Abstract
Electronic cigarettes are a recent development in tobacco harm reduction. They are marketed as less harmful alternatives to smoking. Awareness and use of these devices has grown exponentially in recent years, with millions of people currently using them. This systematic review appraises existing laboratory and clinical research on the potential risks from electronic cigarette use, compared with the well-established devastating effects of smoking tobacco cigarettes. Currently available evidence indicates that electronic cigarettes are by far a less harmful alternative to smoking and significant health benefits are expected in smokers who switch from tobacco to electronic cigarettes. Research will help make electronic cigarettes more effective as smoking substitutes and will better define and further reduce residual risks from use to as low as possible, by establishing appropriate quality control and standards.
Keywords: electronic cigarettes, e-liquid, e-vapor, harm reduction, nicotine, safety, tobacco
Introduction
Complete tobacco cessation is the best outcome for smokers. However, the powerful addictive properties of nicotine and the ritualistic behavior of smoking create a huge hurdle, even for those with a strong desire to quit. Until recently, smokers were left with just two alternatives: either quit or suffer the harmful consequences of continued smoking. This gloomy scenario has allowed the smoking pandemic to escalate, with nearly 6 million deaths annually and a predicted death toll of 1 billion within the 21st century [World Health Organization, 2013]. But a third choice, involving the use of alternative and much safer sources of nicotine with the goal to reduce smoking-related diseases is now available: tobacco harm reduction (THR) [Rodu and Godshall, 2006].
Electronic cigarettes (ECs) are the newest and most promising products for THR [Polosa et al. 2013b]. They are electrically-driven devices consisting of the battery part (usually a lithium battery), and an atomizer where liquid is stored and is aerosolized by applying energy and generating heat to a resistance encircling a wick. The liquid used mainly consists of propylene glycol, glycerol, distilled water, flavorings (that may or may not be approved for food use) and nicotine. Consumers (commonly called ‘vapers’) may choose from several nicotine strengths, including non-nicotine liquids, and a countless list of flavors; this assortment is a characteristic feature that distinguishes ECs from any other THR products. Since their invention in 2003, there has been constant innovation and development of more efficient and appealing products. Currently, there are mainly three types of devices available [Dawkins, 2013], depicted in Figure 1. (1) First-generation devices, generally mimicking the size and look of regular cigarettes and consisting of small lithium batteries and cartomizers (i.e. cartridges, which are usually prefilled with a liquid that bathes the atomizer). Batteries may be disposable (to be used once only) or rechargeable. (2) Second-generation devices, consisting mainly of higher-capacity lithium batteries and atomizers with the ability to refill them with liquid (sold in separate bottles). In the most recent atomizers you can simply change the atomizer head (resistance and wick) while keeping the body of the atomizer, thus reducing the operating costs. (3) Third-generation devices (also called ‘Mods’, from modifications), consisting of very large-capacity lithium batteries with integrated circuits that allow vapers to change the voltage or power (wattage) delivered to the atomizer. These devices can be combined with either second-generation atomizers or with rebuildable atomizers, where the consumers have the ability to prepare their own setup of resistance and wick.
Figure 1.

Examples of electronic cigarette devices currently available on the market.
Awareness and use (vaping) of ECs has increased exponentially in recent years. Data obtained from the HealthStyles survey showed that, in the US, awareness of ECs rose from 40.9–57.9% from 2010 to 2011, with EC use rising from 3.3–6.2% over the same time period [King et al. 2013]. In the United Kingdom, EC use in regular smokers increased from 2.7% in 2010 to 6.7% in 2012 [Dockrell et al. 2013]. Similar findings were obtained from the International Tobacco Control Four-Country Survey [Adkison et al. 2013]. A recent prospective study in Swiss army recruits showed that 12% of smokers who tried ECs progressed to daily use [Douptcheva et al. 2013]. It must be noted that this increase in EC use has occurred despite the concerns raised by public health authorities about the safety and appropriateness of using these products as alternatives to smoking [National Association of Attorneys General, 2013; Food and Drug Administration, 2009; Mayers, 2009].
The popularity of ECs may be due to their ability to deal both with the physical (i.e. nicotine) and the behavioral component of smoking addiction. In particular, sensory stimulation [Rose and Levin, 1991] and simulation of smoking behavior and cigarette manipulation [Hajek et al. 1989] are important determinants of a product’s effectiveness in reducing or completely substituting smoking. These features are generally absent in nicotine replacement therapies (NRTs) and oral medications for nicotine dependence, whereas ECs are unique in that they provide rituals associated with smoking behavior (e.g. hand-to-mouth movement, visible ‘smoke’ exhaled) and sensory stimulation associated with it [Farsalinos et al. 2013b]. This explains why these products can be effective in reducing consumption of tobacco smoking [Bullen et al. 2013; Caponnetto et al. 2013b; Polosa et al. 2011] and are efficient as long-term substitutes of conventional cigarettes [Farsalinos et al. 2013b].
Methods
For this systematic review (Figure 2), we searched the PubMed electronic database by using keywords related to ECs and/or their combination (e-cigarette, electronic cigarette, electronic nicotine delivery systems). We obtained a total of 354 results, and selected 41 studies we judged relevant to research on EC safety/risk profile. Reference lists from these studies were also examined to identify relevant articles. We searched additional information in abstracts presented at scientific congresses (respiratory, cardiovascular, tobacco control, toxicology), and in reports of chemical analyses on EC samples that were available online. We also looked for selected studies on chemicals related to EC ingredients (e.g. nicotine, propylene glycol, glycerol, cinnamaldehyde, microparticles emission, etc.), but not specifically evaluated in EC research. In total, 97 publications were found, from which 15 chemical analyses of single or a limited number of EC samples were excluded because they were discussed in a review paper [Cahn and Siegel, 2011]. In total, 114 studies are cited in this paper.
Figure 2.
Methodology for literature research and selection of studies.
Risk differences compared with conventional cigarettes and the issue of nicotine
Conventional cigarettes are the most common form of nicotine intake. Smoking-related diseases are pathophysiologically attributed to oxidative stress, activation of inflammatory pathways and the toxic effect of more than 4000 chemicals and carcinogens present in tobacco smoke [Environmental Protection Agency, 1992]. In addition, each puff contains >1 × 1015 free radicals [Pryor and Stone, 1993]. All of these chemicals are emitted mostly during the combustion process, which is absent in ECs. Although the addictive potential of nicotine and related compounds is largely documented [Guillem et al. 2005], much less dissemination has been given to the notion that nicotine does not contribute to smoking-related diseases. It is not classified as a carcinogen by the International Agency for Research on Cancer [WHO-IARC, 2004] and does not promote obstructive lung disease. A major misconception, commonly supported even by physicians, is that nicotine promotes cardiovascular disease. However, it has been established that nicotine itself has minimal effect in initiating and promoting atherosclerotic heart disease [Ambrose and Barua, 2004]. It does not promote platelet aggregation [Zevin et al. 1998], does not affect coronary circulation [Nitenberg and Antony, 1999] and does not adversely alter the lipid profile [Ludviksdottir et al. 1999]. An observational study of more than 33,000 smokers found no evidence of increased risk for myocardial infarction or acute stroke after NRT subscription, although follow up was only 56 days [Hubbard et al. 2005]. Up to 5 years of nicotine gum use in the Lung Health Study was unrelated to cardiovascular diseases or other serious side effects [Murray et al. 1996]. A meta-analysis of 35 clinical trials found no evidence of cardiovascular or other life-threatening adverse effects caused by nicotine intake [Greenland et al. 1998]. Even in patients with established cardiovascular disease, nicotine use in the form of NRTs does not increase cardiovascular risk [Woolf et al. 2012; Benowitz and Gourlay, 1997]. It is anticipated that any product delivering nicotine without involving combustion, such as the EC, would confer a significantly lower risk compared with conventional cigarettes and to other nicotine containing combustible products.
The importance of using nicotine in the long-term was recognized several years ago by Russell, indicating that the potential of nicotine delivery systems as long-term alternatives to tobacco should be explored in order to make the elimination of tobacco a realistic future target [Russell, 1991]. However, current regulations restrict the long-term use of pharmaceutical or recreational nicotine products (such as snus) [Le Houezec et al. 2011]. In other words, nicotine intake has been demonized, although evidence suggests that, besides being useful in smoking cessation, it may even have beneficial effects in a variety of disorders such as Parkinson’s disease [Nielsen et al. 2013], depression [McClernon et al. 2006], dementia [Sahakian et al. 1989] and ulcerative colitis [Guslandi, 1999]. Obviously, the addictive potential is an important factor in any decision to endorse nicotine administration; however, it should be considered as slight ‘collateral damage’ with minimal impact to vapers’ health compared with the tremendous benefit of eliminating all disease-related substances coming from tobacco smoking. In fact, smokers are already addicted to nicotine; therefore the use of a ‘cleaner’ form of nicotine delivery would not represent any additional risk of addiction. Surveys have shown that ECs are used as long-term substitutes to smoking [Dawkins et al. 2013; Etter and Bullen, 2012]. Although consumers try to reduce nicotine use with ECs, many are unable to completely stop its intake, indicating an important role for nicotine in the ECs’ effectiveness as a smoking substitute [Farsalinos et al. 2013b].
