Abstract
Paracetamol (acetaminophen) overdose remains the leading cause of death or transplantation due to acute liver failure in many parts of the world. Acetylcysteine has long been recognized as an effective antidote, via oral or intravenous administration, minimizing the risk and severity of acute liver injury if administered sufficiently early after a paracetamol overdose. Despite this, its mechanisms of action remain obscure, and there is uncertainty regarding the optimal dose and duration of treatment. The intravenous infusion protocol was originally developed as a three-step loading regimen; it causes very high early peak plasma concentrations of acetylcysteine whereas the later maintenance infusion is associated with much lower concentrations. This pharmacokinetic profile is associated with two particular concerns: a high rate of occurrence of adverse effects that occur after the initial loading infusion, and the possibility that the maintenance phase of treatment might deliver too low a dose of acetylcysteine for optimum protection against liver injury. Recently described novel administration regimens offer different rates of intravenous acetylcysteine administration in both the loading and maintenance phases. These alternative regimens appear to be well tolerated in small patient groups, but too few clinical data are available to evaluate their comparative efficacy in preventing paracetamol-induced liver injury.
Keywords: acute liver toxicity, overdose, paracetamol
Background
Paracetamol (acetaminophen) overdose was first recognized as a cause of acute liver failure in the 1960s [Davidson and Eastham, 1966]. It has since become the leading cause of death or transplantation due to acute liver failure in the UK, the USA, and elsewhere, and the occurrence of paracetamol liver injury continues to rise [Gow et al. 1999; Brandsaeter et al. 2002; Tessier et al. 2002; Larson et al. 2005; Bernal et al. 2009; Bond et al. 2012]. Acetylcysteine is highly effective in preventing severe liver injury if administered within 8–10 h after a significant paracetamol overdose [Prescott et al. 1979]. However, the standard regimen of intravenous acetylcysteine is associated with a high rate of occurrence of adverse effects, and treatment is normally reserved for patients who have absorbed substantial overdoses of paracetamol and who have a significant risk of acute liver injury [Buckley and Eddleston, 2005; Heard, 2008]. The precise pharmacological basis for the antidotal properties of acetylcysteine is somewhat uncertain, and several different mechanisms have been proposed [Jones, 1998]. Moreover, there is uncertainty concerning the optimal dose and duration of acetylcysteine administration and important international differences exist concerning the precise indications for antidote therapy. The basis for the prevailing acetylcysteine administration protocols is discussed, along with the pharmacological basis to support recently proposed novel administration regimens.
Pathophysiology of aracetamol-induced liver injury
Acute paracetamol administration is associated with significant depletion of glutathione (GSH), attributed to covalent binding of an intermediate paracetamol metabolite, N-acetyl-p-benzoquinone imine (NAPQI) to critical proteins and enzymes within hepatocytes. Restoration of intrahepatic glutathione concentrations in animals minimized the extent of NAPQI covalent binding to hepatocytes, and lessened the severity of the biochemical and histopathological changes observed after paracetamol-induced acute liver injury [Jollow et al. 1973; Mitchell et al. 1973; Potter et al. 1973,1974]. Therefore, NAPQI has been implicated as the toxic intermediate responsible of paracetamol-induced acute liver injury.
Role of glutathione
GSH is a tripeptide that consists of the amino acids cysteine, glycine, and glutamate. It possesses sulfhydryl donor groups and can act as a powerful intracellular reducing agent and antioxidant whilst being converted to its oxidized form, glutathione disulfide (GSSG) The contribution of oxidative stress to drug-induced organ toxicity and its detection in vitro and in vivo have recently been reviewed [Pereira et al. 2012]. The initial metabolic step in the synthesis of GSH involves γ-glutamylcysteine synthetase. The rate and extent of GSH synthesis depends upon the local availability of cysteine, which is normally found in comparatively low concentrations in hepatocytes compared with other tissues [Stipanuk et al. 2006]. L-Cysteine normally does not greatly increase intrahepatic glutathione due to its rapid and extensive catabolism within the gut and circulation [Frimpter, 1966]. Nonetheless, its direct intravenous administration protects against liver damage after paracetamol overdose in man [Prescott, 1978]. However, intravenous administration of large doses is technically difficult due to its poor solubility in aqueous media.
Glutathione donors
Early studies explored the efficacy of alternative sulfhydryl-containing agents. One example is cysteamine, which was shown to prevent paracetamol-induced liver injury in animals [Mitchell et al. 1974]. Prescott and colleagues reported the successful treatment of paracetamol overdose patients with cysteamine, and it was more effective than either penicillamine or methionine [Prescott et al. 1974, 1976]. However, cysteamine administration was frequently associated with very unpleasant adverse effects. Prescott and Matthew first suggested that N-acetylcysteine, which is converted into cysteine in vivo, might protect the liver against paracetamol toxicity [Prescott and Matthew, 1974]. Acetylcysteine was subsequently shown to replenish hepatic GSH stores, and to be more effective than cysteamine and methionine in preventing paracetamol hepatotoxicity and death in animals [Piperno and Berssenbruegge, 1976; Piperno et al. 1978].
