Abstract
Oral immunomodulatory drugs (IMiDs), namely thalidomide, lenalidomide and pomalidomide, interfere with several pathways important for disease progression. Today they play a crucial role in the treatment of multiple myeloma patients, and have considerably improved myeloma outcomes. These agents, and thalidomide in particular, are associated with higher rates of thromboembolic events, both venous and arterial. Individual risk factors for thromboembolic events include advanced age, previous history of thromboembolism, an indwelling central venous catheter, comorbid conditions (e.g. infections, diabetes, cardiac disease, obesity), current or recent immobilization, recent surgery and inherited thrombophilic abnormalities. Cancer therapy and cancer itself also increase the risk of thromboembolic events. The aim of this review is to help clinicians to define the risk of thrombotic events in patients treated with thalidomide and thus to provide practical recommendations to manage thromboprophylaxis in these patients.
Keywords: thalidomide, multiple myeloma, venous thromboembolism, novel agents, aspirin, warfarin, heparin, thromboprophylaxis
Introduction
Thalidomide belongs to the category of immunomodulatory drugs (IMiDs) together with the second-generation lenalidomide and pomalidomide. IMiDs have been shown to block several pathways important for disease progression demonstrating anti-angiogenic, immunomodulatory and apoptotic effects [Anderson, 2005]. After being withdrawn from the world market because of its teratogenicity, thalidomide is today approved for the therapy of some inflammatory and autoimmune disorders, including certain late-stage AIDS symptoms, and plays a crucial role in the therapy of multiple myeloma.
Trials have reported numerous thromboembolic events attributed to thalidomide therapy, especially when thalidomide is used in combination with chemotherapy. Deep vein thrombosis (DVT) and pulmonary embolism (PE) are more common, but occasional arterial thrombotic events have also been detected [Palumbo et al. 2008a; Scarpace et al. 2005].
Although the introduction of IMiDs and thalidomide in cancer therapy, and multiple myeloma in particular, has considerably improved outcome, they have also increased the risk of thromboembolic events. Accurate evaluation of thromboembolic risk and appropriate thromboprophylaxis are recommended to improve the safety profile of the thalidomide-based therapy and optimize patient outcome [Palumbo et al. 2008b].
Which antithrombotic agent is the most appropriate is still a matter of debate; aspirin (ASA), low-molecular weight heparin (LMWH) and warfarin all seem to be effective. This review discusses risk factors for thromboembolism in the general population and, in particular, in multiple myeloma patients receiving thalidomide, and proposes clinical practical recommendations for thromboprophylaxis in this setting.
Brief history of thalidomide
Thalidomide is a synthetic glutamic acid derivative that consists of a chiral center and two amide rings. It is poorly soluble in water, hence no parental preparation is available (Figure 1). This drug exhibits linear and dose-dependent pharmacokinetics that are not affected by age, sex, race, food or smoking status [Weber, 2003].
Figure 1.
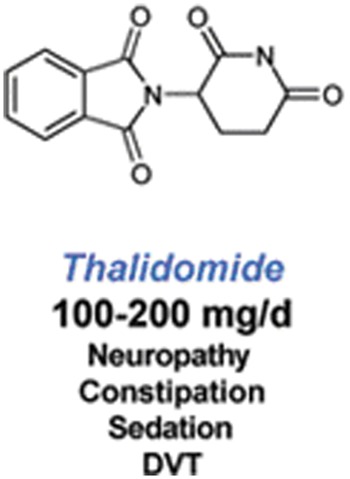
Molecular structure of thalidomide and most common adverse events. DVT, deep vein thrombosis.
Thalidomide and IMiDs inhibit the cytokines tumor necrosis factor-α (TNF-α), interleukins (IL) 1β, 6, 12, and granulocyte macrophage-colony stimulating factor (GM-CSF). TNF-α enhances neo-angiogenesis, and interacts with other proliferative cytokines such as IL-6, known to be involved in the pathogenesis of multiple myeloma [Thomas and Kantarjian, 2001]. Thalidomide also inhibits IL-12 monocyte production, and enhances lymphocytes synthesis of IL-12 determining an enhancement in the cytotoxic activity mediated by natural killer (NK) cells. IL1 β along with TNF-α are implicated in tumorigenesis upregulating the expression of vascular cell adhesion molecules (VCAM) promoting cancer cell adhesion and metastasis [Vidal-Vanaclocha et al. 2000]. IMiDs induce an IL-2-mediated primary T-cell proliferation increasing NK cell numbers and function, thus increasing the activity of NK-dependent cytotoxicity. IMiDs cause a concomitant increase in interferon gamma (IFN-γ) production and decrease in the density of TNF-α-induced cell surface adhesion molecules ICAM-1, VCAM-1 and E-selectin, thus altering the interactions between myeloma cells and the bone marrow microenvironment. Finally, IMiDs activate apoptotic pathways through caspase-8-mediated cell death and, at the mitochondrial level, through the c-jun terminal kinase (JNK)-dependent release of cytochrome-c and Smac into the cytosol of cells [Anderson, 2005].
Some of the above immunomodulatory activities along with anti-angiogenic, antiproliferative and pro-apoptotic properties are thought to mediate the antitumor responses to IMiDs observed in relapsed and refractory multiple myeloma and some solid tumor cancers [Teo, 2005].
Thalidomide was launched by Grünenthal on 1 October 1957; at first it was used as tranquilizer and painkiller and was effective against insomnia, coughs, colds and headaches. It was also found to be an effective antiemetic, with an inhibitory effect on morning sickness during pregnancy. In the early 1960s the drug was withdrawn from the world market due to the discovery of its teratogenic effect (phocomelia). It has then been selectively reintroduced for use in various disorders thought to have an autoimmune or inflammatory basis.
The drug has been used in a variety of dermatologic conditions during the past few decades, including erythema nodosum leprosum, prurigo nodularis, actinic prurigo, discoid lupus erythematosus, aphthous stomatitis, Behçet’s syndrome and graft-versus-host disease [Tseng et al. 1996]. Researchers continue to investigate thalidomide for use in treating a variety of diseases and conditions, and nowadays it has become one of the most widely used drugs to treat multiple myeloma. Moreover, thalidomide has shown promising results for the treatment of inflammatory diseases, HIV-related mouth and throat ulcers and Kaposi’s sarcoma, but further investigation is needed.