Nicotine overdose or intoxication is unlikely to occur with vaping, since the amount consumed [Farsalinos et al. 2013c] and absorbed [Nides et al. 2014; Dawkins and Corcoran, 2013] is quite low. Moreover, although not yet proven, it is expected that vapers will self-titrate their nicotine intake in a similar way to tobacco cigarettes [Benowitz et al. 1998]. Last, but not least, there is evidence suggesting that nicotine cannot be delivered as fast and effectively from ECs compared to tobacco cigarettes [Farsalinos et al. 2014]. Therefore, it seems that ECs have a huge theoretical advantage in terms of health risks compared with conventional cigarettes due to the absence of toxic chemicals that are generated in vast quantities by combustion. Furthermore, nicotine delivery by ECs is unlikely to represent a significant safety issue, particularly when considering they are intended to replace tobacco cigarettes, the most efficient nicotine delivery product.
Studies on the safety/risk profile of ECs
Findings on the safety/risk profile of ECs have just started to accumulate. However, this research must be considered work in progress given that the safety/risk of any product reflects an evolving body of knowledge and also because the product itself is undergoing constant development.
Existing studies about the safety/risk profile of ECs can be divided into chemical, toxicological and clinical studies (Table 1). Obviously, clinical studies are the most informative, but also the most demanding because of several methodological, logistical, ethical and financial challenges. In particular, exploring safety/risk profile in cohorts of well-characterized users in the long-term is required to address the potential of future disease development, but it would take hundreds of users to be followed for a substantial number of years before any conclusions are made. Therefore, most research is currently focused on in vitro effects, with clinical studies confined into evaluation of short-term use or pathophysiological mechanisms of smoking-related diseases.
Table 1.
Types of studies performed to determine safety and to estimate risk from EC use.
| Type of studies | Research subject | Advantages | Disadvantages |
|---|---|---|---|
| Chemical studies | Evaluate the chemical composition of liquids and/or aerosol. Examine environmental exposure (passive ‘vaping’). | Easier and faster to perform. Less expensive. Could realistically be implemented for regulatory purposes. | Usually targeted on specific chemicals. Unknown effects of flavorings when inhaled. No validated protocols for vapor production. Provide no objective evidence about the end results (effects) of use (besides by applying theoretical models). |
| Toxicological studies | Evaluate the effects on cell cultures or experimental animals. | Provide some information about the effects from use. | Difficult to interpret the results in terms of human in vivo effects. More expensive than chemical studies. Need to test aerosol and not liquid. Standards for exposure protocols have not been clearly defined. |
| Clinical studies | Studies on human in vivo effects. | Provide definite and objective evidence about the effects of use. | Difficult and expensive to perform. Long-term follow up is needed due to the expected lag from initiation of use to possible development of any clinically evident disease. For now, limited to acute effects from use. |
Chemical studies
Chemical studies are relatively simple and cheap to perform and provide quick results. However, there are several disadvantages with this approach. Research is usually focused on the known specific chemicals (generally those known to be toxic from studies of cigarette smoke) and fails to address unknown, potentially toxic contaminants that could be detected in the liquid or the emitted aerosol. Problems may also arise from the detection of the chemicals in flavors. Such substances, although approved for use in the food industry, have largely unknown effects when heated and inhaled; thus, information on the presence of such substances is difficult to interpret in terms of in vivo effects. In fact, chemical studies do not provide any objective information about the effects of use; they can only be used to calculate the risk based on theoretical models and on already established safety levels determined by health authorities. An overview of the chemical studies performed on ECs is displayed in Table 2.
Table 2.
Summary of chemical toxicity findings.
| Study | What was investigated? | What were the key findings? |
|
|---|---|---|---|
| Liquid | Vapor | ||
| Laugesen [2009] | Evaluation of 62 toxicants in the EC vapour from Ruyan 16 mg and mainstream tobacco smoke using a standard smoking machine protocol. | N/A | No acrolein, but small quantities of acetaldehyde and formaldehyde found. Traces of TSNAs (NNN, NNK, and NAT) detected. CO, metals, carcinogenic PAHs and phenols not found in EC vapour. Acetaldehyde and formaldehyde from tobacco smoke were 55 and 5 times higher, respectively. |
| Westenberger [2009] | Evaluation of toxicants in EC cartridges from two popular US brands. | TSNAs and certain tobacco specific impurities were detected in both products at very low levels. Diethylene glycol was identified in one cartridge. | N/A |
| Hadwiger et al. [2010] | Evaluation of four refill solutions and six replacement cartridges advertised as containing Cialis or rimonambant. | Small amounts of amino-tandalafil and rimonambant present in all products tested. | N/A |
| Cahn and Siegel [2011] | Overview of 16 chemical toxicity studies of EC liquids/vapours. | TSNAs levels in ECs 500- to 1400-fold lower than those in conventional cigarettes and similar to those in NRTs. Other chemicals found very low levels, which are not expected to result in significant harm. | |
| Pellegrino et al. [2012] | Evaluation of PM fractions and PAHs in the vapour generated from cartomizers of an Italian EC brand. | N/A | PM fractions were found, but levels were 6–18 times lower compared with conventional cigarettes. Traces of PAHs detected. |
| Kim and Shin [2013] | TSNAs (NNN, NNK, NAT, and NAB) content in 105 refill liquids from 11 EC brands purchased in Korean shops. | Total TSNAs averaged 12.99 ng/ml EC liquid; daily total TSNA exposure from conventional cigarettes estimated to be up to 1800 times higher. | N/A |
| Etter et al. [2013] | Nicotine degradation products, ethylene glycol and diethylene glycol evaluation of 20 EC refill liquids from 10 popular brands | The levels of nicotine degradation products represented 0–4.4% of those for nicotine, but for most samples the level was 1–2%. Neither ethylene glycol nor diethylene glycol were detected. | N/A |
| Goniewicz et al. [2013] | Vapours generated from 12 brands of ECs and a medicinal nicotine inhaler using a modified smoking machine protocol | N/A | Carbonyl compounds (formaldehyde, acetaldehyde and acrolein), VOCs (toluene and trace levels of xylene), trace levels of TSNAs (NNN and NNK) and very low levels of metals (cadmium, nickel and lead) were found in almost all examined EC vapours. Trace amounts of formaldehyde, acetaldehyde, cadmium, nickel and lead were also detected from the Nicorette inhalator. Compared with conventional cigarette, formaldehyde, acetaldehyde and acrolein were 9–450 times lower; toluene levels 120 times lower; and NNN and NNK levels 380 and 40 times lower respectively. |
| Williams et al. [2013] | Vapour generated from cartomizers of a popular EC brand using a standard smoking machine protocol | N/A | Trace levels of several metals (including tin, copper, silver, iron, nickel, aluminium, chromium, lead) were found, some of them at higher level compared with conventional cigarettes. Silica particles were also detected. Number of microparticles from 10 EC puffs were 880 times lower compared with one tobacco cigarette. |
| Burstyn [2014] | Systematic review of 35 chemical toxicity studies/technical reports of EC liquids/vapours. | No evidence of levels of contaminants that may be associated with risk to health. These include acrolein, formaldehyde, TSNAs, and metals. Concern about contamination of the liquid by a nontrivial quantity of ethylene glycol or diethylene glycol remains confined to a single sample of an early technology product and has not been replicated. | |
Abbreviations. CO, carbon monoxide; EC, electronic cigarette; NAT, N-Nitrosoanatabine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N-Nitrosonornicotine; PAHs, polycyclic aromatic hydrocarbons; PM, particulate matter; TSNAs, tobacco-specific nitrosamines; VOCs, volatile organic carbons.
Laugesen performed the first studies evaluating the chemical composition of EC aerosols [Laugesen, 2008, 2009]. The temperature of the resistance of the tested EC was 54oC during activation, which is approximately 5–10% of the temperature of a burning tobacco cigarette. Toxic chemicals such as heavy metals, carcinogenic polycyclic aromatic hydrocarbons and phenols were not detected, with the exception of trivial amounts of mercury (0.17 ng per EC) and traces of formaldehyde and acetaldehyde. Laugesen evaluated emissions based on a toxicant emissions score and reported a score of 0 in ECs compared with a score of 100–134 for tobacco cigarettes (Figure 3). The US Food and Drug Administration (FDA) also performed chemical analyses on 18 commercially available products in 2009 [Westenberger, 2009]. They detected the presence of tobacco-specific nitrosamines (TSNAs) but did not declare the levels found. Small amounts of diethylene glycol were also found in one sample, which was unlikely to cause any harm from normal use. Another study identified small amounts of amino-tandalafil and rimonambant in EC liquids [Hadwiger et al. 2010]. Subsequently, several laboratories performed similar tests, mostly on liquids, with Cahn and Siegel publishing a review on the chemical analyses of ECs and comparing the findings with tobacco cigarettes and other tobacco products [Cahn and Siegel, 2011]. They reported that TSNA levels were similar to those measured in pharmaceutical NRTs. The authors concluded that, based on chemical analysis, ECs are far less harmful compared with tobacco cigarettes. The most comprehensive study on TSNAs has been performed recently by a South Korean group, evaluating 105 liquids obtained from local retailers [Kim and Shin, 2013]. On average, they found 12.99 ηg TSNAs per ml of liquid, with the amount of daily exposure to the users estimated to be similar to users of NRTs [Farsalinos et al. 2013d]. The estimated daily exposure to nitrosamines from tobacco cigarettes (average consumption of 15 cigarettes per day) is estimated to be up to 1800 times higher compared with EC use (Table 3). Etter and colleagues evaluated the accuracy of nicotine labeling and the presence of nicotine impurities and degradation products in 20 EC liquid samples [Etter et al. 2013]. They found that nicotine levels were 85–121% of what was labeled, while nicotine degradation products were present at levels of 0–4.4%. Although in some samples the levels were higher than those specified in European Pharmacopoeia, they are not expected to cause any measurable harm to users.