Acetylcysteine: pharmacodynamic properties
Acetylcysteine prevents GSH depletion and minimizes hepatocyte injury caused by a number of different toxins [Dawson et al. 1984]. Animal studies of paracetamol-induced liver injury suggest that a primary mechanism of acetylcysteine is the provision of cysteine to stimulate replenishment of GSH to allow detoxification of NAPQI [Viña et al. 1980; Lin and Levy, 1981; Massey and Racz, 1981]. Perhaps more importantly, at a later stage it can also prevent toxicity by reversing the oxidation and arylation of critical hepatic proteins and enzymes [Davies et al. 1986]. This action may contribute to the finding that acetylcysteine may improve liver function and lessen the severity of acute injury in patients with established paracetamol-induced liver failure, even when administered late after ingestion at a time when NAPQI concentrations would be expected to be negligible [Keays et al. 1991]. These findings suggest that the therapeutic mechanism of acetylcysteine may be more complex than restoration of glutathione alone. The potent electron donor properties of acetylcysteine and the consequent ability to lessen oxidative stress might be another independent mechanism that protects against acute liver injury [Acharya and Lau-Cam, 2010].
Intravenous acetylcysteine regimen
The early clinical trials of acetylcysteine in paracetamol-poisoned patients demonstrated virtually complete protection against severe liver damage if it was given within 10 h after the overdose [Prescott et al. 1977]. The intravenous infusion regimen was developed with the objective of giving the highest tolerated dose as rapidly as possible in view of the short time window of therapeutic efficacy. No pharmacokinetic data were available at that time. Subsequently, Prescott and colleagues noted that ‘the dosage schedule for intravenous N-acetylcysteine should probably be modified since adverse reactions invariably occur early when plasma concentrations are at their highest, and liver damage was prevented just as effectively at the lowest as at the highest Cmax’; the range of Cmax, maximum plasma acetylcysteine concentrations, were reported as 304–875 mg/liter [Prescott et al. 1989]. Despite these observations, the original infusion regimen developed in Edinburgh has persisted with virtually no alteration for more than 30 years.
The dose of acetylcysteine is based on patient weight, and the original protocol consists of a sequential intravenous loading regimen: 150 mg/kg over 15 min, followed by 50 mg/kg over 4 h, and then 100 mg/kg over 16 h, so that a total dose of 300 mg/kg is delivered over 20 h 15 min [Prescott et al. 1979]. Following its original inception, a wealth of clinical experience has accrued to support the efficacy of this regimen, and it remains the standard treatment for paracetamol poisoning in the UK and elsewhere.
Adverse effects of acetylcysteine
The reported rate of occurrence of adverse effects varies according to whether data are collected prospectively or retrospectively. One prospective study found that around 40% of patients treated with intravenous acetylcysteine developed significant adverse effects; these included nonimmunoglobulin E anaphylaxis, so-called ‘anaphylactoid’ reactions in 15% and gastrointestinal symptoms in 25% [Waring et al. 2008b]. Detailed evaluation of a subset of this patient population found that anaphylaxis is characterized clinically by symptoms of flushing, hypotension, and erythema [Pakravan et al. 2008]. Such reactions are around three times commoner in patients with asthma, although of similar severity, and wheeze and laryngeal oedema have been reported in severe cases [Schmidt and Dalhoff, 2001]. The mechanism appears related to acetylcysteine concentration-dependent histamine release but, in contrast to true anaphylaxis, it is independent of tryptase and immunoglobulin E [Pakravan et al. 2008; Coulson and Thompson, 2010]. Occurrence of anaphylaxis may require that acetylcysteine administration is temporarily withheld for a sufficient time to allow plasma concentrations to decline [Kerr et al. 2005]. In many cases, the reaction will resolve spontaneously, although antihistamine administration is effective and used in more severe cases. These reactions do not have an allergic basis, so that acetylcysteine can normally be safely reintroduced.
Other adverse effects include nausea, vomiting, generalized malaise, and abdominal pain, which appear dose dependent and consistent with the adverse effects observed after other sulphydryl-containing drugs. Gastrointestinal symptoms appear attributable to a direct local effect, and are particularly prominent after oral acetylcysteine administration, although localized histamine release may also be important. Gastrointestinal adverse effects do not normally require specific intervention, but in severe cases the infusion may need to be stopped temporarily and an antiemetic administered. It should be remembered that most patients with severe paracetamol poisoning will develop nausea and vomiting, at least in part due to coingestion of ethanol or opioids [Waring et al. 2008a; Waring and Benhalim, 2008].
The rate of occurrence of adverse effects depends on the route and timing of acetylcysteine administration. For example, gastrointestinal symptoms were more common after oral than intravenous administration, 33 of 145 (22.8%) versus 27 of 306 (8.8%), but anaphylactic reactions were more common after intravenous administration, 18 of 306 (5.9%) versus 3 of 145 (2.1%) [Bebarta et al. 2010]. Also, the rate of occurrence of anaphylactic reactions is highest in patients with low paracetamol concentrations, for example those that present late after ingestion [Schmidt and Dalhoff, 2001; Waring et al. 2008b].