Thalidomide is a known teratogenic agent, and its use is absolutely contraindicated in pregnant women. Sedation, constipation and pruritus are its most frequently noted side effects. A sensorimotor peripheral neuropathy usually of hands and feet may occur, particularly after prolonged exposure. Less common effects include peripheral edema, tremors, bradycardia, hypothyroidism and rarely neutropenia and hepatic enzyme elevation [Weber, 2003]. Venous thromboembolism (VTE) events are commonly associated with the use of thalidomide [Scarpace et al. 2005]. Deep vein thrombosis (DVT) is the predominant event, occurring in veins deep within the muscles of the leg and in the pelvis; DVT occurs when part or all of a thrombus breaks away from the blood vessel wall; this clot or embolus is sometime carried in the direction of blood flow, to the pulmonary vessels. For this reason patients with DVT are at risk of PE as well, a life-threatening event. PE is a potentially lethal complication of DVT, leading to sudden death in one fourth of all cases [Heit et al. 1999].
Arterial thromboembolism is a sudden interruption of blood flow to an organ or body part due to a clot (embolus); it is increasingly being recognized as a complication of cancer therapy, although the underlying mechanisms are poorly understood [Lacy, 2010].
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice; without appropriate anticoagulant treatment, most patients with AF are at increased risk of cardioembolic stroke. Coronary artery disease (CAD) is the most common form of cardiovascular disease. In CAD, atherosclerosis damages the coronary artery wall, predisposing to thrombus formation. The symptoms and severity of acute coronary syndromes (unstable angina and myocardial infarction) vary depending on the degree to which thrombi occlude the coronary arteries [Fuster et al. 2005; Bassand et al. 2007; Rosamond et al. 2008; Lloyd-Jones et al. 2010].
Thalidomide and multiple myeloma
Thalidomide received the approval by the United States Food and Drug Administration (FDA) for use in combination with high-dose dexamethasone as treatment for newly diagnosed multiple myeloma in 2006. In 2008, the European Medicines Agency (EMEA) granted approval for thalidomide in the treatment of newly diagnosed multiple myeloma in combination with melphalan and prednisone.
Thalidomide has been shown to have therapeutic activity as a single agent in patients with relapsed and/or refractory multiple myeloma. Thalidomide monotherapy can induce remissions in about one third of relapsing or refractory patients [Dimopoulos et al. 2003; Barlogie et al. 2001; Yakoub-Agha et al. 2002; Neben et al. 2002; Kumar et al. 2003] and combinations of thalidomide with dexamethasone and/or chemotherapy can further improve response rates in these patients [Dimopoulos et al. 2003; Kropff et al. 2003]. Studies have shown good efficacy of thalidomide treatment in untreated patients [Dimopoulos et al. 2003].
Thalidomide has a wide spectrum of biological effects, including some that have been hypothesized to promote thrombosis, such as transient reduction of soluble thrombomodulin levels during the first month of therapy [Corso et al. 2004] and restoration of endothelial cell PAR-1 expression after damage from cytotoxic agents such as doxorubicin [Kaushal et al. 2004]. The latter finding may in part explain the markedly higher thromboembolic risk seen with thalidomide–anthracycline combination therapy compared with thalidomide monotherapy, since IMiDs do not appear to cause endothelial injury themselves [Streetly et al. 2005]. Previous studies showed that single-agent thalidomide is not associated with an increased risk of thrombosis [Barlogie et al. 2001]. In relapsed/refractory multiple myeloma the incidence of VTE is <2% [Barlogie et al. 2001] and in newly diagnosed multiple myeloma the incidence of VTE is 3.5%. Combining thalidomide with dexamethasone has been clearly shown to increase the incidence of VTE; previous studies in newly diagnosed and relapsed/refractory multiple myeloma reported an incidence of 8–26% [Osman et al. 2001; Corso et al. 2004]. A significant increase in the risk of VTE during treatment with thalidomide and anthracyclines has been reported, especially during the first months of therapy [Barlogie et al. 2006]. With regards to alkylating agents, a phase II trial involving 50 elderly patients receiving melphalan, thalidomide and dexamethasone as initial therapy the incidence of VTE was around 9% [Dimopoulos et al. 2006]. In a randomized control trial assessing response rates of elderly patients with newly diagnosed multiple myeloma to melphalan, prednisone and thalidomide (MPT) as compared with melphalan–prednisone (MP) therapy, the incidence of VTE was 17% in the MPT arm and 2% in the MP arm [Palumbo et al. 2006]. The highest incidence of VTE (58%) in myeloma therapy was reported in a phase II trial evaluating the use of doxorubicin, vincristine and dexamethasone with thalidomide both in newly diagnosed and in relapsed/refractory multiple myeloma patients [Baz et al. 2005].
Risk factors for thromboembolism in the general population
Thromboembolism is typical of older age, with an incidence of less than 2% in subjects younger than 55 years and more than 5% in patients older than 65 years [Silverstein et al. 1998].
The cause of thromboembolism is multifactorial and is often a consequence of a combination of risk factors. Thromboembolic risk factors can be categorized into individual and disease-related risk factors [Samama, 2000; Heit et al. 2000, 2002; Ageno et al. 2006; Bauer, 2001].
Individual risk factors include older age, comorbid conditions (such as obesity, infection, renal disease, pulmonary disease and arterial thromboembolism), heritable prothrombotic mutations, the presence of a central venous catheter (CVC) or pacemaker, previous history of thrombosis, pregnancy/puerperium and use of drugs.
Inherited thrombophilic abnormalities may include antithrombin III deficiencies, protein C and S deficiencies, activated protein C resistance, factor V Leiden mutation, prothrombin gene mutation and high levels of homocystein.