Figure 3.
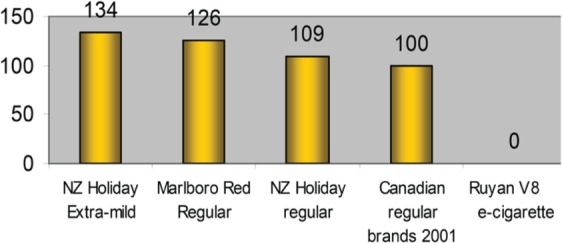
Toxic emissions score, adjusted for nicotine, for electronic cigarette and popular cigarette brands. (Reproduced with permission from Laugesen [2009])
Table 3.
Levels of nitrosamines found in electronic and tobacco cigarettes. Prepared based on information from Laugesen [2009], Cahn and Siegel [2011] and Kim and Shin [2013].
| Product | Total nitrosamines levels (ng) | Daily exposure (ng) | Ratio4 |
|---|---|---|---|
| Electronic cigarette (per ml) | 13 | 521 | 1 |
| Nicotine gum (per piece) | 2 | 482 | 0.92 |
| Winston (per cigarette) | 3365 | 50 4753 | 971 |
| Newport (per cigarette) | 3885 | 50 7753 | 976 |
| Marlboro (per cigarette) | 6260 | 93 9003 | 1806 |
| Camel (per cigarette) | 5191 | 77 8653 | 1497 |
Based on average daily use of 4ml liquid
Based on maximum recommended consumption of 24 pieces per day
Based on consumption of 15 cigarettes per day
Difference (number-fold) between electronic cigarette and all other products in daily exposure to nitrosamines
Besides the evaluation for the presence of TSNAs, analyses have been performed for the detection of carbonyl compounds. It is known that the thermal degradation of propylene glycol and glycerol can lead to the emission of toxic compounds such as aldehydes [Antal et al. 1985; Stein et al. 1983]. Goniewicz and colleagues evaluated the emission of 15 carbonyls from 12 brands of ECs (mostly first-generation) [Goniewicz et al. 2013]. In order to produce vapor, researchers used a smoking machine and followed a regime of 1.8-second puffs with a very short 10-second interpuff interval, which does not represent realistic use [Farsalinos et al. 2013c]; although the puff duration was low, interpuff interval was remarkably short, which could potentially lead to overheating. In addition, the same puff number was used in all devices tested, although there was a significant difference in the design and liquid content between devices. Despite these limitations, out of 15 carbonyls, only 3 were detected (formaldehyde, acetaldehyde and acrolein); levels were 9–450 times lower compared with emissions from tobacco cigarettes (derived from existing literature but not tested in the same experiment). Formaldehyde and acetaldehyde were also emitted from the nicotine inhalator, although at lower levels. In addition, they examined for the presence of 11 volatile organic carbons and found only trace levels of toluene (at levels from 0.2–6.3 µg per 150 puffs) and xylene (from 0.1–0.2 µg per 150 puffs) in 10 of the samples; toluene levels were 120 times lower compared with tobacco cigarettes (again derived from existing literature but not tested in the same experiment).
Given that ECs have several metal parts in direct contact with the e-liquid, it is quite obvious to expect some contamination with metals in the vapor. Goniewicz and colleagues examined samples for the presence of 12 metals and found nickel, cadmium and lead emitted [Goniewicz et al. 2013]; the levels of nickel were similar to those present in a pharmaceutical nicotine inhalator, while lead and cadmium were present at 2–3 times higher levels compared with the inhalator. Still, the absolute levels were very low (few nanograms per 150 puffs). Williams et al. [2013] focused their research on the presence of heavy metals and silicate particles emitted from ECs. They tested poor quality first-generation cartomisers and found several metals emitted in the aerosol of the EC, specifying that in some cases the levels were higher compared with conventional cigarettes. As mentioned earlier, it is not unusual to find trace levels of metals in the vapor generated by these products under experimental conditions that bear little relevance to their normal use; however, it is unlikely that such small amounts pose a serious threat to users’ health. Even if all the aerosol was absorbed by the consumer (which is not the case since most of the aerosol is visibly exhaled), an average user would be exposed to 4–40 times lower amounts for most metals than the maximum daily dose allowance from impurities in medicinal products [US Pharmacopeia, 2013]. Silicate particles were also found in the EC aerosol. Such particles come from the wick material, however the authors did not clarify whether crystalline silica oxide particles were found, which are responsible for respiratory disease. In total, the number of microparticles (< 1000 nm) estimated to be inhaled by EC users from 10 puffs were 880 times lower compared with one tobacco cigarette. Similar findings concerning microparticles were reported by Pellegrino and colleagues who found that, for each particulate matter fraction, conventional cigarettes released 6–18 times higher amounts compared with the EC tested [Pellegrino et al. 2012].
Burstyn has recently reviewed current data on the chemistry of aerosols and the liquids of ECs (including reports which were not peer-reviewed) and estimated the risk to consumers based on workplace exposure standards (i.e. Threshold Limit Values [TLVs]) [Burstyn, 2014]. After reviewing all available evidence, the author concluded that there was no evidence that vaping produced inhalable exposure to contaminants of aerosol that would warrant health concerns. He added that surveillance of use is recommended due to the high levels of propylene glycol and glycerol inhaled (which are not considered contaminants but ingredients of the EC liquid). There are limited data on the chronic inhalation of these chemicals by humans, although there is some evidence from toxicological studies (which are discussed later in this paper).
In conclusion, chemical studies have found that exposure to toxic chemicals from ECs is far lower compared with tobacco cigarettes. Besides comparing the levels of specific chemicals released from tobacco and ECs, it should be taken into consideration that the vast majority of the >4000 chemicals present in tobacco smoke are completely absent from ECs. Obviously, surveillance of use is warranted in order to objectively evaluate the in vivo effects and because the effects of inhaling flavoring substances approved for food use are largely unknown.
Toxicological studies
To date, only a handful of toxicological studies have been performed on ECs, mostly cytotoxicity studies on established cell lines. The cytotoxicity approach also has its flaws. Findings cannot be directly applied to the in vivo situation and there is always the risk of over- (as well as under-)estimating the interpretation of the toxic effects in these investigational models. An ample degree of results variability is to be expected from different cell lines and, sometimes, also within the same cell line. Comparing the potential cytotoxicity effects of EC vapor with those resulting from the exposure of cigarette smoke should be mandatory, but standards for vapor production and exposure protocols have not been clearly defined.
Bahl and colleagues [Bahl et al. 2012] performed cytotoxicity tests on 36 EC liquids, in human embryonic stem cells, mouse neural stem cells and human pulmonary fibroblasts and found that stem cells were more sensitive to the effects of the liquids, with 15 samples being moderately cytotoxic and 12 samples being highly cytotoxic. Propylene glycol and glycerol were not cytotoxic, but a correlation between cytotoxicity and the number and height of the flavoring peaks in high-performance liquid chromatography was noted. Investigations were just restricted to the effect of EC liquids and not to their vapors, thus limiting the importance of the study findings; this is not a trivial issue considering that the intended use of these products is by inhalation only and that it is unlikely that flavoring substances in the EC liquids will still be present in the aerosol in the same amount due to differences in evaporation temperature [Romagna et al. 2013]. Regrettably, a set of experiments with cigarette smoke extracts as comparator was not included. Of note, the authors emphasized that the study could have underestimated the cytotoxicity by 100 times because when they added the EC liquids to the cell, medium final concentration was 1%. However, cells were cultured for 48 hours with continuous exposure to the liquid, while in real use the lungs come in contact with aerosol instead of liquid, the contact lasts for 1–2 seconds per puff and most of the aerosol is visibly exhaled. Finally, Cinnamon Ceylon, the liquid found to be mostly cytotoxic in this study, was not a refill liquid but a concentrated flavor which is not used in ECs unless it is diluted to 3–5%.
Romagna and colleagues [Romagna et al. 2013] performed the first cytotoxicity study of EC vapor on fibroblast cells. They used a standardized ISO 10993-5 protocol, which is used for regulatory purposes of medical devices and products. They tested the vapor of 21 liquid samples containing the same amount of nicotine (9 mg/ml), generated by a commercially available EC device. Cells were incubated for 24 hours with each of these vapors and with smoke from a conventional cigarette. Only one sample was found to be marginally cytotoxic, whereas cigarette smoke was highly cytotoxic (approximately 795% more cytotoxic), even when the extract was diluted up to 25% of the original concentration.
The same group also investigated the cytotoxic potential of 20 EC liquid samples in cardiomyoblasts [Farsalinos et al. 2013a]. Vapor was produced by using a commercially available EC device. Samples contained a wide range of nicotine concentrations. A base liquid mixture of propylene glycol and glycerol (no nicotine and no flavorings) was also included as an additional experimental control. Four of the samples examined were made by using cured tobacco leaves in a steeping process, allowing them to impregnate a mixture of propylene glycol and glycerol for several days before being filtered and bottled for use. Of note, this was the first study which evaluated a limited number of samples with an EC device delivering higher voltage and energy to the atomizer (third-generation device). In total, four samples were found to be cytotoxic; three of them were liquids made by using cured tobacco leaves, with cytotoxicity observed at both 100% and 50% extract concentration, while one sample (cinnamon flavor) was marginally cytotoxic at 100% extract concentration only. In comparison, smoke from three tobacco cigarettes was highly cytotoxic, with toxicity observed even when the extract was diluted to 12.5%. The samples made with tobacco leaves were three times less cytotoxic compared with cigarette smoke; this was probably due to the absence of combustion and the significantly lower temperature of evaporation in EC use. Concerning high-voltage EC use, the authors found slightly reduced cell viability without any of the samples being cytotoxic according to the ISO 10993-5 definition. Finally, no association between cell survival and the amount of nicotine present in the liquids was noted.