Indications for acetylcysteine
Acute overdose at a single time point
After single time-point ingestion, the extent of paracetamol exposure is estimated from the serum paracetamol concentration at a known time after the overdose, and compared with a standard nomogram. In the UK, the standard treatment nomogram, or so-called ‘200-line’ is drawn from a paracetamol concentration of 200 mg/liter at 4 h to 30 mg/liter at 15 h, based on an exponential decay equivalent to a 4 h half-life. Concentrations above the nomogram line are associated with a higher rate of occurrence of acute liver injury, and this is used as an indicator for acetylcysteine administration. For theoretical reasons, some patients may have increased susceptibility to paracetamol-induced acute liver injury; namely those with protein calorie malnutrition, chronic excess ethanol consumption, or previously receiving drugs capable of inducing hepatic enzyme activity, including St John’s wort, carbamazepine, phenytoin, and rifampicin. If any of these risk factors is present, then treatment is more aggressive and acetylcysteine is administered if paracetamol concentrations are higher than a nomogram drawn at 50% of the standard treatment line, the so-called ‘100-line’ or ‘high-risk treatment line’ [Waring, 2012].
The nomogram was originally validated up to 15 h after paracetamol ingestion, but is widely extrapolated to 24 h in routine clinical practice. Discretion is normally applied to take account of patient reporting error, so that acetylcysteine is often administered when paracetamol concentrations lie close to the appropriate treatment line, particularly because the nomogram between 15 and 24 h represents paracetamol concentrations that are very close to the reported laboratory detection limits. After more than 24 h, the decision to administer acetylcysteine is normally based upon clinical features, presence of risk factors, the reported dose, detectable paracetamol concentrations, and biochemical evidence of established liver injury.
Prolonged or multiple time-point ingestion (‘staggered’ overdose)
The nomogram is valid only in patients who present soon after paracetamol ingestion at a single time point, and cannot be applied in other situations. Specifically, it does not apply to patients who present after ingestion of multiple paracetamol doses at different time points or ingestion over more than 1 h, a so-called staggered overdose. In these circumstances, the decision to administer acetylcysteine is usually based on the stated paracetamol dose in the previous 24 h being greater than 12 g, or greater than 150 mg/kg if less than 12 g. Acetylcysteine may be administered at a lower threshold in the presence of risk factors, or if there are clinical or laboratory indicators of established acute liver injury, or if the patient history cannot reliably be ascertained.
An international perspective
In the UK, the decision to use the standard versus high-risk nomogram is based on a subjective evaluation of whether various risk factors are present, and there are no clinical data to support the validity of this approach. In the USA and Australasia, a single nomogram is used to determine the need for acetylcysteine without any additional risk factor assessment; this so-called ‘150-line’ is drawn intermediate to the UK standard and high-risk nomogram lines [Rumack et al. 1981]. Modifications to the original ‘200-line’ were based on a conservative approach, rather than any clinical evidence. Nonetheless, the general approach to risk assessment is broadly similar in many parts of the world insofar as the decision to administer acetylcysteine is based on the extent of paracetamol exposure. One notable exception is in Denmark, where acetylcysteine is routinely administered to all patients after paracetamol overdose irrespective of the extent of drug exposure [Schmidt et al. 2002]. The Danish approach is not widely accepted due to the very low rate of liver injury after minor paracetamol overdose, offset against a higher rate of occurrence of anaphylactic reactions to acetylcysteine in patients with comparatively low paracetamol concentrations [Waring et al. 2008b].
Issues with the existing infusion regimen
Inadequate acetylcysteine delivery in late treatment phases
In a pharmacokinetic study of 17 patients with severe paracetamol poisoning treated with the standard Prescott regimen the mean volume of distribution of acetylcysteine was 0.5 liter/kg and the mean elimination half-life was 5.7 h [Prescott et al. 1989]. Confirmatory data using the same regimen in paracetamol overdose patients found that peak plasma acetylcysteine concentrations occur soon after the initial 15 m loading infusion and then progressively decline despite maintenance administration (Figure 1). The pharmacokinetic profile is associated with progressively declining plasma concentrations despite the 16 h maintenance infusion part of the treatment regimen. This raises a concern that acetylcysteine administration at 6.25 mg/kg/h might be insufficient to fully replenish liver glutathione stores and to offer complete protection in the later phases of treatment. Whilst paracetamol concentrations are usually significantly diminished towards the end of the 20 h 15 min infusion, this does not necessarily indicate a lesser need for acetylcysteine at that time. NAPQI is highly reactive and is expected to disappear soon after paracetamol concentrations have fallen. Nonetheless, paracetamol protein adducts are noted to be more persistent than paracetamol itself, and their clearance is enhanced by acetylcysteine administration [James et al. 2009]. Somewhat paradoxically, in one study it was found that very high acetylcysteine concentrations were associated with impaired recovery from paracetamol-induced acute liver injury, suggesting that a therapeutic range of desirable acetylcysteine concentrations might exist [Yang et al. 2009].
Figure 1.
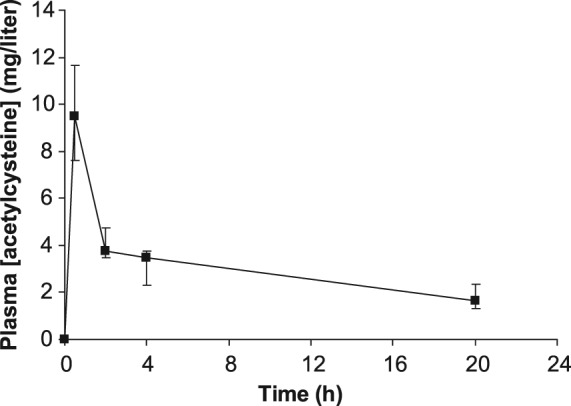
Plasma acetylcysteine concentrations in 22 patients treated with the standard Prescott regimen after paracetamol overdose, presented as median and 95% confidence intervals [Pakravan et al. 2008].
