Abstract
The recent development of new oral anticoagulants (NOACs) offers the possibility of efficacy, relative safety and convenience compared with warfarin. This could lead to greater patient compliance, with easier management and improved provision of thromboprophylaxis. Safety whilst using NOACs should be focused on bleeding cases, surgery or on the management of patients receiving anticoagulant therapy with concomitant impairment of renal function, especially since many NOACs are dependent on renal excretion. Thus, if the clearance creatinine indicates severe renal impairment, NOACS will be contraindicated or their dose needs to be changed. In patients who need surgery, there are published protocols of management, depending on the severity of the intervention and renal function. In the case of severe hemorrhage, requiring rapid reversal of the anticoagulant effect and in the absence of specific antidotes, alternatives such as one of the nonspecific haemostatic agents must be considered. Clinical evaluation in bleeding situations and a meticulous risk–benefit appraisal for NOACs is needed, and these procoagulant agents and patients must be monitored closely. This article provides an overview of the pharmacology and potential risks, as well as the efficacy and safety of NOACs.
Keywords: apixaban, bleeding, dabigatran, new oral anticoagulants, rivaroxaban
Introduction
In recent years, several new oral anticoagulants (NOACs) have been introduced and more drugs are currently under development. These drugs have given patients and providers alternatives to heparin and warfarin, mainly for prophylaxis against stroke in patients with atrial fibrillation (AF), prophylaxis/treatment of venous thromboembolism (VTE) and in treatment of acute coronary syndrome (ACS) [De Caterina, 2009; Geerts et al. 2008; Kearon et al. 2008].
The NOACs differ from vitamin K antagonists (VKAs) in their action mechanism because of direct inhibition of proteins of the coagulation cascade. They have more predictable pharmacokinetics leading to fixed and convenient dosing regimens and no need for routine monitoring, as well as in a rapid onset of action [Eriksson et al. 2009], and importantly, high efficacy and low risk of bleeding [Poulsen et al. 2012]. Some of their limitations are the higher cost, limited monitoring (if needed, as only qualitative measures available) and the lack of a specific antidote [Miesbach and Seifried, 2012].
However, NOACs may not be suitable for everyone. Renal function is known to deteriorate with age, and many patients with impaired renal function or patients with chronic kidney disease may require anticoagulation to prevent AF or VTE [Go et al. 2009; Soliman et al. 2010; ROCKET AF Study Investigators, 2010]. Renal impairment is related to an increased risk of arterial and venous thrombosis [Pavord and Myers, 2011] and is a major risk factor for bleeding during anticoagulation regardless of the indication [Pisters et al. 2010]. In most of the clinical trials of NOACs, patients with severe renal impairment were excluded [ROCKET AF Study Investigators, 2010; Connolly et al. 2009, 2011]. Moreover, the use of NOACs also involves management in other situations, such as elective/urgent surgery or major bleeding cases. This review will summarize the evidence in relation to the safe use of the NOACs and provide an overview of the approach to management in special situations, such as renal impairment, overdosage, active bleeding and perioperative management.
Risks of new oral anticoagulants
Pharmacological characteristics of new oral anticoagulants
The NOACs fall into two broad categories: the oral direct factor Xa (FXa) inhibitors (rivaroxaban and apixaban) and the oral direct thrombin inhibitor (dabigatran etexilate, the prodrug of dabigatran). Other direct FXa inhibitors being investigated in clinical trials are edoxaban and betrixaban [Perez et al. 2013], and are pending approval at the moment.
Impaired renal function will affect dabigatran pharmacokinetics the most, given its predominant excretion via the renal route, whereas betrixaban would be affected the least, due to its metabolism being mainly altered by changes in liver function. The pharmacokinetic data of these agents are summarized in Table 1 [Lip et al. 2012; Perez et al. 2013; Poulsen et al. 2012].
Table 1.
Pharmacokinetics characteristics and indications of the new oral anticoagulants compared with warfarin.