A recent study evaluated in more detail the cytotoxic potential of eight cinnamon-flavored EC liquids in human embryonic stem cells and human pulmonary fibroblasts [Behar et al. 2014]. The authors found that the flavoring substance predominantly present was cinnamaldehyde, which is approved for food use. They observed significant cytotoxic effects, mostly on stem cells but also on fibroblasts, with cytotoxicity associated with the amount of cinnamaldehyde present in the liquid. However, major methodological issues arose from this study. Once again, cytotoxicity was just restricted to EC liquids and not to their vapors. Moreover, the authors mentioned that the amount of cinnamaldehyde differed between liquids by up to 100 times, and this raises the suspicion of testing concentrated flavor rather than refills. By searching the internet and contacting manufacturers, based on the names of samples and suppliers mentioned in the manuscript, it was found that at least four of their samples were not refills but concentrated flavors. Surprisingly, the levels of cinnamaldehyde found to be cytotoxic were about 400 times lower than those currently approved for use [Environmental Protection Agency, 2000].
Few animal studies have been performed to evaluate the potential harm of humectants in EC liquids (i.e. propylene glycol and glycerol) when given by inhalation. Robertson and colleagues tested the effects on primates of inhaling propylene glycol vapor for several months and found no evidence of toxicity on any organ (including the lungs) after post-mortem examination of the animals [Robertson et al. 1947]. Similar observations were made in a recent study in rats and dogs [Werley et al. 2011]. Concerns have been raised in human use, based on studies of people exposed to theatrical fog [Varughese et al. 2005; American Chemistry Council, 2003] or propylene glycol used in the aviation industry [Wieslander et al. 2001]. Irritation of the respiratory tract was found, but no permanent lung injury or other long-term health implications were detected. It should be reminded that, in these circumstances, nonpharmaceutical purity propylene glycol is used and in some cases oils are added, making it difficult to interpret the results in the context of EC use. Evidence for the potential harm of inhaled glycerol is sparse. A study using Sprague–Dawley rats found minimal to mild squamous metaplasia of the epiglottis epithelium in the high-dose group only, without any changes observed in lungs or other organs [Renne et al. 1992]. No comparative set of experiments with cigarette smoke was included, but it is well known that exposure to tobacco smoke in similar animal models leads to dramatic changes in the lungs, liver and kidneys [Czekaj et al. 2002].
In conclusion, toxicological studies have shown significantly lower adverse effects of EC vapor compared with cigarette smoke. Characteristically, the studies performed by using the liquids in their original liquid form have found less favorable results; however, no comparison with tobacco smoke was performed in any of these studies, and they cannot be considered relevant to EC use since the samples were not tested in the form consumed by vapers. More research is needed, including studies on different cell lines such as lung epithelial cells. In addition, it is probably necessary to evaluate a huge number of liquids with different flavors since a minority of them, in an unpredictable manner, appear to raise some concerns when tested in the aerosol form produced by using an EC device.
Clinical studies and research surveys
Clinical trials can be very informative, but they require monitoring of hundreds of users for many years to adequately explore the safety/risk profile of the products under investigation. Research surveys of EC users, on the other hand, can quickly provide information about the potential harm of these products and are much cheaper to run. However, self-reported data, highly self-selected study populations, and the cross-sectional design are some of the most common limitations of research surveys. Taken together, findings from surveys and follow-up studies of vapers have shown that EC use is relatively safe.
Polosa and colleagues followed up smokers for 24 months, after a 6-month period of intervention during which ECs were given [Polosa et al. 2013a]. Only mild symptoms such as mouth and throat irritation and dry cough were observed. Farsalinos and colleagues retrospectively evaluated a group of 111 EC users who had completely quit smoking and were daily EC users for a median period of 8 months [Farsalinos et al. 2013b]. Throat irritation and cough were the most commonly reported side effects. Similar findings have been observed in surveys [Dawkins et al. 2013; Etter et al. 2011]. However, it is expected that dedicated users who have more positive experiences and fewer side effects compared with the general population participate in such studies, therefore interpretation should be done with caution. The only two existing randomized controlled trials have also included detailed EC safety analysis. The ECLAT study [Caponnetto et al. 2013b], a three-arm, controlled, randomized, clinical trial designed to compare efficacy and safety of a first-generation device with 7.2, 5.4, or 0 mg nicotine cartridges, reported clinically significant progressive health improvements already by week two of continuous use of the device, and no serious adverse events (i.e. major depression, abnormal behavior or any event requiring an unscheduled visit to the family practitioner or hospitalization) occurred during the study. The ASCEND study [Bullen et al. 2013], a three-arm, controlled, randomized, clinical trial designed to compare the efficacy and safety of a first-generation device (with or without nicotine) with nicotine patches, reported no serious adverse events in any of the three study groups.
Few clinical studies have been performed to evaluate the short-term in vivo effects of EC use in current or former smokers. Vardavas and colleagues evaluated the acute effects of using an EC for 5 minutes on respiratory function [Vardavas et al. 2012]. Although they did not report the results of commonly-used spirometry parameters, they found that a sensitive measure of airways resistance and nitric oxide levels in exhaled breath were adversely affected. Similar elevations in respiratory resistance were reported by other research groups [Palamidas et al. 2013; Gennimata et al. 2012], who also documented some bizarre elevation in exhaled carbon monoxide levels after EC use; this finding has been challenged by several other studies [Farsalinos et al. 2013f; Nides et al. 2014; Van Staden et al. 2013]. Schober and colleagues found that EC use led to elevated exhaled nitric oxide [Schober et al. 2013], contradicting the findings from Vardavas and colleagues [Vardavas et al. 2012]. Characteristically, none of the above studies performed any comparative tests after smoking tobacco cigarettes. Flouris and colleagues found that only smoking had an acute adverse effect on respiratory function [Flouris et al. 2013]; no difference was observed after the group of smokers was exposed to active or passive EC use.
Two studies have evaluated the short-term effects of ECs on the cardiovascular system. Farsalinos and colleagues evaluated the acute effects of using ECs with an 11 mg/ml nicotine-containing liquid on hemodynamics and left ventricular function, in comparison with the effects of cigarette smoking [Farsalinos et al. 2012]. They found that EC use resulted in a slight elevation in diastolic blood pressure while, after smoking, both systolic and diastolic blood pressure and heart rate were significantly elevated. Obviously, this was due to the relatively low nicotine content of the EC (which is considered medium strength). Diastolic dysfunction was observed in smokers after smoking, which was in line with findings from previous studies. However, no adverse effects were observed in EC users after using the device ad lib for 7 minutes. Another study by the same group [Farsalinos et al. 2013f], evaluated the acute effects of EC use on coronary flow. In particular, they measured the flow velocity reserve of the left anterior descending coronary artery by echocardiography after intravenous infusion of adenosine, representing the maximal ability of the artery to deliver blood to the myocardium. Smoking was associated with a decline in flow velocity reserve by 16% and an elevation in resistance to flow by 19%. On the contrary, no difference was observed in any of these parameters after using the EC. Blood carboxyhemoglobin levels were also measured in participants; baseline values were significantly higher in smokers compared with vapers and were further elevated after smoking but were not altered after EC use. Similar observations for carboxyhemoglobin levels were observed by Van Staden and colleagues [Van Staden et al. 2013].
A clinical case report of a smoker suffering from chronic idiopathic neutrophilia was published. According to that report [Farsalinos and Romagna, 2013], switching from smoking to EC use led to a reversal of the condition after 6 months. In addition, C-reactive protein levels, which were consistently elevated for more than 6 years, decreased to normal levels. Another case report of a patient with lipoid pneumonia was published, with the condition attributed to glycerin-based EC liquids used by the patient [McCauley et al. 2012]. However, glycerin is an alcohol (polyol) and thus it is impossible to cause lipoid pneumonia. Only oil-based liquids could be the cause for this condition; such liquids should not be used with ECs.
One study evaluated the acute effects of tobacco and EC use on white blood cell count [Flouris et al. 2012]. Smoking one tobacco cigarette caused an immediate elevation in white blood cells, neutrophils and lymphocytes, indicating acute inflammatory distress. On the contrary, no differences were observed after using ECs.
In conclusion, clinical studies evaluating the effects of short-term EC use on selected cardiovascular and respiratory functional outcomes have shown that even if some harmful effects of vaping are reported, these are considerably milder compared with smoking conventional cigarettes. However, it is difficult to assess the prognostic implications of these studies; longer-term data are needed before any definite conclusions are made.
Passive vaping
Passive smoking is an established risk factor for a variety of diseases [Barnoya and Navas-Acien, 2013]. Therefore, it is important from a public health perspective to examine the impact of EC use on bystanders. Indirect data can be derived from chemical studies in vapor mentioned above, which show that the potential of any significant adverse effects on bystanders is minimal. In fact, since sidestream exposure is nonexistent in EC (aerosol is produced only during activation of the device, while tobacco cigarettes emit smoke even when no puffs are taken), such studies are undoubtedly overestimating the risk of environmental exposure.
