Abstract
The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guideline E14 recommends ‘Thorough QT Study’ as a standard assessment of drug-induced QT interval prolongation. At the same time, the value of drug-induced QTc prolongation as a surrogate marker for risk of life-threatening polymorphic ventricular tachycardia known as torsades des pointes remains controversial. Beat-to-beat variability of QT interval was recently proposed as an alternative metric. The following review addresses mechanisms of beat-to-beat QT variability, methods of QT interval variability measurements, and its prognostic value in clinical studies.
Keywords: drug safety, QT interval, QT variability, ventricular tachyarrhythmias
Introduction
Prolonged corrected QT interval (QTc) is a well recognized sign of long QT syndromes, manifest by life-threatening polymorphic ventricular tachycardia known as torsades des pointes (TdP) [Moss et al. 1991]. In the 1990s, TdP cases were described in patients taking QT-prolonging drugs [DuBuske, 1999; Kamisako et al. 1995; MacConnell and Stanners, 1991; Monahan et al. 1990], resulting in important changes in regulations of new drugs approval by the United States Federal Drug Administration (FDA). Recommended by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guideline E14, the ‘Thorough QT Study’ (TQT) became a standard of assessment of QT interval prolongation due to a study drug [Rock et al. 2009]. In addition, prolonged QTc has been shown to be associated with increased risk of sudden cardiac arrest (SCA) in the general population [Straus et al. 2006; Algra et al. 1991] and in patients who have had a myocardial infarction [Schwartz and Wolf, 1978].
At the same time, the value of drug-induced QTc prolongation as a surrogate marker for risk of TdP remains controversial [Carlsson, 2008; Thomsen et al. 2006; Shah and Hondeghem, 2005; Roden, 2004]. Beat-to-beat variability of QT interval was recently proposed as an alternative metric [Hinterseer et al. 2008; Thomsen et al. 2004; Hondeghem et al. 2001]. This review addresses mechanisms of beat-to-beat QT variability, methods of QT interval variability measurements, and its prognostic value in clinical studies.
Measurement of beat-to-beat QT interval variability
Early works [Speranza et al. 1993; Nollo et al. 1992] assessed variability of repolarization by measuring standard deviation of QT, R peak to T end, and R peak to T peak intervals, and QT and RT spectra in healthy volunteers. In 1989–1993, Merri and colleagues utilized the first portion of the QT interval (ending at the T peak) to quantify repolarization, and described the relation between RR and R peak to T peak intervals (RTpeak/RR slope) in healthy volunteers and patients with long QT syndrome [Merri et al. 1993, 1992, 1989]. In 1997, Berger and colleagues proposed the beat-to-beat QT variability index (QTVI), which quantifies the magnitude of QT interval variation, normalized by both the mean QT duration and the magnitude of heart rate variation [Berger et al. 1997]. QTVI is calculated as follows:
QTv is the QT interval variance, QTm is the mean QT interval, RRv is the RR interval variance, and RRm is the mean RR interval.
Such normalization of beat-to-beat temporal lability of repolarization proved to be very useful. Importantly, the QT variability method by Berger is insensitive to possible inaccuracies in QT interval measurements because it directly measures temporal beat-to-beat lability of repolarization by stretching or compressing the JT segment of every beat in studied epoch to match template. The recommended duration of recording to determine QT variability is 256 s.
Following Berger’s approach, multiple studies over more than a decade showed the predictive value of beat-to-beat QT variability for risk stratification of SCA [Piccirillo et al. 2007; Jensen et al. 2005; Haigney et al. 2004; Atiga et al. 1998; Berger et al. 1997]. Vos and colleagues proposed to quantify a short-term variability of repolarization (STVQT), calculated on Poincaré plots of 30 consecutive QT intervals [Hinterseer et al. 2008; Oosterhoff et al. 2007; Thomsen et al. 2004] as follows (D is the QT interval):
This formula represents the average distance to the line of identity for 30 points in a Poincaré plot. The predictive value of STVQT and the value of the method for drug safety assessment has been shown in animal models [Baumert et al. 2011, 2008; Carlsson, 2008; Bilchick et al. 2004; Berger et al. 1997] and clinical studies [Cheng et al. 2009; Diaz et al. 2004; DuBuske, 1999].