Uncertainty regarding the correct acetylcysteine dose
The optimal acetylcysteine concentrations to protect against acute liver injury are unknown. Novel data from preclinical and clinical studies are required to define the target therapeutic range and so allow a more rational approach to determining an effective acetylcysteine administration regimen. Soon after the intravenous regimen was implemented in the UK, an oral formulation of acetylcysteine was being explored as an antidote in the USA in a prospective multicentre open-label study. The US Food and Drug Administration (FDA) stipulated that a lower nomogram line be used to provide a safety factor, and this was redrawn 25% lower than in the original Prescott nomogram [Rumack et al. 1981]. Moreover, the FDA considered the 20 h 15 min protocol too short because a paracetamol half-life of up to 12 h had been observed and, therefore, a study duration of 72 h was stipulated. On the basis of this open-label study, oral acetylcysteine was approved in the USA for the treatment of paracetamol overdose in 1985. The prolonged duration of therapy was questioned, and treatment for 20–48 h in selected patients proved equally effective [Betten et al. 2007]. Oral acetylcysteine is delivered in high concentrations to the liver, but lower concentrations in the blood due to first pass metabolism, and it is unclear whether portal or systemic drug delivery is most important in determining antidote efficacy. The intravenous regimen is associated with a shorter duration of treatment and shorter hospital duration than the oral administration regimen [Blackford et al. 2011].
The original 20 h 15 min intravenous infusion protocol involved a total dose of 300 mg/kg total dose, infused as 150 mg/kg over 15 min (600 mg/kg/h), then 50 mg/kg over 4 h (12.5 mg/kg/h), and then 100 mg/kg over 16 h (6.25 mg/kg/h). In contrast, the oral administration protocol involved a total dose of 1330 mg/kg over 72 h, administered as 35 mg/kg/h for 4 h and then 17.5 mg/kg/h for 68 h. The Canadian Acetaminophen Overdose Study examined outcomes in 2086 patients in Canadian hospitals who received intravenous acetylcysteine and compared these with a historical control group of 1962 patients treated with oral acetylcysteine in the USA. Among patients who commenced treatment less than 12 h after overdose, the intravenous regimen was associated with a lower occurrence of acute liver injury (alanine transaminase ≥1000 U/liter), whereas in patients treated more than 18 h after overdose the oral regimen was more effective; the rate of acute liver injury was similar for patients who presented between 12 and 18 h. The bioavailability of oral acetylcysteine is around 80%, but too few data exist to allow comparison of plasma or liver concentrations achieved by intravenous or oral administration [Holdiness, 1991]. The study design was limited by the lack of an effective control group. Nonetheless, it raises the prospect that more intense acetylcysteine treatment might improve clinical outcome in those at greatest risk of acute liver injury [Yarema et al. 2009].
Complexity of preparing and administering acetylcysteine
The dosing regimen for intravenous acetylcysteine is complicated and prone to errors in preparation and administration. The proprietary acetylcysteine solution is 10 mg/ml, and may be subject to potentially hazardous administration of inadequate dosages, and 10-fold excess dose calculation errors [Little et al. 2005; Dart and Rumack, 2012]. Inadvertent administration of a fivefold higher dose during the first and second treatment bags was associated with haemolysis and acute kidney injury in a 21-year-old woman [Mullins and Vitkovitsky, 2011]. Elsewhere, massive inadvertent acetylcysteine overdose was followed by cerebral oedema and seizures [Heard and Schaeffer, 2011]. ST-elevation myocardial infarction and subsequent death due to heart failure followed a 10-fold dose administration error in a 49-year-old man with no previous history of cardiac disease [Elms et al. 2011].
Modifications to the original infusion regimen
Extended duration of infusion
Based on an average paracetamol half-life of 4 h, paracetamol concentrations might be expected to have fallen to negligible or undetectable concentration by around five half lives, or 20 h. However, there is inter-individual variability and the half-life of paracetamol is prolonged in patients who develop liver injury. Thus, acetylcysteine administration may need to be continued for longer than proposed in the original infusion regimen. It is routine practice in the UK and elsewhere to check clinical status, liver biochemistry, and coagulation after completion of the standard 20 h 15 min infusion, and if there is any clinical or laboratory evidence of liver injury, then further acetylcysteine is administered at the same rate as the ‘third bag’, namely 6.25 mg/kg/h, until a significant trend indicating recovery, liver transplant or death [Keays et al. 1991; Yip et al. 1998]; a retrospective study found that administration of acetylcysteine was beneficial, even if delayed to when liver injury had already occurred [Harrison et al. 1990]. Elsewhere, liver biochemistry and coagulation are not routinely rechecked if acetylcysteine is commenced within 8 h of acute paracetamol ingestion [Daly et al. 2008].