Disease-related factors include recent (generally less than 3 months) surgical procedures, including vertebroplasty and kyphoplasty, trauma or hospital admission, neurologic disease with subsequent high degree and prolonged immobilization, chronic clinical conditions (hyperviscosity, chronic renal disease, infections, cardiac disease and malignant neoplasm, such as myeloma, with or without chemotherapy), primary site of cancer (gastrointestinal, brain, lung, gynecologic, renal or hematologic), initial 3–6 months after cancer diagnosis, antiangiogenic therapy (thalidomide, lenalidomide or bevacizumab) and erythropoiesis-stimulating agents
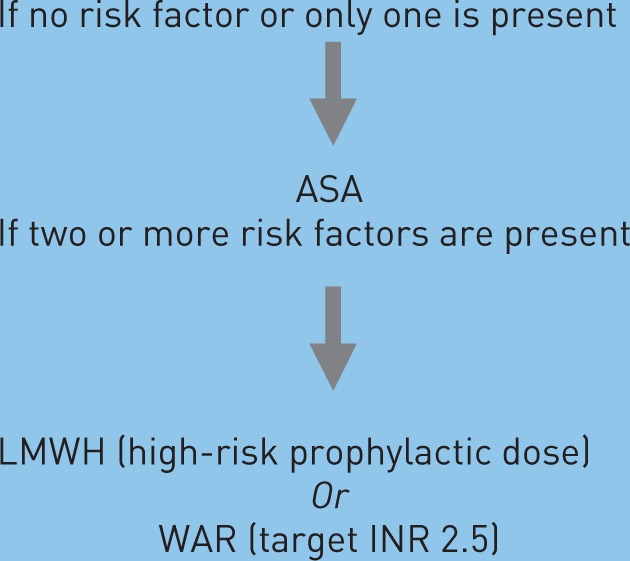
The simultaneous presence of more than one risk factor increases the risk of VTE (Table 1).
Table 1.
Major risk factors for venous thromboembolism (VTE) and suggested actions.
| Risk factors | Action |
|---|---|
Individual
|
|
Myeloma therapy
|
LMWH (high-risk prophylactic dose) Or WAR (target INR 2.5) |
Bleeding
|
Clinicians should consider whether the presence of bleeding risk is sufficient to preclude pharmacological thromboprophylaxis |
ASA, aspirin 81–325 mg once daily; LMWH, low molecular weight heparin, equivalent of enoxaparin 40 mg once daily; PT, prothrombin time; WAR, full-dose warfarin; INR, International Normalized Ratio.
Risk of thromboembolism in patients with cancer
The risk of thromboembolism is 7–10 times higher in patients with cancer compared with the normal population. The risk is considerably higher (28 times) in patients with hematologic cancer [Blom et al. 2005]. The pathophysiology of thrombosis in cancer is a complex process. The interaction between malignant cells and monocyte/macrophage cells stimulates the release of TNF, IL-1 and IL-6, all causing endothelial damage. The interaction between tumor cells and macrophages also activates platelets, factor XII and X. Cysteine protease and tissue factor are highly expressed in cancer cells, have procoagulant activity and can directly activate factor X and VII [Bick, 2003; Prandoni et al. 2005]. The risk is usually higher in the first months after diagnosis. In cancer patients, the risk of thromboembolic events is increased with active therapy, immobilization, surgery and the presence of CVC. In cancer patients undergoing surgery, the risk of DVT is doubled after surgery, and the risk of PE is three times as high as compared with patients without cancer undergoing surgery [White et al. 2003].
Recently, the risk of VTE was also found to be increased by the administration of erythropoiesis-stimulating agents [FDA, 2010]. A previous trial reported an increased incidence of VTE in cancer patients treated with erythropoiesis-stimulating agents, doubled in patients using erythropoietin and treated with thalidomide compared with patients who were treated with thalidomide and not receiving erythropoietin [Anaisse et al. 2012]. To conclude, in cancer patients the risk of developing VTE is higher because of the presence of several risk factors, including cancer therapy itself.
Thromboembolism in multiple myeloma patients treated with thalidomide
Hypercoagulable states have been described in plasma cell dyscrasias without being specifically related to myeloma. The incidence of thromboembolic events in multiple myeloma varies from 3% to 10% [Srkalovic et al. 2004; Rajkumar et al. 2006]. Several mechanisms are implicated, yet the exact mechanisms associated with VTE in multiple myeloma are unclear. M-protein interacts with platelets, enhancing platelet adhesion and aggregation [Eby and Blinder, 2003]. Procoagulant antibody formation, paraprotein interference with fibrin structure, activated protein C resistance and endothelial damage may be considered important factors favoring thromboembolism in patients with multiple myeloma [Zangari et al. 2003]. The interaction between tumor cells and their microenvironment may explain the increased expression of von Willebrand and factor VIII. This close relationship may be responsible for increased secretion of proinflammatory cytokines such as IL-6 and TNF that can activate coagulation pathways [Fox and Kahn, 2005]. Thalidomide can enhance expression of tissue factor and vascular endothelial growth factor, downregulate thrombospondin and cause cytokine-mediated, activated protein C resistance. Thalidomide has been shown to increase the levels of von Willebrand factor and factor VIII. In addition thalidomide regulates the level of the prothrombotic factor COX-2 [Kristinsson, 2010].
In multiple myeloma, published studies showed that thalidomide alone as induction therapy or as maintenance approach after chemotherapy is associated with a low incidence of thromboembolism, below 5% [Rajkumar et al. 2003; Weber et al. 2003; Barlogie et al. 2001; Zonder, 2006] and that the major risk is in the first months after diagnosis. Thalidomide associated with dexamethasone was found to lead to increased thromboembolic risk. In a randomized trial by the Eastern Cooperative Oncology Group including 102 newly diagnosed patients with myeloma, the rate of thromboembolic events was 17% in subjects receiving thalidomide plus dexamethasone and 3% in those receiving dexamethasone alone [Rajkumar et al. 2006].
The VTE risk may decrease in relapsed/ refractory patients because they are more likely to be treated with lower doses of thalidomide [Zangari et al. 2007; Niesvizky and Badros, 2010].