Prognostic value of beat-to-beat QT interval variability
Several prospective observational studies convincingly showed the predictive value of QTVI for risk stratification of SCA. Elevated beat-to-beat QT variability predicted SCA and ventricular arrhythmia in patients with ischemic and nonischemic cardiomyopathy [Piccirillo et al. 2007; Haigney et al. 2004; Atiga et al. 1998], hypertrophic cardiomyopathy [Atiga et al. 2000], myocardial ischemia [Murabayashi et al. 2002], and long QT syndrome [Bilchick et al. 2004]. Specifically, marked elevation of QT variance, rather than a drop in heart rate variance, was responsible for increased QTVI in these conditions.
QTVI was explored in a wide range of diseases and conditions. In children with Kawasaki disease, QTVI was correlated with an inflammatory reaction (body temperature and C-reactive protein) [Kuriki et al. 2011]. Elevated QTVI was found in otherwise healthy men with spinal cord injury [La Fountaine et al. 2011], in healthy individuals with non-dipping blood pressure pattern [Myredal et al. 2010], in patients with type 1 myotonic dystrophy [Magri et al. 2010], and in patients with familial dysautonomia [Nussinovitch et al. 2010], in patients after coronary artery bypass grafting [Myredal et al. 2008], in asymptomatic patients with beta-thalassemia major [Magri et al. 2007] and end-stage renal disease [Gao et al. 2005]. Obesity was characterized with increased QTVI, whereas bariatric surgery and progressive weight loss was shown associated with improvement of QTVI [Alam et al. 2009]. QT variability was shown to correlate with the severity of obstructive sleep apnea and blood oxygenation during sleep [Baumert et al. 2008]. Increased QT variability was demonstrated in patients with schizophrenia during acute psychosis [Bar et al. 2007b], in patients with panic disorder [Yeragani et al. 2002], depression [Yeragani et al. 2000], and in acute alcohol withdrawal [Bar et al. 2007a]. Predominantly decreased heart-rate variance and out-of-proportion unchanged or mildly increased QT variance was usually observed in such cases, while a true dramatic increase in QT variance was rather infrequent.
Mechanisms of beat-to-beat QT interval variability
Increased QT variability observed in patients with heart failure has been considered a sign of an increased sympathetic tone in the ventricles of the heart. However, only recently, direct evidence of augmented sympathetic tone was obtained in an experiment in dogs implanted with a data transmitter that monitored simultaneously integrated left stellate-ganglion nervous activity, integrated vagus nerve activity, and ECG [Piccirillo et al. 2009]. In healthy dogs, QTVI correlated inversely with integrated vagus nerve activity, whereas, during heart failure QTVI correlated directly with integrated left stellate-ganglion nervous activity.
Another direct proof of correlation between sympathetic activation and QT variability was shown in the study of resting norepinephrine spillover into the coronary sinus [Baumert et al. 2011]. In patients with hypertension, QT variability and cardiac norepinephrine spillover into the coronary sinus were increased (compared with normotensive controls) and significantly correlated.
Electrical restitution, which reflects adaptation of the action potential duration to changes in cycle length, is another important mechanism of QT variability [Franz et al. 1988]. Our recent finding of increased intracardiac QT variability in patients with structural heart disease and implanted cardioverters–defibrillators confirmed that repolarization lability may be present throughout the ventricles [Tereshchenko et al. 2009b].
Modeling studies help us to understand mechanisms of QT variability on a cellular level [Pueyo et al. 2010; Romero et al. 2009]. A combination of instabilities in action potential duration restitution [Pastore et al. 1999; Nolasco and Dahlen, 1968] and intracellular calcium dynamics [Diaz et al. 2004], along with anatomical and dynamically generated instabilities as a response of a nonlinear medium to periodic excitation [Garfinkel, 2007; Echebarria and Karma, 2002], may produce both alternating [Rosenbaum et al. 1994] and nonalternating [Shusterman et al. 2006] repolarization lability. Stochastic prolongation of action potential duration may be an important mechanism of ventricular tachyarrhythmia as well [Tanskanen et al. 2005a].