Few studies have focused on second-hand vaping. McAuley and colleagues [McAuley et al. 2012], although mentioning indoor air quality in the title of their study and finding minimal health-related impact, did not in fact evaluate second-hand vaping because aerosol was produced from an EC device and was evaluated without previously being inhaled by any user. Moreover, there were some problems with cross-contamination with tobacco cigarette smoke, which made the results somewhat questionable, at least for some of the parameters tested. Schripp and colleagues [Schripp et al. 2013] evaluated the emissions from an EC by asking a volunteer to use three different EC devices in a closed 8 m3 chamber. From a selection of 20 chemicals analyzed, only formaldehyde, acrolein, isoprene, acetaldehyde and acetic acid were detected. The levels were 5–40 times lower compared with emissions from a conventional cigarette. For formaldehyde, the authors specifically mentioned that the levels were continuously rising from the time the volunteer entered the room, even before he started using the EC. Moreover, no acute elevation was observed when the smoker used the three EC devices, contrary to the acute elevation and spiking of levels when a tobacco cigarette was lit. The authors concluded that formaldehyde was not emitted from the ECs but was due to human contamination, since low amounts of formaldehyde of endogenous origin can be found in exhaled breath [Riess et al. 2010]. Romagna and colleagues [Romagna et al. 2012] evaluated chemicals released in a realistic setting of a 60 m3 room, by asking five smokers to smoke ad lib for 5 hours and five vapers to use ECs ad lib for a similar period of time on two separate days. Nicotine, acrolein, toluene, xylene and polycyclic aromatic hydrocarbons were detected in room air after the smoking session, with the amount of total organic carbon (TOC) reaching to 6.66 mg/m3. In contrast, after the EC session, only glycerol was detected in minimal levels (72 µg/m3), while TOC reached a maximum level of 0.73 mg/m3. Characteristically, the amount of TOC accumulated after 5 hours of EC use was similar to the amount found after just 11 minutes of smoking. The study on heavy metals mentioned previously [Williams et al. 2013] could also be used to examine any potential risk of bystanders’ exposure to toxic metals. The levels of heavy metals found in vapor were minimal, and considering the dispersion of these molecules in the whole room air, it is unlikely that any of these metals could be present in measurable quantities in the environment. Therefore, the risk for bystanders would be literally nonexistent. Contrary to that, Schober and colleagues [Schober et al. 2013] found that levels of aluminum were raised by 2.4 times in a 45 m3 room where volunteers were asked to use ECs for 2 hours. This is a highly unexpected finding which cannot be supported by the findings of the study by Williams and colleagues [Williams et al. 2013]; because the levels found in the latter could not result in such elevation of the environmental levels of aluminum, unless nothing is retained in or absorbed from the lungs. Moreover, Schober and colleagues [Schober et al. 2013] found that levels of polycyclic aromatic hydrocarbons (PAHs) were raised by 20% after EC use. However, a major methodological problem of this study is that control environmental measurements were performed on a separate day and not on the same day of EC use. This is a major limitation, because the levels of environmental PAHs have significant diurnal and day-to-day variations [Ravindra et al. 2008]; therefore, it is highly likely that the differences in levels of PAHs (which are mainly products of combustion and are not expected to be emitted from EC use) represented changes due to environmental conditions and not due to EC use. Bertholon and colleagues [Bertholon et al. 2013] examined the EC aerosol exhaled from a user, in comparison with exhaled smoke from a smoker. The authors found that particle size diameters were 0.29–0.033µm. They observed that the half life of EC aerosol was 11 seconds compared with 20 minutes for cigarette smoke, indicating that risk of passive vaping exposure is significantly lower compared with passive smoking.
The recent findings by Czogala and colleagues [Czogala et al. 2013] led to similar conclusions. The authors compared the emissions of electronic and conventional cigarettes generated by experienced dual users in a ventilated full-sized room and found that ECs may emit detectable amounts of nicotine (depending on the specific EC brand tested), but no carbon monoxide and volatile organic carbons. However, the average ambient levels of nicotine of ECs were 10 times lower than those of conventional cigarettes (3.32 ± 2.49 versus 31.60 ± 6.91 μg/m3).
In his review and comparison with TLVs, Burstyn found that emissions from ECs to the environment are not expected to pose any measurable risk for bystanders [Burstyn, 2014].
An issue that needs further clarification relates to the findings of microparticles emitted from ECs. In most studies, these findings are presented in a way implying that the risk is similar to environmental or smoking microparticles. In reality, it is not just the size but the composition of the microparticles that matters. Environmental microparticles are mainly carbon, metal, acid and organic microparticles, many of which result from combustion and are commonly called particulate matter. Particulate matter exposure is definitely associated with lung and cardiovascular disease [Peters, 2005; Seaton et al. 1995]. In the case of ECs, microparticles are expected to consist mostly of propylene glycol, glycerol, water and nicotine droplets. Metal and silica nanoparticles may also be present [Williams et al. 2013], but, in general, emissions from ECs are incomparable to environmental particulate matter or cigarette smoke microparticles.
Flouris and colleagues [Flouris et al. 2013] performed the only clinical study evaluating the respiratory effects of passive vaping compared with passive smoking. Researchers found significant adverse effects in spirometry parameters after being exposed to passive smoking for 1 hour, while no adverse effects were observed after exposure to passive vaping.
Although evaluating the effects of passive vaping requires further work, based on the existing evidence from environmental exposure and chemical analyses of vapor, it is safe to conclude that the effects of EC use on bystanders are minimal compared with conventional cigarettes.
Miscellaneous safety issues
Specific subpopulations: psychiatric and chronic obstructive pulmonary disorder patients
A challenging population subgroup with unique smoking patterns is that of psychiatric patients and in particular schizophrenic patients. This subpopulation is characterized by a very high smoking prevalence [De Leon and Diaz, 2005] with an excess of smoking-related mortality [Brown et al. 2000]. Currently, only NRTs are recommended to treat nicotine dependence in this specific subpopulation, but in general they are not particularly effective [Aubin et al. 2012]. ECs could be used as an alternative to smoking products in this group. Caponnetto and colleagues performed a prospective 12-month pilot study to evaluate the efficacy of EC use in smoking reduction and cessation in a group of 14 patients with schizophrenia [Caponnetto et al. 2013a]. In 50% of participants, smoking consumption went from 30 to 15 cigarettes per day at 52 weeks of follow up, while 14.3% managed to quit smoking. Importantly, no deterioration in their psychiatric condition was observed, and side effects were mild and temporary. The results were promising although an outdated EC device was used in this study.
There is also anecdotal evidence that successful smoking cessation could be attained by using an EC in smokers with other psychiatric conditions such as depression [Caponnetto et al. 2011a]. Both patients described in this case series stated that EC use was well tolerated and no adverse events were reported.
Considering that first-line oral medications for nicotine addiction are contraindicated in such patients (prescribing information for bupropion and varenicline carry a ‘black-box’ warning for certain psychiatric conditions), ECs may be a promising tool in these challenging patient groups.
Another subpopulation that may benefit from regular EC use is that of respiratory patients with chronic obstructive pulmonary disease (COPD), a progressive disease characterized by a persistent inflammatory response to tobacco smoke that generally leads to decline in lung function, respiratory failure, cor pulmonale and death. Consequently, smoking cessation plays a crucial part in the management of COPD patients. However, the available evidence in the medical literature indicates that COPD patients who smoke respond poorly to smoking cessation efforts [Schiller and Ni, 2006]. To date, no formal efficacy and safety assessment of EC use in COPD patients has been conducted. There is only evidence from a case report of inveterate smokers with COPD and a documented history of recurring relapses, who eventually quit tobacco smoking on their own by using an EC [Caponnetto et al. 2011b]. Significant improvement in quality of life and reduction in the number of disease exacerbations were noted. EC use was well tolerated with no reported adverse events.
Accidental nicotine exposure
Accidental ingestion of nicotine, especially by children, or skin contact with large amounts of liquid or highly concentrated nicotine solution can be an issue. However, the historically referenced lethal dose of 60 mg has recently been challenged in a review by Mayer [Mayer, 2013]; he found that the lethal levels currently reproduced in every document originated from dubious experiments performed in the 19th century. Based on post-mortem studies, he suggested that the acute dose associated with a lethal outcome would be 500–1000 mg. Taking into account that voluminous vomiting is the first and characteristic symptom of nicotine ingestion, it seems that far higher levels of nicotine need to be ingested in order to have lethal consequences.
A surveillance system of adverse events has been developed by the FDA, which identifies safety concerns in relation to tobacco products. Since 2008, 47 adverse events were reported for ECs [Chen, 2013]. Eight of them were serious events such as hospitalizations for pneumonia, heart failure, seizures and hypotension and burns. A case of second-degree burns was caused by a battery explosion, which is generally a problem observed in lithium batteries and has occurred in other products (such as mobile phones). The author emphasized that the reported events were not necessarily associated with EC use but may have been related to pre-existing conditions or other causes. No condition was characteristically associated with EC use.
A recent review of the California Poison Control System database from 2010 to 2012 identified 35 cases (14 children) associated with EC exposure (accidental exposure in 25 cases) [Cantrell, 2013]. A total of five patients were evaluated in an emergency department and all were discharged within 4 hours. Nausea, vomiting, dizziness and oral irritation were most commonly reported. Taken together, data from surveillance systems of adverse events suggest that short-term adverse effects and accidental exposures to EC cartridges are unlikely to result in serious toxicity.