There appears to be a striking increase in the risk of developing thromboembolic events during treatment with thalidomide–anthracycline combination regimens, particularly during the first months of therapy [Barlogie et al. 2006]. The risk of developing thromboembolism during therapy with thalidomide-alkylating agent combination therapy may be increased as well, but not to the same degree as with anthracyclines. The main chemotherapeutic regimens including thalidomide and the incidence of thromboembolic events are summarized in Table 2.
Table 2.
Incidences of thromboembolism with regimens including thalidomide plus alkylating agents.
| Regimen | Prophylaxis | Incidence | Reference |
|---|---|---|---|
| THAL 300 mg days 1–4, 17–20 | None | 10% | Dimopoulos et al. [2006] |
| DEX 12 mg/m2 days 1–4, 17–20 | |||
| MEL 8 mg/m2 days 1–4 | None* | 20%* | Palumbo et al. [2006] |
| MEL 4 mg/m2 days 1–7 | |||
| PRED 40 mg/m2 days 1–7 | |||
| THAL 100 mg/day until progression | |||
| MEL 0.25 mg/kg days 1–4 | None | 12% | Facon et al. [2007] |
| PRED 2 mg/kg days 1–4 | |||
| THAL 100-400 mg/day until end of MP | |||
| MEL 0.2 mg/kg days 1–4 | NR | 6% | Hulin et al. [2009] |
| PRED 2 mg/kg days 1–4 | |||
| THAL100 mg/day§ | |||
| MEL: 0.25 mg/kg days 1–4 | 40% | 8% | Waage et al. [2010] |
| PRED: 100 mg/day days 1–4 for 6-week cycles until plateau | |||
| THAL: 400 mg/day until plateau, reduced to 200 mg/day until progression | |||
| MEL: 0.25 mg/kg | NR | 10% | Wijermans et al. [2010] |
| PRED: 1 mg/kg days 1–5 | |||
| THAL: 200 mg/day for eight 4-week cycles, followed by 50 mg/day until relapse | |||
| MEL: 9 mg/m2 days 1–4 | Yes | 22.4% | Beksac et al. [2011] |
| PRED: 60 mg/m2 days 1–4 | |||
| THAL: 100 mg/day for eight 6-week cycles, followed by100 mg/day until relapse | |||
| BOR: 1.3 mg/m2 days 1, 4, 8, 11, 22, 25, 29 and 32 during cycles 1–4; days 1, 8, 15 and 22 during cycles 5–9 | Yes | 5% | Palumbo et al. [2010] |
| MEL: 9 mg/m2 days 1–4 | |||
| PRED: 60 mg/m2 days 1–4 | |||
| THAL: 50 mg daily for nine 6-week cycles | |||
| CTX 500 mg orally days 1, 8, 15 | None | 3.2% | Sidra et al. [2006] |
| DEX 40 mg days 1–4, 15–18 | |||
| THAL 100–200 mg/day | |||
| CTX 300 mg/m2 each week | Warfarin | 11.5% | Kyriakou et al. [2005] |
| DEX 40 mg/day for 4 days each month 1 mg/day | |||
| THAL ≤300 mg/day | |||
| CTX 50 mg/day | None | 7% | García-Sanz et al. [2004] |
| DEX 40 mg/day for 4 days every 3 weeks | |||
| THAL 200–800 mg/day | |||
| THAL 400 mg/day days 1–5, 14–18 | None | 4% | Dimopoulos et al. [2004] |
| DEX 20 mg/m2 days 1–4, 15–18 | |||
| CTX 150 mg/m2 orally every 12 hours, days 1-5 | |||
| THAL 100–400 mg/day | None | 3% | Hovenga et al. [2005] |
| CTX 100–150 mg/day | |||
| DEX 20 mg/m2 days 1–4, 9–12, 17–20 | None | 9% | Kropff et al. [2003] |
| THAL 100–400 mg/day | |||
| CTX 300 mg/m2 every 12 hours, days 1-3 |
After addition of enoxaparin 40 mg daily, incidence decreased to 3%, resulting in overall incidence of 12% for patients receiving MP + thalidomide on this study.
A dose reduction to 50 mg per day of thalidomide or placebo was allowed at the investigator’s discretion in the event of patient intolerance to the 100 mg/day dose, especially in case of mild or moderate peripheral neuropathy (grade 1 or 2).
BOR, bortezomib; CTX, cyclophosphamide; DEX, dexamethasone; MEL, melphalan; MP, melphalan + prednisone; NR, not reported; PRED, prednisone; THAL, thalidomide.
In conclusion, myeloma therapy-related risk factors include use of doxorubicin, high-dose steroids and combination chemotherapy [Bauer, 2001; Snowden et al. 2011].
Thromboprophylaxis approaches available
Several classes of agents have been used for prophylaxis of VTE. Most commonly used pharmacologic agents for thromboprophylaxis of VTE include: ASA, unfractionated heparin (UH; standard, low-dose or adjusted-dose), oral anticoagulants such as warfarin and LMWH.
To date, only one randomized study is currently available. This study compared the use of ASA and fixed low-dose warfarin versus LMWH to prevent thromboembolism in myeloma patients receiving thalidomide [Palumbo et al. 2011]; 667 patients with previously untreated myeloma who received thalidomide-containing regimens (with or without bortezomib), were randomly assigned to receive ASA (100 mg/day), warfarin (1.25 mg/day) or LMWH (enoxaparin 40 mg/day). There were 6.5% who had serious thromboembolic events, acute cardiovascular events or sudden death during the first 6 months (6.4% in the ASA group, 8.2% in the warfarin group and 5.0% in the LMWH group). Compared with LMWH, the absolute differences were 1.3% in the ASA group and 3.2% in the warfarin group. The risk of thromboembolism was 1.38 times higher in patients treated with thalidomide without bortezomib, suggesting a protective role of bortezomib against VTE. ASA and warfarin showed similar efficacy compared with LMWH, except in elderly patients, where warfarin was shown to be less effective than LMWH.