Notably, mechanistic studies, elucidating mechanisms of increased QT variability, showed differences between heart failure and structural heart disease, in comparison to the healthy state. Apparently, QT variability is influenced by multiple factors that play different roles depending on the substrate, and the presence and degree of structural and electrical remodeling (Figure 1). The correlation between sympathetic tone and QT variability is very strong in the state of high sympathetic activation, but might be much weaker in other conditions when autonomic balance is unaffected. Accordingly, changes in ion-channel functions, whether inherited, or drug induced, could be the major driver of elevated QT variability in some, but not other, cases.
Figure 1.
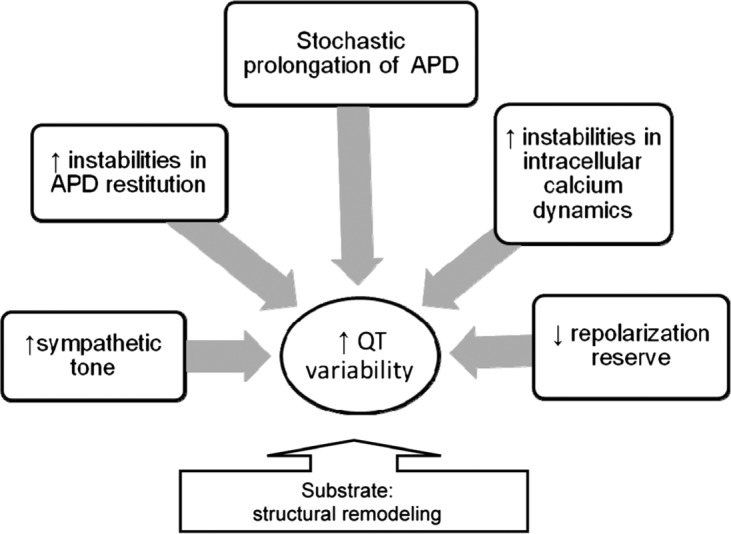
Factors influencing QT interval variability. APD, action potential duration.
Mechanisms by which elevated QT variability translates into ventricular tachyarrhythmia are less understood. It is known that early afterdepolarizations might lead to triggered activity, as well as to re-entry polymorphic ventricular tachycardia, including TdP, and ventricular fibrillation. Cardiac myocyte modeling studies [Tanskanen et al. 2005b] have shown an increased rate of early afterdepolarizations in conditions of a stochastic mechanism of L-type Ca-channel gating. We speculate that beat-to-beat changes in Ca-channel gating properties might manifest by elevated repolarization lability and result in more frequent afterdepolarizations: an important mechanism of arrhythmogenesis.
Beat-to-beat QT interval variability and drugs
Prognostic value of QT variability in patients on drugs affecting QT variability
Clinical data on the effect of drugs on QT variability are mainly limited to the effect of class III antiarrhytymic drugs (AADs). The mechanisms of action of class III AADs are complex. Amiodarone blocks rapidly and slowly activating delayed rectifier K+ currents (IKr and IKs), Na+ currents (INa), L-type Ca2+ currents (ICaL), and adrenergic receptors [Kodama et al. 1999, 1997]. Sotalol is both a β-blocker and Ikr channel blocker. Ibutilide blocks Ikr channels, but activates slow inward sodium currents [Murray, 1998].
The substrate is extremely important when considering the effect of drugs on repolarization and QT variability. Structural heart disease decreases [Maltsev et al. 2007; Maltsev and Undrovinas, 2006] the repolarization reserve [Roden, 2008a, 2008b] of the myocardium. Amiodarone-induced TdP is more common in patients with structural heart diseases [Schrickel et al. 2006a, 2006b]. Risk stratification in such patients is difficult, and usually these patients are excluded from QT variability studies [Tereshchenko et al. 2009b]. In our study, the predictive value of QTVI in multivariate analysis was shown with extended follow up [Tereshchenko et al. 2009a]. QT variability was highly predictive in patients with paroxysmal atrial fibrillation on class III AADs. Consistently, another class III AAD, ibutilide, demonstrated increased QT variability only with enriched fluctuations in heart rate [Cheng et al. 2009]. Importantly, the response to class III AADs, as well as the response to other medications, varies among individuals [Fenichel et al. 2004], which underscores the importance of efforts towards future individualized medicine.
Drug overdose is another potentially life-threatening clinical scenario, for which predictive value of QT variability might be of interest, but has not been explored. A QT-heart rate nomogram has recently been proposed to identify patients who have had a drug overdose at risk of TdP, and showed an advantage over the QTc metric [Waring et al. 2010].