Notwithstanding, avoiding preventable contact with highly concentrated nicotine solution remains important; this can be achieved by specific labeling of the products, child-proof caps and proper education of consumers. There is no evidence that nicotine-containing EC liquids should be treated in any different way compared with other consumer products used every day in households (such as bleach, washing machine powder, etc.).
Electrical accidents and fires
The electronic equipment of ECs may be the cause for accidents. ECs are mainly composed of lithium batteries. There have been reports of explosions of batteries, caused either by prolonged charging and use of improper chargers or by design defects. Similar accidents have occurred with batteries of other popular devices, such as mobile phones. Therefore, this does not occur specifically with ECs, however, quality standards of production should be used in order to avoid such accidents.
Smoking is a major cause of residential fires. Between 2008 and 2010, an estimated annual average of 7600 smoking-related fires occurred in residential buildings in the US [US Fire Administration, 2012]. They account for only 2% of all residential building fires but for 14% of fire deaths. Since ECs are activated only when used by the person and there is no combustion involved, there is the potential to avoid the risk of smoking-related fires.
Use by youngsters and nonsmokers
Although beyond the scope of this review, it is important to briefly discuss the potential for addiction from EC use. It should be acknowledged that nicotine is addictive, although recent studies have shown that several other chemicals present in tobacco are associated with a significant enhancement of the addictiveness of nicotine [Lotfipour et al. 2011; Rose, 2006; Guillem et al. 2005]. Still, nicotine intake should not be recommended to nonsmokers. Smokers are already addicted to nicotine, thus ECs will be a cleaner form of nicotine intake, while at the same time they will maintain their sensory stimulation and motor simulation of smoking; these are important aspects of the addiction to smoking. Regulatory authorities have expressed concern about EC use by youngsters or by never-smokers, with ECs becoming a gateway to smoking or becoming a new form of addiction. However, such concerns are unsubstantiated; research has shown that EC use by youngsters is virtually nonexistent unless they are smokers. Camenga and colleagues [Camenga et al. 2013] examined the use of ECs and tobacco in a group of adolescents, in a survey conducted in three waves. In the first wave of the survey (February 2010), 1719 adolescents were surveyed from which only one nonsmoker was found to be using ECs. In the second and third wave of the surveys, only five nonsmoking adolescents were using ECs. In fact, these are adolescents who reported first ever use of ECs in the past 30 days; therefore they were not necessarily regular or daily EC consumers. The increased prevalence of EC use from 0.9% in 2010 to 2.3% in 2011 concerned smoking adolescents, therefore it should be considered a positive finding that smokers are experimenting with the significantly less harmful ECs. Similarly, the Medicines and Healthcare Products Regulatory Agency (MHRA) found that less than 1% of EC users are never-smokers [MHRA, 2013]. Data from the Centers for Disease Control [2013] National Youth Tobacco Survey reported doubling in EC experimentation by 13–18 year old students from 1.1% in 2011 to 2.1% in 2012; however, 90.6% of them were smokers. From the whole population, only 0.5% were nonsmokers experimenting with ECs. Once again, participants were asked about ever experimenting with an EC in the past 30 days, not regular or daily EC use. Recently, a survey of more than 75,000 students in South Korea was published [Lee et al. 2013]. Although they found that 12.6% of them were daily smokers (8.6% were using only tobacco cigarettes and 3.6% were using both tobacco and ECs), only 0.6% of nonsmokers had used ECs in the past 30 days. Although the above mentioned data have been used as arguments to support the fact that a new epidemic of nicotine addiction through the use of ECs is appearing, in reality they are showing that any experimentation with ECs is done by smokers. This is in fact a positive finding, and could lead to reduced smoking prevalence through adoption of EC use. Therefore, ECs could serve as gateway from smoking; on the contrary, there is no evidence indicating that they could be a gateway to smoking. It is promising to see that penetration of EC use in youngsters is virtually nonexistent, especially when you take into consideration that there is currently no official regulation in most countries to prohibit the access to ECs by youngsters.
Conclusion
Existing evidence indicates that EC use is by far a less harmful alternative to smoking. There is no tobacco and no combustion involved in EC use; therefore, regular vapers may avoid several harmful toxic chemicals that are typically present in the smoke of tobacco cigarettes. Indeed, some toxic chemicals are released in the EC vapor as well, but their levels are substantially lower compared with tobacco smoke, and in some cases (such as nitrosamines) are comparable with the amounts found in pharmaceutical nicotine products. Surveys, clinical, chemistry and toxicology data have often been mispresented or misinterpreted by health authorities and tobacco regulators, in such a way that the potential for harmful consequences of EC use has been largely exaggerated [Polosa and Caponnetto, 2013]. It is obvious that some residual risk associated with EC use may be present, but this is probably trivial compared with the devastating consequences of smoking. Moreover, ECs are recommended to smokers or former smokers only, as a substitute for conventional cigarettes or to prevent smoking relapse; thus, any risk should be estimated relative to the risk of continuing or relapsing back to smoking and the low efficacy of currently approved medications for smoking cessation should be taken into consideration [Moore et al. 2009; Rigotti et al. 2010; Yudkin et al. 2003]. Nonetheless, more research is needed in several areas, such as atomizer design and materials to further reduce toxic emissions and improve nicotine delivery, and liquid ingredients to determine the relative risk of the variety of compounds (mostly flavorings) inhaled. Regulations need to be implemented in order to maintain the current situation of minimal penetration of EC use in nonsmokers and youngsters, while manufacturers should be forced to provide proof for the quality of the ingredients used and to perform tests on the efficiency and safety of their products. However, any regulatory decisions should not compromise the variability of choices for consumers and should make sure that ECs are more easily accessible compared with their main competitor, the tobacco cigarette. Consumers deserve, and should make, informed decisions and research will definitely promote this. In particular, current data on safety evaluation and risk assessment of ECs is sufficient enough to avert restrictive regulatory measures as a consequence of an irrational application of the precautionary principle [Saitta et al. 2014].
ECs are a revolutionary product in tobacco harm reduction. Although they emit vapor, which resembles smoke, there is literally no fire (combustion) and no ‘fire’ (suspicion or evidence that they may be the cause for disease in a similar way to tobacco cigarettes). Due to their unique characteristics, ECs represent a historical opportunity to save millions of lives and significantly reduce the burden of smoking-related diseases worldwide.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Riccardo Polosa is a Professor of Medicine and is supported by the University of Catania, Italy. He has received lecture fees and research funding from GlaxoSmithKline and Pfizer, manufacturers of stop smoking medications. He has also served as a consultant for Pfizer and Arbi Group Srl (Milano, Italy), the distributor of Categoria™ e-Cigarettes. His research on electronic cigarettes is currently supported by LIAF (Lega Italiana AntiFumo).
Konstantinos Farsalinos is a researcher at Onassis Cardiac Surgery Center. He has never been funded by the pharmaceutical or the tobacco industry. For some of his studies, the institution has received financial compensation from electronic cigarette companies for the studies’ cost. His salary is currently being paid by a scholarship grant from the Hellenic Society of Cardiology.
Contributor Information
Konstantinos E. Farsalinos, Onassis Cardiac Surgery Center, Sygrou 356, Kallithea 17674, Greece
Riccardo Polosa, Centro per la Prevenzione e Cura del Tabagismo (CPCT) and Institute of Internal Medicine, Università di Catania, Catania, Italy.
References
- Adkison S., O’Connor R., Bansal-Travers M., Hyland A., Borland R., Yong H.H., et al. (2013) Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med 44: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose J., Barua R. (2004) The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737 [DOI] [PubMed] [Google Scholar]
- American Chemistry Council (2003) Ethylene Glycols: Considerations Against Use in Theatrical Fogs/Mist and Artificial Smoke. Available at: http://www.americanchemistry.com/ProductsTechnology/Ethylene-Glycols-2/PDF-Ethylene-Glycols-Fog-Information-Sheet.pdf (Accessed: 20 November 2013).