| Characteristics | Warfarin | Dabigatran | Apixaban | Rivaroxaban | Betrixaban | Edoxaban |
|---|---|---|---|---|---|---|
| Molecular weight (Da) | 308 | 628 | 460 | 436 | 452 | 548 |
| Bioavailability (%) | 98 | 6-7 | 66 | 63–79 | 40–80ª | 50ª |
| tmax (h) | 72-120 | 2-3 | 1–3 | 2–4 | NR | 1–3 |
| t½ (h) | 20-60 | 7-17 | 8–15 | 7–13 | 5ª | 9–11 |
| Protein binding (%) | 99 | 35 | 87 | 95 | NR | 54 |
| Food effect | Yes | Delayed absorption | No | Delayed absorption | No | No |
| Dosing regimen | once daily | twice daily | twice daily | once daily | once daily | once daily |
| Metabolism/elimination | 100% liver | 80% renal | 25% renal | 1/3 renal | 5% renal | 35% renal |
| 20% liver | 75% fecal | 2/3 liver | 95% liver | 65% liver | ||
| Substrate CYP | 2C9, 3A4 | No | 3A4 | 3A4, 2J2 | No | 3A4 |
| Substrate P-gp | No | Yes | Yes | Yes | No | yes |
| Food interaction | Yes | No | No | No | No | NR |
| Monitoring required | INR | No | No | No | No | No |
| Target | II, VII, IX, X, | II | Xa | Xa | Xa | Xa |
| P-S, P-C | ||||||
| Antidote | Yes | No | No | No | No | No |
| Typical effective dose | INR guided | 150 mg or 220 mg once daily (VTE prophylaxis)* | 2.5 bid (VTE prophylaxis)* | 10 mg once daily (VTE prophylaxis)* 15 mg twice daily (1–21) followed by 20 mg once daily (DVT treatment/prevention of recurrent VTE)$20 mg once daily (AF)‡ | In development | 30 mg (VTE prophylaxis) |
| 75 mg or 150 mg twice daily (AF) ‡ | ||||||
| Approved indications | Approved for VTE prevention and treatment of the thromboembolic complications associated with AF and cardiac valve replacement, and secondary prevention after MI | Approved for VTE prevention after elective hip or knee replacement in adults and for prevention of stroke and systemic embolism in patients with nonvalvular AF | Approved for VTE prevention after elective hip or knee replacement in adults. Stroke prevention and systemic emolization in nonvalvular AF | Approved for VTE prevention after elective hip or knee replacement in adults, for prevention of stroke and systemic embolism in patients with nonvalvular AF, and for treatment of acute DVT and prevention of VTE recurrence | Has not been approved yet | Only approved in Japan for VTE prophylaxis joint replacement |
Adapted from Poulsen et al. [2012], Lip et al. [2012] and Perez et al. [2013].
ACS, acute coronary syndrome; AF, atrial fibrillation; CYP, cytochrome P450; DVT, deep vein thrombosis; INR, international normalized ratio; MI, myocardial infarction; NR, not reported; P-C, protein C; P-gp, P glycoprotein; P-S, protein S; t½, terminal elimination half life; tmax, time to reach maximal plasma concentration; VTE, venous thromboembolism.
Approved dose for VTE prevention in adult patients undergoing elective hip or knee replacement surgery.
Approved dose for treatment of acute DVT and prevention of recurrent DVT and PE.
Approved dose for prevention of stroke and systemic embolism in adult patients with nonvalvular AF.
Stroke prevention in atrial fibrillation
The NOACs appear to be as good as, and possibly better than, warfarin for the reduction of stroke and systemic embolism in AF, with similar or less major bleeding and consistently lower risk of intracranial bleeding. Both safety and efficacy of VKAs depend on anticoagulation control in the warfarin arms, with a wide variability in clinical care (the phase III trials, RE-LY [Connolly et al. 2009] ARISTOTLE [Granger et al. 2011] and ROCKET [Patel et al. 2011] reported overall mean time in therapeutic range of 64%, 62% and 55% respectively); the benefit of NOACs was enhanced in the prevention of AF. Ancillary analyses [Wallentin et al. 2010, 2013] have shown benefits in the reduction of both stroke and bleeding events with NOACs across the range of predicted time in therapeutic range (TTR), showing that they are not simply superior or noninferior to warfarin because of poor international normalized ratio (INR) control.
It is worth highlighting that no direct comparison of the NOACs has been done, and it is not likely that such head-to-head trials will be attempted. As all NOACs have been compared with warfarin, this was used as a common comparator for indirect comparisons. Notwithstanding the limitations of such an approach (clinical studies are not absolutely comparable in terms of population characteristics) and given the lack of trials directly comparing NOACs, indirect comparisons offer some insights.
However, special attention should also be paid to populations excluded from the clinical trials, such as older people, patients with renal or liver impaired function and people who are underweight or obese; for example, the average weight in major phase III trials is 80 kg and weight is one of the criteria to be considered for dose adjustment of apixaban [Gallego et al. 2013].
Indeed, ageing results in the deterioration of some physiological functions, such as liver or renal function and a lower catabolism, which may require lower drug doses. If we add some comorbidities and polypharmacy, which are common among older people, we have a patient population more prone to complications when anticoagulated. Therefore, renal function should be closely monitored and further data regarding the use of NOACs in patients with impaired renal function are awaited [Poulsen et al. 2012].
NOACs may potentially cause bleeding complications in patients with reduced drug excretion or metabolism due to physiological function impairment, such as renal or liver functions. Pharmacokinetic studies and data from clinical studies [ROCKET AF Study Investigators, 2010; Connolly et al. 2009, 2011; Jover et al. 2012; Poulsen et al. 2012] have provided information on how to guide dosing in patients with renal impairment evaluated by the estimated glomerular filtration rate or calculation of the creatinine clearance (CLcr), as summarized in Table 2. Regarding patients with liver function impairment, their higher bleeding risk is due to a lower drug metabolism, in addition to a decrease in coagulation factor synthesis.