QT variability as a future tool for drug safety assessment
In experiments, increased beat-to-beat variability of action potential duration was increased during exposure of hearts to cisapride, ziprasidone, quinidine and monofloxacin, but not ranolazine or Phenobarbital [Wu et al. 2004]. While previous experiments and clinical studies showed the usefulness of QT variability for quantification of risk in patients with cardiac problems, it is important to emphasize that standardized assessment of drug-induced changes in temporal QT variability was not performed. Further studies are needed before considering regulatory use of beat-to-beat QT variability because assessment of drug-induced changes in beat-to-beat QT variability currently remains a research tool only.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Berger holds a patent on the QT variability algorithm.
Contributor Information
Larisa G. Tereshchenko, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ronald D. Berger, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Carnegie 592, 600 N. Wolfe St., Baltimore, MD 21287, USA.
References
- Alam I., Lewis M.J., Lewis K.E., Stephens J.W., Baxter J.N. (2009) Influence of bariatric surgery on indices of cardiac autonomic control. Auton Neurosci 151: 168–173 [DOI] [PubMed] [Google Scholar]
- Algra A., Tijssen J.G., Roelandt J.R., Pool J., Lubsen J. (1991) QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 83: 1888–1894 [DOI] [PubMed] [Google Scholar]
- Atiga W.L., Calkins H., Lawrence J.H., Tomaselli G.F., Smith J.M., Berger R.D. (1998) Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 9: 899–908 [DOI] [PubMed] [Google Scholar]
- Atiga W.L., Fananapazir L., McAreavey D., Calkins H., Berger R.D. (2000) Temporal repolarization lability in hypertrophic cardiomyopathy caused by beta-myosin heavy-chain gene mutations. Circulation 101: 1237–1242 [DOI] [PubMed] [Google Scholar]
- Bar K.J., Boettger M.K., Koschke M., Boettger S., Groteluschen M., Voss A., et al. (2007a) Increased QT interval variability index in acute alcohol withdrawal. Drug Alcohol Depend 89: 259–266 [DOI] [PubMed] [Google Scholar]
- Bar K.J., Koschke M., Boettger M.K., Berger S., Kabisch A., Sauer H., et al. (2007b) Acute psychosis leads to increased QT variability in patients suffering from schizophrenia. Schizophr Res 95: 115–123 [DOI] [PubMed] [Google Scholar]
- Baumert M., Schlaich M.P., Nalivaiko E., Lambert E., Sari C.I., Kaye D.M., et al. (2011) Relation between QT interval variability and cardiac sympathetic activity in hypertension. Am J Physiol Heart Circ Physiol 300: H1412–H1417 [DOI] [PubMed] [Google Scholar]
- Baumert M., Smith J., Catcheside P., McEvoy R.D., Abbott D., Sanders P., et al. (2008) Variability of QT interval duration in obstructive sleep apnea: an indicator of disease severity. Sleep 31: 959–966 [PMC free article] [PubMed] [Google Scholar]
- Berger R.D., Kasper E.K., Baughman K.L., Marban E., Calkins H., Tomaselli G.F. (1997) Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 96: 1557–1565 [DOI] [PubMed] [Google Scholar]
- Bilchick K., Viitasalo M., Oikarinen L., Fetics B., Tomaselli G., Swan H., et al. (2004) Temporal repolarization lability differences among genotyped patients with the long QT syndrome. Am J Cardiol 94: 1312–1316 [DOI] [PubMed] [Google Scholar]
- Carlsson L. (2008) The anaesthetised methoxamine-sensitised rabbit model of torsades de pointes. Pharmacol Ther 119: 160–167 [DOI] [PubMed] [Google Scholar]
- Cheng A., Dalal D., Fetics B.J., Angkeow P., Spragg D.D., Calkins H., et al. (2009) Ibutilide-induced changes in the temporal lability of ventricular repolarization in patients with and without structural heart disease. J Cardiovasc Electrophysiol 20: 873–879 [DOI] [PubMed] [Google Scholar]
- Diaz M.E., O’Neill S.C., Eisner D.A. (2004) Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res 94: 650–656 [DOI] [PubMed] [Google Scholar]