- Antal M., Mok W., Roy J., T-Raissi A. (1985) Pyrolytic sources of hydrocarbons from biomass. J Anal Appl Pyrol 8: 291–303 [Google Scholar]
- Aubin H., Rollema H., Svensson T., Winterer G. (2012) Smoking, quitting, and psychiatric disease: A review. Neurosci Biobehav Rev 36: 271–284 [DOI] [PubMed] [Google Scholar]
- Bahl V., Lin S., Xu N., Davis B., Wang Y., Talbot P. (2012) Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 34: 529–537 [DOI] [PubMed] [Google Scholar]
- Barnoya J., Navas-Acien A. (2013) Protecting the world from secondhand tobacco smoke exposure: where do we stand and where do we go from here? Nicotine Tob Res 15: 789–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar R., Davis B., Wang Y., Bahl V., Lin S., Talbot P. (2014) Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro 28: 198–208 [DOI] [PubMed] [Google Scholar]
- Benowitz N., Gourlay S. (1997) Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 29: 1422–1431 [DOI] [PubMed] [Google Scholar]
- Benowitz N., Zevin S., Jacob P., III (1998) Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther 287: 958–962 [PubMed] [Google Scholar]
- Bertholon J., Becquemin M., Roy M., Roy F., Ledur D., Annesi Maesano I., et al. (2013) Comparison of the aerosol produced by electronic cigarettes with conventional cigarettes and the shisha. Rev Mal Respir 30: 752–757 [DOI] [PubMed] [Google Scholar]
- Brown S., Inskip H., Barraclough B. (2000) Causes of the excess mortality of schizophrenia. Br J Psychiatry 177: 212–217 [DOI] [PubMed] [Google Scholar]
- Bullen C., Howe C., Laugesen M., McRobbie H., Parag V., Williman J., et al. (2013) Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 382: 1629–1637 [DOI] [PubMed] [Google Scholar]
- Burstyn I. (2014) Peering through the mist: Systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn Z., Siegel M. (2011) Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy 32: 16–31 [DOI] [PubMed] [Google Scholar]
- Camenga D., Delmerico J., Kong G., Cavallo D., Hyland A., Cummings K., et al. (2013) Trends in use of electronic nicotine delivery systems by adolescents. Addict Behav 39(1): 338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell F. (2013) Adverse effects of e-cigarette exposures. J Community Health 15 December 2013. (Epub ahead of print). DOI: 10.1007/s10900-013-9807-5 [DOI] [PubMed] [Google Scholar]
- Caponnetto P., Auditore R., Russo C., Cappello G., Polosa R. (2013a) Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health 10: 446–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P., Campagna D., Cibella F., Morjaria J., Caruso M., Russo C., et al. (2013b) EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 8: e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P., Polosa R., Auditore R., Russo C., Campagna D. (2011a) Smoking cessation with e-cigarettes in smokers with a documented history of depression and recurring relapses. Int J Clin Med 2: 281–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P., Polosa R., Russo C., Leotta C., Campagna D. (2011b) Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: a case series. J Med Case Rep 5: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2013) Notes from the field: electronic cigarette use among middle and high school students - United States, 2011-2012. MMWR Morb Mortal Wkly Rep 62: 729–730 [PMC free article] [PubMed] [Google Scholar]
- Chen I. (2013) FDA summary of adverse events on electronic cigarettes. Nicotine Tob Res 15: 615–616 [DOI] [PubMed] [Google Scholar]
- Czekaj P., Pałasz A., Lebda-Wyborny T., Nowaczyk-Dura G., Karczewska W., Florek E., et al. (2002) Morphological changes in lungs, placenta, liver and kidneys of pregnant rats exposed to cigarette smoke. Int Arch Occup Environ Health 75 (Suppl): S27–S35 [DOI] [PubMed] [Google Scholar]
- Czogala J., Goniewicz M., Fidelus B., Zielinska-Danch W., Travers M., Sobczak A. (2013) Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res (11 December 2011 (Epub ahead of print). DOI: 10.1093/ntr/ntt203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L. (2013) Electronic cigarettes: what are they and are they effective? E-Cigarette Summit, London, UK: (oral presentation). Available at: http://e-cigarette-summit.com/wp-content/uploads/2013/12/Summit-Presentations.pdf [accessed 22 December 2013]. [Google Scholar]
- Dawkins L., Corcoran O. (2013) Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 231: 401–407 [DOI] [PubMed] [Google Scholar]
- Dawkins L., Turnern J., Roberts A., Soar K. (2013) ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction 108: 1115–1125 [DOI] [PubMed] [Google Scholar]
- De Leon J., Diaz F. (2005). A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 76: 1351–1357 [DOI] [PubMed] [Google Scholar]
- Dockrell M., Morison R., Bauld L., McNeill A. (2013) E-Cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res 15: 1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douptcheva N., Gmel G., Studer J., Deline S., Etter J.F. (2013) Use of electronic cigarettes among young Swiss men. J Epidemiol Community Health 67: 1075–1076 [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (1992) EPA Report/600/6-90/006F. Respiratory health effects of passive smoking: lung cancer and other disorders. Washington, DC. Available at: http://oaspub.epa.gov/eims/eimscomm.getfile?p_download_id=36793 (Accessed: 20 November 2013). [Google Scholar]
- Environmental Protection Agency (2000) Cinnamaldehyde (040506) fact sheet. Available at: http://www.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-040506_1-Oct-98.pdf (Accessed: 20 November 2013).
- Etter J., Bullen C. (2011) Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 106: 2017–2028 [DOI] [PubMed] [Google Scholar]
- Etter J., Zäther E., Svensson S. (2013) Analysis of refill liquids for electronic cigarettes. Addiction 108: 1671–1679 [DOI] [PubMed] [Google Scholar]
- Farsalinos K., Romagna G. (2013) Chronic idiopathic neutrophilia in a smoker, relieved after smoking cessation with the use of electronic cigarette: a case report. Clin Med Insights Case Rep 6: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Romagna G., Allifranchini E., Ripamonti E., Bocchietto E., Todeschi S., et al.(2013a) Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health 10: 5146–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. (2013b) Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst Abuse 7: 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. (2013c) Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health 10: 2500–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Romagna G., Voudris V. (2013d) Authors miss the opportunity to discuss important public health implications. J Chromatogr A 1312: 155–156 [DOI] [PubMed] [Google Scholar]
- Farsalinos K., Spyrou A., Tsimopoulou K., Stefopoulos C., Romagna G., Voudris V. (2014). Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Tsiapras D., Kyrzopoulos S., Savvopoulou M., Avramidou E., Vasilopoulou D., et al. (2012) Acute effects of using an electronic nicotine-delivery device (e-cigarette) on myocardial function: comparison with the effects of regular cigarettes. Eur Heart J 33(Abstract Supplement): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Tsiapras D., Kyrzopoulos S., Stefopoulos C., Spyrou A., Tsakalou M., et al. (2013f) Immediate effects of electronic cigarette use on coronary circulation and blood carboxyhemoglobin levels: comparison with cigarette smoking. Eur Heart J 34(Abstract Supplement): 1322933568 [Google Scholar]
- Flouris A., Chorti M., Poulianiti K., Jamurtas A., Kostikas K., Tzatzarakis M., et al. (2013) Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 25: 91–101 [DOI] [PubMed] [Google Scholar]
- Flouris A., Poulianiti K., Chorti M., Jamurtas A., Kouretas D., Owolabi E., et al. (2012) Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol 50: 3600–3603 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2009) FDA and Public health experts warn about electronic cigarettes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm173222.htm (Accessed: 20 November 2013).
- Gennimata S., Palamidas A., Kaltsakas G., Tsikrika S., Vakali S., Gratziou C., et al. (2012) Acute effect of e-cigarette on pulmonary function in healthy subjects and smokers. Presented at the European Respiratory Society’s Annual Congress, Poster P1053. Available at: https://www.ersnetsecure.org/public/prg_congres.abstract?ww_i_presentation=59718 (Accessed: 20 November 2013). [Google Scholar]
- Goniewicz M., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., et al. (2013) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. DOI: 10.1136/tobaccocontrol-2012-050859. (Published online: 6 March 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S., Satterfield M., Lanes S. (1998) A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Safety 18: 297–308 [DOI] [PubMed] [Google Scholar]
- Guillem K., Vouillac C., Azar M., Parsons L., Koob G., Cador M., et al. (2005) Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci 25: 8593–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guslandi M. (1999) Nicotine treatment for ulcerative colitis. Br J Clin Pharmacol 48: 481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger M., Trehy M., Ye W., Moore T., Allgire J., Westenberger B. (2010) Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A 1217: 7547–7555 [DOI] [PubMed] [Google Scholar]
- Hajek P., Jarvis M., Belcher M., Sutherland G., Feyerabend C. (1989) Effect of smoke-free cigarettes on 24 h cigarette withdrawal: a double-blind placebo-controlled study. Psychopharmacology (Berl) 97: 99–102 [DOI] [PubMed] [Google Scholar]
- Hubbard R., Lewis S., Smith C., Godfrey C., Smeeth L., Farrington P., et al. (2005) Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control 14: 416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Shin H. (2013) Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1291: 48–55 [DOI] [PubMed] [Google Scholar]
- King B., Alam S., Promoff G., Arrazola R., Dube S. (2013) Awareness and ever use of electronic cigarettes among US adults, 2010–2011. Nicotine Tob Res 15(9): 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen M. (2008) Safety Report on the Ruyan® e-cigarette Cartridge and Inhaled Aerosol. Available at: http://www.healthnz.co.nz/RuyanCartridgeReport30-Oct-08.pdf (Accessed: 18 November 2013).
- Laugesen M. (2009). Ruyan®E-cigarette Bench-top tests. Society for Research on Nicotine and Tobacco (SRNT) Dublin, Poster 5-11. Available at: http://www.healthnz.co.nz/DublinEcigBenchtopHandout.pdf [accessed 20 November 2013].
- Le Houezec J., McNeill A., Britton J. (2011) Tobacco, nicotine and harm reduction. Drug Alcohol Rev 30: 119–123 [DOI] [PubMed] [Google Scholar]
- Lee S., Grana R., Glantz S. (2013) Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. DOI: 10.1016/j.jadohealth.2013.11.003. (Published online: 22 November 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S., Arnold M., Hogenkamp D., Gee K., Belluzzi J., Leslie F. (2011) The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology 61: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lúdvíksdóttir D., Blöndal T., Franzon M., Gudmundsson T., Säwe U. (1999) Effects of nicotine nasal spray on atherogenic and thrombogenic factors during smoking cessation. J Intern Med 246: 61–66 [DOI] [PubMed] [Google Scholar]
- Mayer B. (2013). How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol 88: 5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers M. (2009) FDA acts to protect public health from electronic cigarettes. Campaign for Tobacco-Free Kids statement. Available at: http://www.tobaccofreekids.org/press_releases/post/id_1166 (Accessed: 20 November 2013).
