3/Pages/vol3n37.aspx (accessed 18 August 2011).
- Stevenson D.D. (1988) Oral challenges to detect aspirin and sulphite sensitivity in asthma. N Eng Reg Allergy Proc 9: 135–145 [DOI] [PubMed] [Google Scholar]
- Stevenson D.D., Simon R.A. (2006) Selection of patients for ASA desensitisation treatment. J Allergy Clin Immunol 118: 801–804 [DOI] [PubMed] [Google Scholar]
- Stevenson D.D., Szczeklik A. (2006) Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol 118: 773–786 [DOI] [PubMed] [Google Scholar]
- Sweetman S.C. (ed.) (2009). Martindale: The Complete Drug Reference 36th edn, Pharmaceutical Press: London [Google Scholar]
- TenBrook J.A., Jr, Wolf M.P., Hoffman S.N., Rosenwasser L.J., Konstam M.A., Salem D.N., Wong J.B. (2004) Should beta-blockers be given to patients with heart disease and peanut- induced anaphylaxis? A decision analysis. J Allergy Clin Immunol 113: 977–982 [DOI] [PubMed] [Google Scholar]
- Thomson Healthcare (2010) Micromedex database: Drugdex drug evaluation. ASA 143.
- Toogood J.H. (1987) Beta-blocker therapy and the risk of anaphylaxis. Can Med Assoc J 136: 929–933 [PMC free article] [PubMed] [Google Scholar]
- Vally H., Taylor M.L., Thompson P.J. (2002) The prevalence of aspirin intolerance asthma (AIA) in Australian asthmatic patients. Thorax 57: 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.T., Nagy C.S., Krinzman S.J., MacLean J.A., Bloch K.J. (2000) Rapid oral challenge-desensitisation for patients with ASA-related urticaria-angioedema. J Allergy Clin Immunol 105: 997–1001 [DOI] [PubMed] [Google Scholar]
- Working Group of the Resuscitation Council (UK). (2008) Emergency treatment of anaphylactic reactions (guidelines for healthcare providers), Resuscitation Council (UK): London: [DOI] [PubMed] [Google Scholar]