- DuBuske L.M. (1999) Second-generation antihistamines: the risk of ventricular arrhythmias. Clin Ther 21: 281–295 [DOI] [PubMed] [Google Scholar]
- Echebarria B., Karma A. (2002) Instability and spatiotemporal dynamics of alternans in paced cardiac tissue. Phys Rev Lett 88: 208101. [DOI] [PubMed] [Google Scholar]
- Fenichel R.R., Malik M., Antzelevitch C., Sanguinetti M., Roden D.M., Priori S.G., et al. (2004) Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol 15: 475–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M.R., Swerdlow C.D., Liem L.B., Schaefer J. (1988) Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest 82: 972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S.A., Johansson M., Hammaren A., Nordberg M., Friberg P. (2005) Reproducibility of methods for assessing baroreflex sensitivity and temporal QT variability in end-stage renal disease and healthy subjects. Clin Auton Res 15: 21–28 [DOI] [PubMed] [Google Scholar]
- Garfinkel A. (2007) Eight (or more) kinds of alternans. J Electrocardiol 40: S70–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigney M.C., Zareba W., Gentlesk P.J., Goldstein R.E., Illovsky M., McNitt S., et al. (2004) QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol 44: 1481–1487 [DOI] [PubMed] [Google Scholar]
- Hinterseer M., Thomsen M.B., Beckmann B.M., Pfeufer A., Schimpf R., Wichmann H.E., et al. (2008) Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: a case control pilot study. Eur Heart J 29: 185–190 [DOI] [PubMed] [Google Scholar]
- Hondeghem L.M., Carlsson L., Duker G. (2001) Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation 103: 2004–2013 [DOI] [PubMed] [Google Scholar]
- Jensen B.T., Abildstrom S.Z., Larroude C.E., Agner E., Torp-Pedersen C., Nyvad O., et al. (2005) QT dynamics in risk stratification after myocardial infarction. Heart Rhythm 2: 357–364 [DOI] [PubMed] [Google Scholar]
- Kamisako T., Adachi Y., Nakagawa H., Yamamoto T. (1995) Torsades de pointes associated with terfenadine in a case of liver cirrhosis and hepatocellular carcinoma. Intern Med 34: 92–95 [DOI] [PubMed] [Google Scholar]
- Kodama I., Kamiya K., Toyama J. (1997) Cellular electropharmacology of amiodarone. Cardiovasc Res 35: 13–29 [DOI] [PubMed] [Google Scholar]
- Kodama I., Kamiya K., Toyama J. (1999) Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol 84: 20R–28R [DOI] [PubMed] [Google Scholar]
- Kuriki M., Fujino M., Tanaka K.I., Horio K., Kusuki H., Hosoi M., et al. (2011) Ventricular repolarization lability in children with Kawasaki dDisease. Pediatr Cardiol 32: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fountaine M.F., Wecht J.M., Rosado-Rivera D., Cirnigliaro C.M., Spungen A.M., Bauman W.A. (2011) The QT Variability Index and cardiac autonomic modulation: perspectives from apparently healthy men with spinal cord injury. Cardiology 117: 253–259 [DOI] [PubMed] [Google Scholar]
- MacConnell T.J., Stanners A.J. (1991) Torsades de pointes complicating treatment with terfenadine. BMJ 302: 1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri D., Piccirillo G., Bucci E., Pignatelli G., Cauti F.M., Morino S., et al. (2010) Increased temporal dispersion of myocardial repolarization in myotonic dystrophy type 1 beyond the cardiac conduction system. Int J Cardiol 26 November [Epubahead of print]. [DOI] [PubMed] [Google Scholar]
- Magri D., Sciomer S., Fedele F., Gualdi G., Casciani E., Pugliese P., et al. (2007) Increased QT variability in young asymptomatic patients with beta-thalassemia major. Eur J Haematol 79: 322–329 [DOI] [PubMed] [Google Scholar]
- Maltsev V.A., Silverman N., Sabbah H.N., Undrovinas A.I. (2007) Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 9: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev V.A., Undrovinas A.I. (2006) A multi-modal composition of the late Na+ current in human ventricular cardiomyocytes. Cardiovasc Res 69: 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merri M., Alberti M., Moss A.J. (1993) Dynamic analysis of ventricular repolarization duration from 24-hour Holter recordings. IEEE Trans Biomed Eng 40: 1219–1225 [DOI] [PubMed] [Google Scholar]