- McAuley T., Hopke P., Zhao J., Babaian S. (2012) Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol 24: 850–857 [DOI] [PubMed] [Google Scholar]
- McCauley L., Markin C., Hosmer D. (2012) An unexpected consequence of electronic cigarette use. Chest 141(4): 1110–1113 [DOI] [PubMed] [Google Scholar]
- McClernon F., Hiott F., Westman E., Rose J., Levin E. (2006) Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 189: 125–133 [DOI] [PubMed] [Google Scholar]
- MHRA Commission on human medicines, Working Group on nicotine containing products (NCPS) (2013). Current use of electronic cigarettes. Available at: http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/con286845.pdf (Accessed: 20 November 2013).
- Moore D., Aveyard P., Connock M., Wang D., Fry-Smith A., Barton P. (2009) Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 338: b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R., Bailey W., Daniels K., Bjornson W., Kurnow K., Connett J., et al. (1996) Safety of nicotine polacrilex gum used by 3,094 participants in the Lung Health Study. Lung Health Study Research Group. Chest 109: 438–445 [DOI] [PubMed] [Google Scholar]
- National Association of Attorneys General (2013) FDA regulation on E-cigarettes. Available at: http://www.naag.org/assets/files/pdf/E%20Cigarette%20Final%20Letter%20(5)(1).pdf (Accessed: 20 November 2013).
- Nides M., Leischow S., Bhatter M., Simmons M. (2014) Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav 38: 265–274 [DOI] [PubMed] [Google Scholar]
- Nielsen S., Franklin G., Longstreth W., Swanson P., Checkoway H. (2013) Nicotine from edible Solanaceae and risk of Parkinson disease. Ann Neurol 74: 472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitenberg A., Antony I. (1999) Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol 34: 694–699 [DOI] [PubMed] [Google Scholar]
- Palamidas A., Gennimata S., Kaltsakas G., Tsikrika S., Vakali S., Gratziou C., et al. (2013) Acute effect of an e-cigarette with and without nicotine on lung function. Presented at the European Respiratory Society’s Annual Congress, Poster P1054. Available at: http://www.ersnet.org/learning_resources_player/abstract_print_13/files/100.pdf (Accessed: 20 November 2013). [Google Scholar]
- Pellegrino R., Tinghino B., Mangiaracina G., Marani A., Vitali M., Protano C., et al. (2012) Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig 24: 279–288 [PubMed] [Google Scholar]
- Peters A. (2005) Particulate matter and heart disease: evidence from epidemiological studies. Toxicol Appl Pharmacol 207: 477–482 [DOI] [PubMed] [Google Scholar]
- Polosa R., Caponnetto P. (2013) Time for evidence-based e-cigarette regulation. Lancet Oncol 14: 582–583 [DOI] [PubMed] [Google Scholar]
- Polosa R., Caponnetto P., Morjaria J., Papale G., Campagna D., Russo C. (2011) Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health 11: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R., Morjaria J., Caponnetto P., Campagna D., Russo C., Alamo A., et al. (2013a) Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. DOI: 10.1007/s11739-013-0977-z (Published online: July 2013). [DOI] [PubMed] [Google Scholar]
- Polosa R., Rodu B., Caponnetto P., Maglia M., Raciti C. (2013b) A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W., Stone K. (1993) Oxidants in cigarette smoke: radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci 686: 12–28 [DOI] [PubMed] [Google Scholar]
- Ravindra K., Wauters E., Van Grieken R. (2008) Variation in particulate PAHs levels and their relation with the transboundary movement of the air masses. Sci Total Environ 396: 100–110 [DOI] [PubMed] [Google Scholar]
- Renne R., Wehner A., Greenspan B., Deford H., Ragan H., Westenberg R., et al. (1992) 2-Week and 13-Week Inhalation Studies of Aerosolized Glycerol in Rats. Inhal Toxicol 4: 95–111 [Google Scholar]
- Riess U., Tegtbur U., Fauck C., Fuhrmann F., Markewitz D., Salthammer T. (2010) Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NOx in exhaled human breath. Anal Chim Acta 669: 53–62 [DOI] [PubMed] [Google Scholar]
- Rigotti N., Pipe A., Benowitz N., Arteaga C., Garza D., Tonstad S. (2010) Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: A randomized trial. Circulation 121: 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson O., Loosli C., Puck T., Wise H., Lemon H., Lester W., Jr (1947) Tests for the chronic toxicity of propylene glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration. J Pharmacol Exp Ther 91: 52–76 [PubMed] [Google Scholar]
- Rodu B., Godshall W. (2006) Tobacco harm reduction: An alternative cessation strategy for inveterate smokers. Harm Reduct J 3: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagna G., Allifranchini E., Bocchietto E., Todeschi S., Esposito M., Farsalinos K. (2013) Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol 25: 354–361 [DOI] [PubMed] [Google Scholar]
- Romagna G., Zabarini L., Barbiero L., Bocchietto E., Todeschi S., Caravati E., et al. (2012) Characterization of chemicals released to the environment by electronic cigarettes use (ClearStream-Air project): is passive vaping a reality? SRNT Europe Annual Congress, Helsinki, Finland. Poster RRP18. Available at: http://www.srnteurope.org/assets/srnt-e2012abstractbook.pdf [accessed 20 November 2013]. [Google Scholar]
- Rose J. (2006) Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 184: 274–285 [DOI] [PubMed] [Google Scholar]
- Rose J., Levin E. (1991) Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict 86: 605–609 [DOI] [PubMed] [Google Scholar]
- Russell M. (1991) The future of nicotine replacement. Br J Addict 86: 653–658 [DOI] [PubMed] [Google Scholar]
- Sahakian B., Jones G., Levy R., Gray J., Warburton D.(1989) The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry 154: 797–800 [DOI] [PubMed] [Google Scholar]
- Saitta D., Ferro G., Polosa R. (2014) Achieving appropriate regulations for electronic cigarettes. Ther Adv Chronic Dis 3 February 2014 (Epub ahead of print). DOI: 10.1177/2040622314521271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J., Ni H. (2006) Cigarette smoking and smoking cessation among persons with chronic obstructive pulmonary disease. Am J Health Promot 20: 319–323 [DOI] [PubMed] [Google Scholar]
- Schober W., Szendrei K., Matzen W., Osiander-Fuchs H., Heitmann D., Schettgen T., et al. (2013) Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. DOI: 10.1016/j.ijheh.2013.11.003. (Published online: 6 December 2013). [DOI] [PubMed] [Google Scholar]
- Schripp T., Markewitz D., Uhde E., Salthammer T. (2013) Does e-cigarette consumption cause passive vaping? Indoor Air 23: 25–31 [DOI] [PubMed] [Google Scholar]
- Seaton A., MacNee W., Donaldson K., Godden D. (1995) Particulate air pollution and acute health effects. Lancet 345: 176-178 [DOI] [PubMed] [Google Scholar]
- Stein Y., Antal M., Jones M. (1983) A study of the gas-phase pyrolysis of glycerol. J Anal Appl Pyrol 4: 283–296 [Google Scholar]
- US Fire Administration (2012) Smoking-related Fires in residential buildings (2008-2010). Topical Fire Report Series 13. Available at: http://www.usfa.fema.gov/downloads/pdf/statistics/v13i6.pdf (Accessed: 20 November 2013).
- US Pharmacopeia (2013) Elemental impurities limits. Available at: http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/key-issues/c232_final.pdf (Accessed: 20 November 2013).
- Van Staden S., Groenewald M., Engelbrecht R., Becker P., Hazelhurst L. (2013) Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. S Afr Med J 103: 865–868 [DOI] [PubMed] [Google Scholar]
- Vardavas C., Anagnostopoulos N., Kougias M., Evangelopoulou V., Connolly G., Behrakis P. (2012) Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141: 1400–1406 [DOI] [PubMed] [Google Scholar]
- Varughese S., Teschke K., Brauer M., Chow Y., van Netten C., Kennedy S. (2005) Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med 47: 411–418 [DOI] [PubMed] [Google Scholar]
- Werley M., McDonald P., Lilly P., Kirkpatrick D., Wallery J., Byron P., et al. (2011) Non-clinical safety and pharmacokinetic evaluations of propylene glycol aerosol in Sprague-Dawley rats and Beagle dogs. Toxicology 287: 76–90 [DOI] [PubMed] [Google Scholar]
- Westenberger B. (2009) Evaluation of e-Cigarettes. St.Louis, MO: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis. Available at: http://www.fda.gov/downloads/drugs/Scienceresearch/UCM173250.pdf (Accessed: November 10, 2013).
- WHO-IARC (2004) IARC monographs on the evaluation of carcinogenic risks to humans. Volume 83, tobacco smoke and involuntary smoking. Available at: http://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf. (Accessed: 20 November 2013). [PMC free article] [PubMed]
- Wieslander G., Norbäck D., Lindgren T. (2001) Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58: 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Villarreal A., Bozhilov K., Lin S., Talbot P. (2013) Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 8: e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2013) Tobacco fact sheet No 339. Updated July 2013. Available at: http://www.who.int/mediacentre/factsheets/fs339/en/ (Accessed: 18 November 2013).
- Woolf K., Zabad M., Post J., McNitt S., Williams G., Bisognano J. (2012) Effect of nicotine replacement therapy on cardiovascular outcomes after acute coronary syndromes. Am J Cardiol 110: 968–970 [DOI] [PubMed] [Google Scholar]
- Yudkin P., Hey K., Roberts S., Welch S., Murphy M., Walton R. (2003) Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 327: 28–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin S., Benowitz N., Jacob P. (1998) Dose-related cardiovascular and endocrine effects of transdermal nicotine. Clin Pharmacol Ther 64: 87–95 [DOI] [PubMed] [Google Scholar]