‘Slow loading’ regimen
In the USA, an intravenous formulation of acetylcysteine was approved in 2004. The FDA approved an infusion regimen based on that of Prescott and colleagues, but subject to minor modification in that the initial 150 mg/kg loading infusion should be administered over 60 rather than 15 min, resulting in a 21 h infusion regimen. This slower loading regimen was intended to minimize the occurrence of early adverse effects thought to be attributable to high peak acetylcysteine concentrations. Few comparative data exist between the ‘slow loading’ and standard Prescott regimen. In a randomized controlled, prospective study, adverse effects were analysed at predetermined time points during both infusion regimens and no difference was found [Kerr et al. 2005]. However, a limitation was the lack of data collection in the very early period after commencement of the infusion.
Maximum weight-based dose
A second and more recent modification in the UK is that the 300 mg/kg dose includes a maximum body weight of 110 kg to take into account the theoretical consideration that acetylcysteine is distributed predominantly within the aqueous compartment, and this is unlikely to increase in proportion to body weight beyond a certain point [Thanacoody et al. 2008].
Earlier initiation, two-step regimens
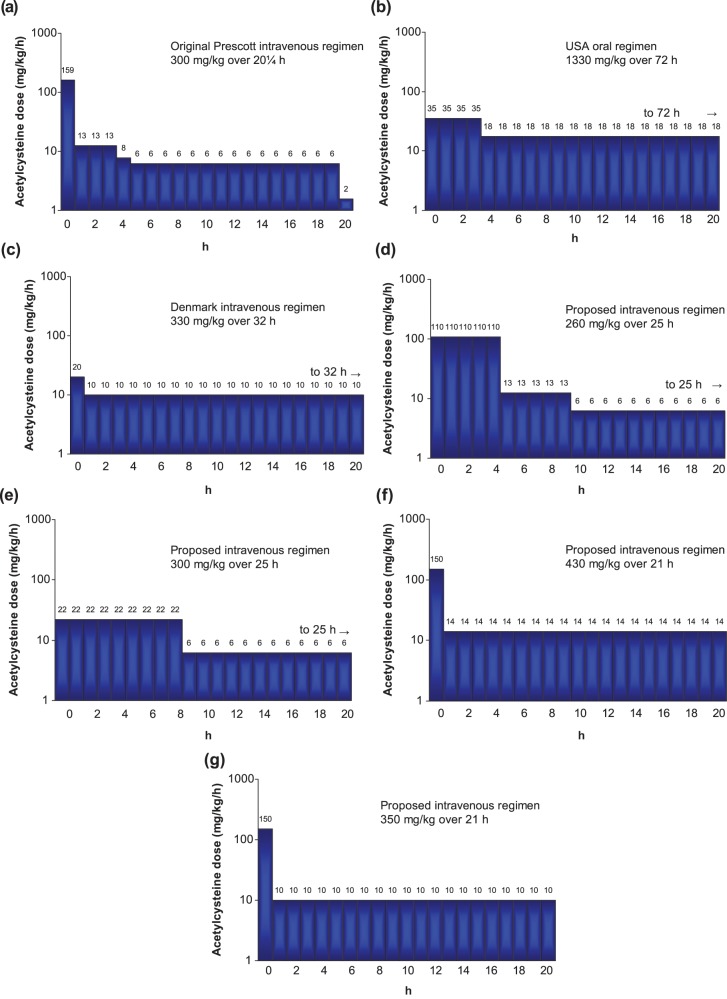
Simulation of different intravenous acetylcysteine regimens using published pharmacokinetic data has suggested different approaches to delivering the same overall dose. Two schemes were proposed to allow lower initial concentrations to be maintained for longer periods [Prescott et al. 1989]. Shen and colleagues recently suggested that rather than waiting for paracetamol concentrations, treatment could be commenced immediately using one of two possible strategies [Shen et al. 2011]. Firstly, intravenous administration of 110 mg/kg over the first 5 h (22 mg/kg/h) followed by the last two phases of the conventional regimen: 12.5 mg/kg/h for 4 h then 6.25 mg/kg/h for 16 h. Second, intravenous administration of 200 mg/kg over 9 h (22.6 mg/kg/h) followed by the last phase of the conventional regimen; 6.25 mg/kg/h for 16 h. Both of these regimens might result in a similar area under the concentration–time curve as the standard regimen, and would dramatically reduce the early peak acetylcysteine concentrations (Figure 2). The latter might also offer a simpler regimen to prepare and administer, thereby reducing dose administration error. Earlier initiation of acetylcysteine is associated with a lesser occurrence of anaphylactic reactions in patients with paracetamol poisoning [Zyoud et al. 2010].
Figure 2.
Schematic representation of several acetylcysteine administration regimens that are in widespread clinical use: (a) [Prescott et al. 1977], (b) [Rumack et al. 1981], and (c) [Schmidt and Dalhoff, 2001]. Recently proposed regimens shown in panels (d) and (e) [Shen et al. 2011], (f) [Johnson et al. 2011], and (g) [Oakley et al. 2011].