LMWH
The recommended dosage of LMWH in thalidomide treated patients is within the prophylactic range [Zangari et al. 2004]. The use of LMWH is more appropriate for cytopenic (mainly thrombocytopenic) patients. Nevertheless, this type of prophylaxis may increase the hemorrhagic risk and patients should be carefully monitored. Additional limiting aspects are the high cost of treatment, the lower patient compliance because of the need for self-injection and also the risk of renal impairment [Palumbo et al. 2011]. Indeed, most of LMWH is cleared via the renal route and LMWH therapy should be adjusted when the creatinine clearance is <30 ml/min to avoid drug accumulation and consequently an increase risk of bleeding. Kidney function is often altered in patients with multiple myeloma, thus a constant close monitoring is necessary when administering LMWH to these patients [Dimopoulos et al. 2008].
Aspirin
ASA showed to reduce the risk of arterial complication [Cohen et al. 1994] but its role in venous thromboprophylaxis remains controversial. Although LMWH and warfarin-type prophylaxes are considered more efficient, there are data demonstrating that ASA reduces the risk of VTE in multiple myeloma patients treated with thalidomide [Hovens et al. 2006; Niesvizky et al. 2007]. One trial has reported a protective role of ASA in first-line therapy of patients with multiple myeloma treated with thalidomide and anthracyclines. The rate of thrombosis dramatically decreased (from 55% to 18%) when ASA was administered [Baz et al. 2005]. A significant risk reduction in both arterial and venous thrombosis with ASA was seen also in other similar studies [Pulmonary Embolism Prevention (PEP) Trial Collaborative Group, 2000].
Warfarin
Warfarin is an oral anticoagulant commonly prescribed to treat VTE and to decrease the risk of VTE in AF [Ansell et al. 2008]. Warfarin therapy is complicated by the wide interindividual variation in response and dose requirements for adequate anticoagulation. Optimal warfarin therapy is achieved by maintaining the anticoagulation response, International Normalized Ratio (INR), within a narrow therapeutic range of 2.0–3.0 for most indications. In multiple myeloma, warfarin-type drugs increase the incidence of hemorrhage when the INR is above 3. This bleeding risk is further increased in patients who become thrombocytopenic particularly with a platelet count below 80 g/l. Owing to the unpredictable pharmacokinetic and pharmacodynamic responses to warfarin, initiation of therapy is the most clinically challenging phase as the optimal dose is often determined iteratively, guided by INR [Landefeld and Beyth, 1993]. Low-dose warfarin corresponds to a dose of 2.5–5 mg/day, while full dose warfarin corresponds to the dose adjusted according to INR (target 2.0–3.0). Fixed low-dose warfarin corresponds to warfarin 1–1.25 mg/day.
How to manage thromboembolic prophylaxis in patients receiving thalidomide-containing regimens
Patients receiving thalidomide with chemotherapy or dexamethasone are at high risk for thrombosis and warrant prophylaxis [Lyman et al. 2007, 2007]. The choice of thromboprophylaxis should be based on the presence of individual and myeloma-related risk factors. Patients may be divided into two subcategories: standard and high-risk patients. If none of these risk factors is present, the patient is considered at standard risk. The simultaneous presence of more than one risk factor exponentially increases the risk of VTE [Palumbo et al. 2008b]. Myeloma-related risk factors such as the combination of thalidomide with high-dose dexamethasone or doxorubicin or multi-agent chemotherapy should be considered as high-risk factors (Table 1).
In newly diagnosed myeloma patients treated with single-agent thalidomide, thromboprophylaxis is not usually recommended, while it is suggested when thalidomide is combined with other agents. The risk of VTE is much lower at relapse, so antithrombotic prophylaxis may be suggested only in high-risk patients in this setting. LMWH or full-dose warfarin should be administered in all patients who receive thalidomide with high-dose dexamethasone or doxorubicin or multi-agent chemotherapy.
LMWH prophylaxis is widely used, while warfarin is scarcely used in the routine clinical practice because of the risk of hemorrhage in patients receiving active chemotherapy that could increase the risk of thrombocytopenia. The correct dose of LMWH to administer for thromboprophylaxis is defined according to the patients’ weight; monitoring of the anti-Xa level is not required except in the case of extremes of body weight (<50 kg, >100 kg), prosthetic heart valves or impaired renal function.
A prophylactic anti-Xa level for LMWH is considered 0.1–0.3 IU/ml 4 hours after a dose of LMWH, in the case of prosthetic heart valves it is higher 0.8–1.2 IU/ml.
In case of thromboprophylaxis using dalteparin, the dose to administer is 2500–5000 units daily, while enoxaparin is 40 mg once daily.
Renal impairment may be a limit in the use of LMWH prophylaxis, due to the renal elimination of the drug, the consequent risk of accumulation and increased risk of bleeding. In this case, full-dose warfarin is recommended.
In patients presenting with a standard risk to develop VTE or treated with thalidomide as single-agent, anti-aggregation therapy with low-dose ASA should be considered. In the case of the presence of additional risk factors, which increase the risk of VTE, even in patients receiving thalidomide as a single agent, LWMH or full-dose warfarin are recommended (Table 1). Thromboprophylaxis should be continued for 3–6 months. Treatment with LMWH should be given for at least 6 months. Indefinite treatment should be considered for selected patients with active cancer, such as those with active disease and receiving chemotherapy. Anticoagulation should be avoided in the presence of intracranial bleeding, recent surgery, preexisting bleeding diathesis such as thrombocytopenia, platelet count <50,000/µl or coagulopathy.
For myeloma patients, constant monitoring of platelet count during chemotherapy and antithrombotic prophylaxis is necessary.
Rivaroxaban and dabigatran etexilate are the first new oral anticoagulants recently approved in the European Union, Canada and many other countries worldwide for the prevention of VTE in adult patients undergoing elective hip or knee replacement surgery [Friedman, 2009]. Fondaparinux is the first agent of a new class of selective factor Xa inhibitors (pentasaccharides). This drug was first approved for VTE prophylaxis in patients undergoing major orthopedic procedures and more recently was also approved to manage PE and DVT [Buller et al. 2003, 2004].