- Merri M., Benhorin J., Alberti M., Locati E., Moss A.J. (1989) Electrocardiographic quantitation of ventricular repolarization. Circulation 80: 1301–1308 [DOI] [PubMed] [Google Scholar]
- Merri M., Moss A.J., Benhorin J., Locati E.H., Alberti M., Badilini F. (1992) Relation between ventricular repolarization duration and cardiac cycle length during 24-hour Holter recordings. Findings in normal patients and patients with long QT syndrome. Circulation 85: 1816–1821 [DOI] [PubMed] [Google Scholar]
- Monahan B.P., Ferguson C.L., Killeavy E.S., Lloyd B.K., Troy J., Cantilena L.R., Jr (1990) Torsades de pointes occurring in association with terfenadine use. JAMA 264: 2788–2790 [PubMed] [Google Scholar]
- Moss A.J., Schwartz P.J., Crampton R.S., Tzivoni D., Locati E.H., MacCluer J., et al. (1991) The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 84: 1136–1144 [DOI] [PubMed] [Google Scholar]
- Murabayashi T., Fetics B., Kass D., Nevo E., Gramatikov B., Berger R.D. (2002) Beat-to-beat QT interval variability associated with acute myocardial ischemia. J Electrocardiol 35: 19–25 [DOI] [PubMed] [Google Scholar]
- Murray K.T. (1998) Ibutilide. Circulation 97: 493–497 [DOI] [PubMed] [Google Scholar]
- Myredal A., Friberg P., Johansson M. (2010) Elevated myocardial repolarization lability and arterial baroreflex dysfunction in healthy individuals with nondipping blood pressure pattern. Am J Hypertens 23: 255–259 [DOI] [PubMed] [Google Scholar]
- Myredal A., Karlsson A.K., Johansson M. (2008) Elevated temporal lability of myocardial repolarization after coronary artery bypass grafting. J Electrocardiol 41: 698–702 [DOI] [PubMed] [Google Scholar]
- Nolasco J.B., Dahlen R.W. (1968) A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol 25: 191–196 [DOI] [PubMed] [Google Scholar]
- Nollo G., Speranza G., Grasso R., Bonamini R., Mangiardi L., Antolini R. (1992) Spontaneous beat-to-beat variability of the ventricular repolarization duration. J Electrocardiol 25: 9–17 [DOI] [PubMed] [Google Scholar]
- Nussinovitch U., Katz U., Nussinovitch M., Nussinovitch N. (2010) Beat-to-beat QT interval dynamics and variability in familial dysautonomia. Pediatr Cardiol 31: 80–84 [DOI] [PubMed] [Google Scholar]
- Oosterhoff P., Oros A., Vos M.A. (2007) Beat-to-beat variability of repolarization: a new parameter to determine arrhythmic risk of an individual or identify proarrhythmic drugs. Anadolu Kardiyol Derg 7(Suppl. 1): 73–78 [PubMed] [Google Scholar]
- Pastore J.M., Girouard S.D., Laurita K.R., Akar F.G., Rosenbaum D.S. (1999) Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394 [DOI] [PubMed] [Google Scholar]
- Piccirillo G., Magri D., Matera S., Magnanti M., Torrini A., Pasquazzi E., et al. (2007) QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J 28: 1344–1350 [DOI] [PubMed] [Google Scholar]
- Piccirillo G., Magri D., Ogawa M., Song J., Chong V.J., Han S., et al. (2009) Autonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogs. J Am Coll Cardiol 54: 840–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo E., Husti Z., Hornyik T., Baczko I., Laguna P., Varro A., et al. (2010) Mechanisms of ventricular rate adaptation as a predictor of arrhythmic risk. Am J Physiol Heart Circ Physiol 298: H1577–H1587 [DOI] [PubMed] [Google Scholar]
- Rock E.P., Finkle J., Fingert H.J., Booth B.P., Garnett C.E., Grant S., et al. (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157: 827–836 [DOI] [PubMed] [Google Scholar]
- Roden D.M. (2004) Drug-induced prolongation of the QT interval. N Engl J Med 350: 1013–1022 [DOI] [PubMed] [Google Scholar]
- Roden D.M. (2008a) Cellular basis of drug-induced torsades de pointes. Br J Pharmacol 154: 1502–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden D.M. (2008b) Repolarization reserve: a moving target. Circulation 118: 981–982 [DOI] [PubMed] [Google Scholar]