Increased maintenance infusion
Another approach was the administration of the conventional ‘first bag’ 150 mg/kg loading dose over 1 h, but then followed by an infusion of 14 mg/kg/h for 20 h. Implementation of this regimen over a 2-year period at a single institution resulted in fewer treatment interruptions than might have been expected with the standard regimen, and a similar rate of liver injury in both groups. This modified regimen delivered a higher overall dose of acetylcysteine than the standard regimen (430 mg/kg rather than 300 mg/kg), but the treatment was well tolerated [Johnson et al. 2011].
Two-step infusion in children
A two-stage infusion was also explored in 40 children with paracetamol overdose: administration of intravenous acetylcysteine 150 mg/kg over 1 h was followed by a continuous infusion of 10 mg/kg/h for 20 h [Oakley et al. 2011]. This two step regimen involved a much less complex preparation and administration schedule than the standard infusion. It also delivered a higher overall dose of acetylcysteine (350 mg/kg versus 300 mg/kg) but again this was well tolerated, and no differences in liver toxicity were observed.
Conclusion
Over the past few decades the availability of acetylcysteine has substantially improved the outcome in patients with major paracetamol overdose. However, there has been comparatively little recent progress towards defining its precise mechanisms of action or identifying the optimal dose and duration of therapy. Treatment with intravenous acetylcysteine is associated with relatively frequent adverse effects, and these could undoubtedly be lessened by minor modification of the infusion protocol to avoid very high early peak concentrations. There is also concern that the maintenance administration rate of 6.25 mg/kg/h might be inadequate in patients who present late after paracetamol overdose, and alternative regimens that deliver higher maintenance infusion rates have been proposed. A particular limitation is that the current antidote regimen takes no account of the paracetamol dose ingested. Future research should seek to establish the optimal acetylcysteine dose and duration of treatment that minimizes the occurrence or severity of acute liver injury, whilst avoiding unnecessary hospital-based therapy.
Although the original dosage regimen for intravenous acetylcysteine may have stood the test of time, there is now a strong case for modification to reduce the incidence of adverse effects and to increase the ease of administration. Many of the novel regimens are derived from the Prescott regimen, involving administration of 300 mg/kg over 20 h 15 min; no dose–response data are available to inform the optimum dose. The apparent acceptability of the original regimen and its subsequent modification might suggest that the existing dose is excessive, but the available data involve too few patients to allow meaningful comparisons of safety between the regimens. Clinical outcome measures from novel administration regimens need further evaluation in much larger patient numbers to ensure that they are at least as efficacious as existing protocols.
Acknowledgments
The author would like to express his sincere gratitude to Professor Laurie Prescott for his constructive comments and critique during preparation of this manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares no conflict of interest in reparing this manuscript.
References
- Acharya M., Lau-Cam C. (2010) Comparison of the protective actions of N-acetylcysteine, hypotaurine and taurine against acetaminophen-induced hepatotoxicity in the rat. J Biomed Sci 17(Suppl. 1): S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebarta V., Kao L., Froberg B., Clark R., Lavonas E., Qi M., et al. (2010) A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol 48: 424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal W., Cross T., Auzinger G., Sizer E., Heneghan M., Bowles M., et al. (2009) Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol 50: 306–313 [DOI] [PubMed] [Google Scholar]
- Betten D., Cantrell F., Thomas S., Williams S., Clark R. (2007) A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med 50: 272–279 [DOI] [PubMed] [Google Scholar]
- Blackford M., Felter T., Gothard M., Reed M. (2011) Assessment of the clinical use of intravenous and oral N-acetylcysteine in the treatment of acute acetaminophen poisoning in children: a retrospective review. Clin Ther 33: 1322–1330 [DOI] [PubMed] [Google Scholar]
- Bond G., Ho M., Woodward R. (2012) Trends in hepatic injury associated with unintentional overdose of paracetamol (acetaminophen) in products with and without opioid: an analysis using the National Poison Data System of the American Association of Poison Control Centers, 2000–7. Drug Saf 35: 149–157 [DOI] [PubMed] [Google Scholar]
- Brandsaeter B., Höckerstedt K., Friman S., Ericzon B., Kirkegaard P., Isoniemi H., et al. (2002) Fulminant hepatic failure: outcome after listing for highly urgent liver transplantation: 12 years experience in the Nordic countries. Liver Transpl 8: 1055–1062 [DOI] [PubMed] [Google Scholar]
- Buckley N., Eddleston M. (2005) Paracetamol (acetaminophen) poisoning. Clin Evid (14): 1738–1744 [PubMed] [Google Scholar]