These newer and effective antithrombotic agents with reduced drug interactions and without the need for constant monitoring could optimize patient care and eventually modify the indication for use and duration of thromboprophylaxis, but further investigation is needed.
Conclusion
In conclusion, myeloma patients treated with thalidomide require routine thromboprophylaxis. ASA, LMWH and warfarin showed similar efficacy in reducing serious thromboembolic events, except in elderly patients where fixed low-dose warfarin showed less efficacy than LMWH. Although it could be an attractive option, fixed low-dose warfarin needs more extensive investigation.
The specific thromboprophylaxis should be given according to individual risk factors and the type of therapy. It is crucial to identify patients at high risk of VTE in order to intervene promptly and to administer an appropriate prophylaxis, thus limiting the incidence of serious adverse events.
Acknowledgments
The authors thank the editorial assistant, Giorgio Schirripa.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Antonio Palumbo has received honoraria from Celgene, Janssen-Cilag, Merck, Amgen and served on the advisory committee of Celgene, Janssen-Cilag. Carmela Palladino has no conflicts of interest.
Contributor Information
Antonio Palumbo, Myeloma Unit, Division of Hematology, University of Torino, AOU San Giovanni Battista, Torino, Italy.
Carmela Palladino, Myeloma Unit, Division of Hematology, University of Torino, AOU San Giovanni Battista, Torino, Italy.
References
- Ageno W., Squizzato A., Garcia D., Imberti D. (2006) Epidemiology and risk factors of venous thromboembolism. Semin Thromb Hemost 32: 651–658 [DOI] [PubMed] [Google Scholar]
- Anaissie E., Coleman E., Goodwin J., Kennedy R., Lockhart K., Stewart C., et al. (2012) Prophylactic recombinant erythropoietin therapy and thalidomide are predictors of venous thromboembolism in patients with multiple myeloma: limited effectiveness of thromboprophylaxis. Cancer 118: 549–557 [DOI] [PubMed] [Google Scholar]
- Anderson K. (2005) Lenalidomide and thalidomide: mechanisms of action–similarities and differences. Semin Hematol 42(4 Suppl. 4): S3–S8 [DOI] [PubMed] [Google Scholar]
- Ansell J., Hirsh J., Hylek E., Jacobson A., Crowther M., Palareti G. (2008) Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines (8th Edition). Chest 133: 160S–198S [DOI] [PubMed] [Google Scholar]
- Barlogie B., Desikan R., Eddlemon P., Spencer T., Zeldis J., Munshi N., et al. (2001) Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood 98: 492–494 [DOI] [PubMed] [Google Scholar]
- Barlogie B., Tricot G., Anaissie E., Shaughnessy J., Rasmussen E., van Rhee F., et al. (2006) Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med 354: 1021–1030 [DOI] [PubMed] [Google Scholar]
- Bassand J., Hamm C., Ardissino D., Boersma E., Budaj A., Fernández-Avilés F., et al. (2007) Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 28: 1598–1660 [DOI] [PubMed] [Google Scholar]
- Bauer K. (2001) The thrombophilias: well-defined risk factors with uncertain therapeutic implications. Ann Intern Med 135: 367–373 [DOI] [PubMed] [Google Scholar]
- Baz R., Li L., Kottke-Marchant K., Srkalovic G., McGowan B., Yiannaki E., et al. (2005) The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc 80: 1568–1574 [DOI] [PubMed] [Google Scholar]
- Beksac M., Haznedar R., Firatli-Tuglular T., Ozdogu H., Aydogdu I., Konuk N., et al. (2011) Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol 86: 16–22 [DOI] [PubMed] [Google Scholar]
- Bick R. (2003) Cancer-associated thrombosis. N Engl J Med 349: 109–111 [DOI] [PubMed] [Google Scholar]
- Blom J., Doggen C., Osanto S., Rosendaal F. (2005) Malignancy, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293: 715–722 [DOI] [PubMed] [Google Scholar]
- Buller H., Davidson B., Decousus H., Gallus A., Gent M., Piovella F., et al. (2003) Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 349: 1695–1702. [DOI] [PubMed] [Google Scholar]
- Buller H., Davidson B., Decousus H., Gallus A., Gent M., Piovella F., et al. (2004) Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: A randomized trial. Ann Intern Med 140: 867–873. [DOI] [PubMed] [Google Scholar]
- Cohen A., Skinner J., Kakkar V. (1994) Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ 309: 1213–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso A., Lorenzi A., Terulla V., Airò F., Varettoni M., Mangiacavalli S., et al. (2004) Modification of thrombomodulin plasma levels in refractory myeloma patients during treatment with thalidomide and dexamethasone. Ann Hematol 83: 588–591 [DOI] [PubMed] [Google Scholar]
- Dimopoulos M., Anagnostopoulos A., Terpos E., Repoussis P., Zomas A., Katodritou E., et al. (2006) Primary treatment with pulsed melphalan, dexamethasone and thalidomide for elderly symptomatic patients with multiple myeloma. Haematologica 91: 252–254 [PubMed] [Google Scholar]
- Dimopoulos M., Anagnostopoulos A., Weber D. (2003) Treatment of plasma cell dyscrasias with thalidomide and its derivates. J Clin Oncol 21: 4444–4454 [DOI] [PubMed] [Google Scholar]
- Dimopoulos M., Hamilos G., Zomas A., Gika D., Efstathiou E., Grigoraki V., et al. (2004) Pulsed cyclophosphamide, thalidomide and dexamethasone: an oral regimen for previously treated patients with multiple myeloma. Hematol J 5: 112–117 [DOI] [PubMed] [Google Scholar]
- Dimopoulos M.A., Kastritis E., Rosinol L., Bladé J., Ludwig H. (2008) Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 22: 1485–1493 [DOI] [PubMed] [Google Scholar]