- Romero L., Pueyo E., Fink M., Rodriguez B. (2009) Impact of ionic current variability on human ventricular cellular electrophysiology. Am J Physiol Heart Circ Physiol 297: H1436–H1445 [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.S., Jackson L.E., Smith J.M., Garan H., Ruskin J.N., Cohen R.J. (1994) Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 330: 235–241 [DOI] [PubMed] [Google Scholar]
- Schrickel J.W., Schwab J.O., Yang A., Bielik H., Bitzen A., Luderitz B., et al. (2006a) Pro-arrhythmic effects of amiodarone and concomitant rate-control medication. Europace 8: 403–407 [DOI] [PubMed] [Google Scholar]
- Schrickel J.W., Schwab J.O., Yang A., Bitzen A., Luderitz B., Lewalter T. (2006b) ’Torsade de pointes’ in patients with structural heart disease and atrial fibrillation treated with amiodarone, beta-blockers, and digitalis. Pacing Clin Electrophysiol 29: 363–366 [DOI] [PubMed] [Google Scholar]
- Schwartz P.J., Wolf S. (1978) QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation 57: 1074–1077 [DOI] [PubMed] [Google Scholar]
- Shah R.R., Hondeghem L.M. (2005) Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm 2: 758–772 [DOI] [PubMed] [Google Scholar]
- Shusterman V., Goldberg A., London B. (2006) Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation 113: 2880–2887 [DOI] [PubMed] [Google Scholar]
- Speranza G., Nollo G., Ravelli F., Antolini R. (1993) Beat-to-beat measurement and analysis of the R-T interval in 24 h ECG Holter recordings. Med Biol Eng Comput 31: 487–494 [DOI] [PubMed] [Google Scholar]
- Straus S.M., Kors J.A., De Bruin M.L., van der Hooft C.S., Hofman A., Heeringa J., et al. (2006) Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 47: 362–367 [DOI] [PubMed] [Google Scholar]
- Tanskanen A.J., Greenstein J.L., O’Rourke B., Winslow R.L. (2005a) The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys J 88: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen A.J., Greenstein J.L., O’Rourke B., Winslow R.L. (2005b) The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys J 88: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereshchenko L.G., Fetics B.J., Berger R.D. (2009a) Intracardiac QT variability in patients with structural heart disease on class III antiarrhythmic drugs. J Electrocardiol 42: 505–510 [DOI] [PubMed] [Google Scholar]
- Tereshchenko L.G., Fetics B.J., Domitrovich P.P., Lindsay B.D., Berger R.D. (2009b) Prediction of ventricular tachyarrhythmias by intracardiac repolarization variability analysis. Circ Arrhythm Electrophysiol 2: 276–284 [DOI] [PubMed] [Google Scholar]
- Thomsen M.B., Matz J., Volders P.G., Vos M.A. (2006) Assessing the proarrhythmic potential of drugs: current status of models and surrogate parameters of torsades de pointes arrhythmias. Pharmacol Ther 112: 150–170 [DOI] [PubMed] [Google Scholar]
- Thomsen M.B., Verduyn S.C., Stengl M., Beekman J.D., de Pater G., van Opstal J., et al. (2004) Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation 110: 2453–2459 [DOI] [PubMed] [Google Scholar]
- Waring W.S., Graham A., Gray J., Wilson A.D., Howell C., Bateman D.N. (2010) Evaluation of a QT nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol 70: 881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Shryock J.C., Song Y., Li Y., Antzelevitch C., Belardinelli L. (2004) Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther 310: 599–605 [DOI] [PubMed] [Google Scholar]
- Yeragani V.K., Pohl R., Balon R., Jampala V.C., Jayaraman A. (2002) Twenty-four-hour QT interval variability: increased QT variability during sleep in patients with panic disorder. Neuropsychobiology 46: 1–6 [DOI] [PubMed] [Google Scholar]
- Yeragani V.K., Pohl R., Jampala V.C., Balon R., Ramesh C., Srinivasan K. (2000) Increased QT variability in patients with panic disorder and depression. Psychiatry Res 93: 225–235 [DOI] [PubMed] [Google Scholar]