- Coulson J., Thompson J. (2010) Paracetamol (acetaminophen) attenuates in vitro mast cell and peripheral blood mononucleocyte cell histamine release induced by N-acetylcysteine. Clin Toxicol 48: 111–114 [DOI] [PubMed] [Google Scholar]
- Daly F., Fountain J., Murray L., Graudins A., Buckley N. (2008) Panel of Australian and New Zealand clinical toxicologists. Guidelines for the management of paracetamol poisoning in Australia and New Zealand–explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Med J Aust 188: 296–301 [DOI] [PubMed] [Google Scholar]
- Dart R., Rumack B. (2012) Intravenous acetaminophen in the United States: iatrogenic dosing errors. Pediatrics 129: 349–353 [DOI] [PubMed] [Google Scholar]
- Davidson D., Eastham W. (1966) Acute liver necrosis following overdose of paracetamol. Br Med J 2: 497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D., Tee L., Hampden C., Boobis A. (1986) Adv Exp Med Biol 197: 993–1003 [DOI] [PubMed] [Google Scholar]
- Dawson J., Norbeck K., Anundi I., Moldéus P. (1984) The effectiveness of N-acetylcysteine in isolated hepatocytes, against the toxicity of paracetamol, acrolein, and paraquat. Arch Toxicol 55: 11–15 [DOI] [PubMed] [Google Scholar]
- Elms A., Owen K., Albertson T., Sutter M. (2011) Fatal myocardial infarction associated with intravenous N-acetylcysteine error. Int J Emerg Med 4: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimpter G. (1966) Cystinuria: intravenous administration of 35S cystine and 35S cysteine. Clin Sci 31: 207–214 [PubMed] [Google Scholar]
- Gow P., Smallwood R., Angus P. (1999) Paracetamol overdose in a liver transplantation centre: an 8-year experience. J Gastroenterol Hepatol 14: 817–821 [DOI] [PubMed] [Google Scholar]
- Harrison P., Keays R., Bray G., Alexander G., Williams R. (1990) Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet 335: 1572–1573 [DOI] [PubMed] [Google Scholar]
- Heard K. (2008) Acetylcysteine for acetaminophen poisoning. N Engl J Med 359: 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard K., Schaeffer T. (2011) Massive acetylcysteine overdose associated with cerebral edema and seizures. Clin Toxicol (Phila) 49:423–5 [DOI] [PubMed] [Google Scholar]
- Holdiness M. (1991) Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet 20: 123–134 [DOI] [PubMed] [Google Scholar]
- James L., Letzig L., Simpson P., Capparelli E., Roberts D., Hinson J., et al. (2009) Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos 37: 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., McCammon C., Mullins M., Halcomb S. (2011) Evaluation of a simplified N-acetylcysteine dosing regimen for the treatment of acetaminophen toxicity. Ann Pharmacother 45: 713–720 [DOI] [PubMed] [Google Scholar]
- Jollow D., Mitchell J., Potter W., Davis D., Gillette J., Brodie B. (1973) Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187: 195–202 [PubMed] [Google Scholar]
- Jones A. (1998) Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. J Toxicol Clin Toxicol 36: 277–285 [DOI] [PubMed] [Google Scholar]
- Keays R., Harrison P., Wendon J., Forbes A., Gove C., Alexander G., et al. (1991) Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ 303: 1026–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr F., Dawson A., Whyte I., Buckley N., Murray L., Graudins A., et al. (2005) The Australasian clinical toxicology investigators collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med 45: 402–408 [DOI] [PubMed] [Google Scholar]
- Larson A., Polson J., Fontana R., Davern T., Lalani E., Hynan L., et al. (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42: 1364–1372 [DOI] [PubMed] [Google Scholar]
- Lin J., Levy G. (1981) Sulfate depletion after acetaminophen administration and replenishment by infusion of sodium sulfate or N-acetylcysteine in rats. Biochem Pharmacol 30: 2723–2725 [DOI] [PubMed] [Google Scholar]
- Little M., Murray L., McCoubrie D., Daly F. (2005) A potentially fatal prescribing error in the treatment of paracetamol poisoning. Med J Aust 183: 535–536 [DOI] [PubMed] [Google Scholar]
- Massey T., Racz W. (1981) Effects of N-acetylcysteine on metabolism, covalent binding, and toxicity of acetaminophen in isolated mouse hepatocytes. Toxicol Appl Pharmacol 60: 220–228 [DOI] [PubMed] [Google Scholar]
- Mitchell J., Jollow D., Potter W., Gillette J., Brodie B. (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187: 211–217 [PubMed] [Google Scholar]
- Mitchell J., Thorgeirsson S., Potter W., Jollow D., Keiser H. (1974) Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther 16: 676–684 [DOI] [PubMed] [Google Scholar]
- Mullins M., Vitkovitsky I. (2011) Hemolysis and hemolytic uremic syndrome following five-fold N-acetylcysteine overdose. Clin Toxicol 49: 755–759 [DOI] [PubMed] [Google Scholar]
- Oakley E., Robinson J., Deasy C. (2011) Using 0.45% saline solution and a modified dosing regimen for infusing N-acetylcysteine in children with paracetamol poisoning. Emerg Med Australas 23: 63–67 [DOI] [PubMed] [Google Scholar]
- Pakravan N., Waring W., Sharma S., Ludlam C., Megson I., Bateman D. (2008) Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin Toxicol 46: 697–702 [DOI] [PubMed] [Google Scholar]