- Eby C., Blinder M. (2003) Hemostatic complications associated with paraproteinemias. Curr Hematol Rep 2: 388–394 [PubMed] [Google Scholar]
- Facon T., Mary J., Hulin C., Benboubker L., Attal M., Pegourie B., et al. (2007) Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 370: 1209–1218 [DOI] [PubMed] [Google Scholar]
- FDA (2010) Questions and Answers on Medication Guides for Erythropoiesis-Stimulating Agents (ESAs). Accessed 12 December 2011, at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109380.htm
- Fox E., Kahn S. (2005) The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost 94: 362–365 [DOI] [PubMed] [Google Scholar]
- Friedman R. (2009) New oral anticoagulants for thromboprophylaxis after total hip or knee arthroplasty. Orthopedics 32: 79–84 [DOI] [PubMed] [Google Scholar]
- Fuster V., Moreno P., Fayad Z., Corti R., Badimon J. (2005) Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 46: 937–954 [DOI] [PubMed] [Google Scholar]
- García-Sanz R., González-Porras J., Hernández J., Polo-Zarzuela M., Sureda A., Barrenetxea C., et al. (2004) The oral combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is effective in relapsed/refractory multiple myeloma. Leukemia 18: 856–63 [DOI] [PubMed] [Google Scholar]
- Heit J., O’Fallon W., Petterson T., Lohse C., Silverstein M., Mohr D., et al. (2002) Relative impact of risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med 162: 1245–1248 [DOI] [PubMed] [Google Scholar]
- Heit J., Silverstein M., Mohr D., Petterson T., O’Fallon W., Melton L. (1999) Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 159: 445–453 [DOI] [PubMed] [Google Scholar]
- Heit J., Silverstein M., Mohr D., Petterson T., O’Fallon W., Melton L. (2000) Risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med 160: 809–815 [DOI] [PubMed] [Google Scholar]
- Hovenga S., Daenen S., de Wolf J., van Imhoff G., Kluin-Nelemans H., Sluiter W., et al. (2005) Combined thalidomide and cyclophosphamide treatment for refractory or relapsed multiple myeloma patients: a prospective phase II study. Ann Hematol 84: 311–316 [DOI] [PubMed] [Google Scholar]
- Hovens M., Snoep J., Tamsma J., Huisman M. (2006) Aspirin in the prevention and treatment of venous thromboembolism. J Thromb Haemost 4: 1470–1475 [DOI] [PubMed] [Google Scholar]
- Hulin C., Facon T., Rodon P., Pegourie B., Benboubker L., Doyen C., et al. (2009) Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol 27: 3664–3670 [DOI] [PubMed] [Google Scholar]
- Kaushal V., Kaushal G., Melkaveri S., Mehta P. (2004) Thalidomide protects endothelial cells from doxorubicin-induced apoptosis but alters cell morphology. J Thromb Haemost 2: 327–334 [DOI] [PubMed] [Google Scholar]
- Kristinsson S. (2010) Thrombosis in multiple myeloma. Hematology Am Soc Hematol Educ Program 2010: 437–444. [DOI] [PubMed] [Google Scholar]
- Kropff M., Lang N., Bisping G., Dominé N., Innig G., Hentrich M., et al. (2003) Hyperfractionated cyclophosphamide in combination with pulsed dexamethasone and thalidomide (HyperCDT) in primary refractory or relapsed multiple myeloma. Br J Haematol 122: 607–616 [DOI] [PubMed] [Google Scholar]
- Kumar S., Gertz M., Dispenzieri A., Lacy M., Geyer S., Iturria N., et al. (2003) Response rate, durability of response, and survival after thalidomide therapy for relapsed multiple myeloma. Mayo Clin Proc 78: 34–39 [DOI] [PubMed] [Google Scholar]
- Kyriakou C., Thomson K., D’Sa S., Flory A., Hanslip J., Goldstone A., et al. (2005) Low-dose thalidomide in combination with oral weekly cyclophosphamide and pulsed dexamethasone is a well tolerated and effective regimen in patients with relapsed and refractory multiple myeloma. Br J Haematol 129: 763–770 [DOI] [PubMed] [Google Scholar]
- Lacy M. (2010) Arterial thrombosis complicates myeloma. Blood 116: 2–3 [DOI] [PubMed] [Google Scholar]
- Landefeld C., Beyth R. (1993) Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 95: 315–328 [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D., Adams R., Brown T., Carnethon M., Dai S., De Simone G., et al. (2010) Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215 [DOI] [PubMed] [Google Scholar]
- Lyman G.H., Khorana A.A., Falanga A., Clarke-Pearson D., Flowers C., Jahanzeb M., et al. (2007) American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 25: 5490–5505 [DOI] [PubMed] [Google Scholar]
- Neben K., Moehler T., Benner A., Kraemer A., Egerer G., Ho A., et al. (2002) Dose-dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin Cancer Res 8: 3377–3382 [PubMed] [Google Scholar]
- Niesvizky R., Badros A. (2010) Complications of multiple myeloma therapy, part 2: risk reduction and management of venous thromboembolism, osteonecrosis of the jaw, renal complications, and anemia. J Natl Compr Canc Netw 8: S13–S20 [DOI] [PubMed] [Google Scholar]
- Niesvizky R., Martínez-Baños D., Jalbrzikowski J., Christos P., Furst J., De Sancho M., et al. (2007) Prophylactic low-dose aspirin is effective antithrombotic therapy for combination treatments of thalidomide or lenalidomide in myeloma. Leuk Lymphoma 48: 2330–2337 [DOI] [PubMed] [Google Scholar]
- Osman K., Comenzo R., Rajkumar S. (2001) Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med 344: 1951–1952 [DOI] [PubMed] [Google Scholar]
- Palumbo A., Bringhen S., Caravita T., Merla E., Capparella V., Callea V., et al. (2006) Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 367: 825–831 [DOI] [PubMed] [Google Scholar]
- Palumbo A., Bringhen S., Rossi D., Cavalli M., Larocca A., Ria R., et al. (2010) Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol 28: 5101–5109 [DOI] [PubMed] [Google Scholar]