- Pereira C., Nadanaciva S., Oliveira P., Will Y. (2012) The contribution of oxidative stress to drug-induced organ toxicity and its detection in vitro and in vivo. Expert Opin Drug Metab Toxicol 8: 219–237 [DOI] [PubMed] [Google Scholar]
- Piperno E., Berssenbruegge D. (1976) Reversal of experimental paracetamol toxicosis with N-acetylcysteine. Lancet 2: 738–739 [DOI] [PubMed] [Google Scholar]
- Piperno E., Mosher A., Berssenbruegge D., Winkler J., Smith R. (1978) Pathophysiology of acetaminophen overdosage toxicity: implications for management. Pediatrics 62(Suppl.): 880–889 [PubMed] [Google Scholar]
- Potter W., Davis D., Mitchell J., Jollow D., Gillette J., Brodie B. (1973) Acetaminophen-induced hepatic necrosis. III. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther 187: 203–210 [PubMed] [Google Scholar]
- Potter W., Thorgeirsson S., Jollow D., Mitchell J. (1974) Acetaminophen-induced hepatic necrosis. V. Correlation of hepatic necrosis, covalent binding and glutathione depletion in hamsters. Pharmacology 12: 129–143 [DOI] [PubMed] [Google Scholar]
- Prescott L. (1978) The chief scientist reports. Prevention of hepatic necrosis following paracetamol overdose. Health Bull 36: 204–212 [PubMed] [Google Scholar]
- Prescott L., Donovan J., Jarvie D., Proudfoot A. (1989) The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Prescott L., Illingworth R., Critchley J., Stewart M., Adam R., Proudfoot A. (1979) Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J 2: 1097–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L., Matthew H. (1974) Cysteamine for paracetamol overdosage. Lancet 1: 998. [PubMed] [Google Scholar]
- Prescott L., Newton R., Swainson C., Wright N., Forrest A., Matthew H. (1974) Successful treatment of severe paracetamol overdosage with cysteamine. Lancet 1: 588–592 [DOI] [PubMed] [Google Scholar]
- Prescott L., Park J., Ballantyne A., Adriaenssens P., Proudfoot A. (1977) Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 2: 432–434 [DOI] [PubMed] [Google Scholar]
- Prescott L., Sutherland G., Park J., Smith I., Proudfoot A. (1976) Cysteamine, methionine, and penicillamine in the treatment of paracetamol poisoning. Lancet 2: 109–113 [DOI] [PubMed] [Google Scholar]
- Rumack B., Peterson R., Koch G., Amara I. (1981) Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med 141: 380–385 [DOI] [PubMed] [Google Scholar]
- Schmidt L., Dalhoff K. (2001) Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharmacol 51: 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L., Knudsen T., Dalhoff K., Bendtsen F. (2002) Effect of acetylcysteine on prothrombin index in paracetamol poisoning without hepatocellular injury. Lancet 360: 1151–1152 [DOI] [PubMed] [Google Scholar]
- Shen F., Coulter C., Isbister G., Duffull S. (2011) A dosing regimen for immediate N-acetylcysteine treatment for acute paracetamol overdose. Clin Toxicol 49: 643–647 [DOI] [PubMed] [Google Scholar]
- Stipanuk M., Dominy J., Jr, Lee J., Coloso R. (2006) Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136(6 Suppl.): 1652S–1659S [DOI] [PubMed] [Google Scholar]
- Tessier G., Villeneuve E., Villeneuve J. (2002) Etiology and outcome of acute liver failure: experience from a liver transplantation centre in Montreal. Can J Gastroenterol 16: 672–676 [DOI] [PubMed] [Google Scholar]
- Thanacoody H., Good A., Waring W., Bateman D.(2008) Survey of cases of paracetamol overdose in the UK referred to National Poisons Information Service (NPIS) consultants. Emerg Med J 25: 140–143 [DOI] [PubMed] [Google Scholar]
- Viña J., Romero F., Estrela J., Viña J. (1980) Effect of acetaminophen (paracetamol) and its antagonists on glutathione (GSH) content in rat liver. Biochem Pharmacol 29: 1968–1970 [DOI] [PubMed] [Google Scholar]
- Waring W. (2012) Criteria for acetylcysteine treatment and clinical outcomes after paracetamol poisoning. Exp Rev Clin Pharmacol 5: 311–318 [DOI] [PubMed] [Google Scholar]
- Waring W., Benhalim S. (2008) Serum acetaminophen concentrations after acute overdose are not altered by opioid co-ingestion. J Toxicol Sci 33: 549–553 [DOI] [PubMed] [Google Scholar]
- Waring W., Stephen A., Malkowska A., Robinson O. (2008a) Acute ethanol coingestion confers a lower risk of hepatotoxicity after deliberate acetaminophen overdose. Acad Emerg Med 15: 54–58 [DOI] [PubMed] [Google Scholar]
- Waring W., Stephen A., Robinson O., Dow M., Pettie J. (2008b) Lower incidence of anaphylactoid reactions to N-acetylcysteine in patients with high acetaminophen concentrations after overdose. Clin Toxicol 46: 496–500 [DOI] [PubMed] [Google Scholar]
- Yang R., Miki K., He X., Killeen M., Fink M. (2009) Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care 13: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarema M., Johnson D., Berlin R., Sivilotti M., Nettel-Aguirre A., Brant R., et al. (2009) Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med 54: 606–614 [DOI] [PubMed] [Google Scholar]
- Yip L., Dart R., Hurlbut K. (1998) Intravenous administration of oral N-acetylcysteine. Crit Care Med 26: 40–43 [DOI] [PubMed] [Google Scholar]
- Zyoud S., Awang R., Sulaiman S., Al-Jabi S. (2010) Effects of delay in infusion of N-acetylcysteine on appearance of adverse drug reactions after acetaminophen overdose: a retrospective study. Pharmacoepidemiol Drug Saf 19: 1064–1070 [DOI] [PubMed] [Google Scholar]