- Palumbo A., Cavo M., Bringhen S., Zamagni E., Romano A., Patriarca F., et al. (2011) Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol 29: 986–993 [DOI] [PubMed] [Google Scholar]
- Palumbo A., Facon T., Sonneveld P., Bladè J., Offidani M., Gay F., et al. (2008a) Thalidomide for treatment of multiple myeloma: 10 years later. Blood 111: 3968–3977 [DOI] [PubMed] [Google Scholar]
- Palumbo A., Rajkumar S., Dimopoulos M., Richardson P., San Miguel J., Barlogie B., et al. (2008b) Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 22: 414–423 [DOI] [PubMed] [Google Scholar]
- Prandoni P., Falanga A., Piccioli A. (2005) Cancer and venous thromboembolism. Lancet Oncol 6: 401–410 [DOI] [PubMed] [Google Scholar]
- Pulmonary Embolism Prevention (PEP) Trial Collaborative Group (2000) Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 355: 1295–1302 [PubMed] [Google Scholar]
- Rajkumar S., Blood E., Vesole D., Fonseca R., Greipp P.(2006) Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 24: 431–436 [DOI] [PubMed] [Google Scholar]
- Rajkumar S., Gertz M., Lacy M., Dispenzieri A., Fonseca R., Geyer S., et al. (2003) Thalidomide as initial therapy for early-stage myeloma. Leukemia 17: 775–779 [DOI] [PubMed] [Google Scholar]
- Rosamond W., Flegal K., Furie K., Go A., Greenlund K., Haase N., et al. (2008) Heart disease and stroke statistics - 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146 [DOI] [PubMed] [Google Scholar]
- Samama M. (2000) An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med 160: 3415–3420 [DOI] [PubMed] [Google Scholar]
- Scarpace S., Hahn T., Roy H., Brown K., Paplham P., Chanan-Khan A., et al. (2005) Arterial thrombosis in four patients treated with thalidomide. Leuk Lymphoma 46: 239–242 [DOI] [PubMed] [Google Scholar]
- Sidra G., Williams C., Russell N., Zaman S., Myers B., Byrne J. (2006) Combination chemotherapy with cyclophosphamide, thalidomide and dexamethasone for patients with refractory, newly diagnosed or relapsed myeloma. Haematologica 91: 862–863 [PubMed] [Google Scholar]
- Silverstein M., Heit J., Mohr D., Petterson T., O’Fallon W., Melton L. (1998) Trends in the incidence of deep vein thrombosis and pulmonary embolism. A 25-year population-based study. Arch Intern Med 158: 585–593 [DOI] [PubMed] [Google Scholar]
- Snowden J.A., Ahmedzai S.H., Ashcroft J., D’Sa S., Littlewood T., Low E., et al. (2011) Guidelines for supportive care in multiple myeloma 2011. Br J Haematol 154: 76–103 [DOI] [PubMed] [Google Scholar]
- Srkalovic G., Cameron M., Rybicki L., Deitcher S., Kattke-Marchant K., Hussein M. (2004) Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer 101: 558–566 [DOI] [PubMed] [Google Scholar]
- Streetly M., Hunt B., Parmar K., Jones R., Zeldis J., Schey S. (2005) Markers of endothelial and haemostatic function in the treatment of relapsed myeloma with the immunomodulatory agent Actimid (CC-4047) and their relationship with venous thrombosis. Eur J Haematol 74: 293–296 [DOI] [PubMed] [Google Scholar]
- Teo S. (2005) Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J 7: E14–E19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Kantarjian H. (2001) The revitalization of thalidomide. Ann Oncol 12: 885–886 [DOI] [PubMed] [Google Scholar]
- Tseng S., Pak G., Washenik K., Pomeranz M., Shupack J. (1996) Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol 35: 969–979 [DOI] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F., Fantuzzi G., Mendoza L., Fuentes A., Anasagasti M., Martín J., et al. (2000) IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A 97: 734–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Gimsing P., Fayers P., Abildgaard N., Ahlberg L., Björkstrand B., et al. (2010) Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood 116: 1405–1412 [DOI] [PubMed] [Google Scholar]
- Weber D. (2003) Thalidomide and its derivatives: new promise for multiple myeloma. Cancer Control 10: 375–83 [DOI] [PubMed] [Google Scholar]
- Weber D., Rankin K., Gavino M., Delasalle K., Alexanian R. (2003) Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol 21: 16–19 [DOI] [PubMed] [Google Scholar]
- White R., Zhou H., Romano P. (2003) Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 90: 446–455 [DOI] [PubMed] [Google Scholar]
- Wijermans P., Schaafsma M., Termorshuizen F., Ammerlaan R., Wittebol S., Sinnige H., et al. (2010) Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol 28: 3160–3166 [DOI] [PubMed] [Google Scholar]
- Yakoub-Agha I., Attal M., Dumontet C., Delannoy V., Moreau P., Berthou C., et al. (2002) Thalidomide in patients with advanced multiple myeloma: a study of 83 patients – report of the Intergroupe Francophone du Myelome (IFM). Hematol J 3: 185–192 [DOI] [PubMed] [Google Scholar]
- Zangari M., Barlogie B., Anaissie E., Saghafifar F., Eddlemon P., Jacobson J., et al. (2004) Deep vein thrombosis in patients with multiple myeloma treated with thalidomide and chemotherapy: effects of prophylactic and therapeutic anticoagulation. Br J Haematol 126: 715–721 [DOI] [PubMed] [Google Scholar]
- Zangari M., Elice F., Fink L., Tricot G. (2007) Thrombosis in multiple myeloma. Expert Rev Anticancer Ther 7: 307–315 [DOI] [PubMed] [Google Scholar]
- Zangari M., Saghafifar F., Mehta P., Barlogie B., Fink L., Tricot G. (2003) The blood coagulation mechanism in multiple myeloma. Semin Thromb Hemost 29: 275–282 [DOI] [PubMed] [Google Scholar]
- Zonder J. (2006) Thrombotic complications of myeloma therapy. Hematology Am Soc Hematol Educ Program: 348–355 [DOI] [PubMed] [Google Scholar]