Table 2.
Approved dosing according to indication and renal function.
| Drug and indication | Renal function classification | ||||
|---|---|---|---|---|---|
|
| |||||
| Normal | Mild | Moderate | Severe | End-stage renal disease | |
| CLcr ≥ 90 ml/min | CLcr 60–89 ml/min | CLcr 30–59 ml/min | CLcr 15–29 ml/min | CLcr <15 ml/min | |
| Dabigatran | |||||
| Atrial fibrillation/EMA | 150 mg twice daily | 150 mg twice daily | 110 mg twice daily | Contraindicated | Contraindicated |
| Atrial fibrillation/FDA | 150 mg twice daily | 150 mg twice daily | 150 mg twice daily | 75 mg twice daily$ | Contraindicated |
| VTE prophylaxis joint replacement | 220 mg once daily | 220 mg once daily | 150 mg once daily | Contraindicated | Contraindicated |
| VTE treatment (not yet approved) | 150 mg twice daily | 150 mg twice daily | 150 mg twice daily | Contraindicated | Contraindicated |
| Rivaroxaban | |||||
| Atrial fibrillation | 20 mg once daily | 20 mg once daily | 15 mg once daily | 15 mg once daily | Contraindicated |
| VTE prophylaxis joint replacement | 10 mg once daily | 10 mg once daily | 10 mg once daily | Use with caution | Contraindicated |
| VTE treatment | 15 mg twice daily/20 mg once daily | 15 mg twice daily/20 mg once daily | 15 mg twice daily/15 mg once daily | 15 mg twice daily/15 mg once daily | Contraindicated |
| Apixaban | |||||
| Atrial fibrillation/EMA/FDA | 5 mg twice daily | 5 mg twice daily | 5 mg twice daily | 2.5 mg twice daily | Contraindicated |
| VTE prophylaxis joint replacement | 2.5 mg twice daily | 2.5 mg twice daily | 2.5 mg twice daily | Use with caution | Contraindicated |
| Edoxaban* | |||||
| VTE prophylaxis joint replacement | 30 mg once daily | 30 mg once daily | 30 mg once daily | Contraindicated | Contraindicated |
Adapted from Poulsen et al. [2012] and Turpie et al. [2012].
Only approved in Japan.
Dose based on pharmacokinetic modeling, not on randomized control trials.
CLcr, creatinine clearance; EMA, European Medicines Agency; FDA, US Food and Drug Administration; VTE, venous thromboembolism.
Some concomitant drugs may enhance the antithrombotic response, such as antiplatelet or nonsteroidal anti-inflammatory drugs. Special attention should be given to patients needing a combination of an oral anticoagulant and an antiplatelet agent and efforts should be made to improve patients’ education to prevent them taking nonprescribed drugs. Sparse data are available regarding NOACs as concomitant antiplatelet drugs were avoided in initial clinical trials. Concomitant drugs and extrapolation from VKAs should be done while awaiting specific data.
Combining NOACs with antiplatelet agents is becoming a major issue because of the additional management options that the emerging use of dabigatran, rivaroxaban and apixaban has introduced into clinical practice. Recent papers [Komocsi et al. 2012; Oldgren et al. 2013; Tsu and Dager, 2013] have shown an increase in bleeding events when using NOACs in patients with a recent ACS (unstable angina or myocardial infarction), which was dramatically higher when combined with dual antiplatelet therapy compared with the standard of care with VKAs. This might offset the ischemic benefits in this subgroup of patients, although further studies are welcomed.
Whilst there was some concern over a potential numerical (but nonsignificant) increased risk of myocardial infarctions and events with the use of dabigatran, this may not be evident in ‘real world’ data [Larsen et al. 2013].
NOACs have not been evaluated in patients with mechanical heart valve prosthesis, and one phase II trial (RE-ALIGN) with dabigatran was stopped early due to increased risk of thromboembolism and bleeding [Van de Werf et al. 2012]. As safety and efficacy profiles in such patients cannot be determined, they should remain on warfarin.
Prevention of venous thromboembolism
A VTE event might be life threatening, and thus anticoagulant therapy is needed for two objectives: in the active treatment of the acute episode (for the first 3 months) and the prevention of new events.
Some NOACs can also be used for VTE primary prophylaxis, and some agents, such as rivaroxaban, offer the advantage of a single drug treatment for the whole period [Buller et al. 2012]. However the optimal duration remains uncertain, needing a balance between the estimated risk of recurrence and the risk of bleeding complications. Cases of VTE due to a transient risk factor (e.g. surgery or trauma), have a lower risk of recurrence and therefore do not benefit from prolonged treatment; in contrast, among patients with unprovoked VTE, risk of recurrence has been shown to be highest [Kyrle and Eichinger, 2012].
The risk of VTE increases beyond the time of hospital discharge [Cohen et al. 2013], but unfortunately higher rates of clinically relevant bleeding could arguably not lead to net clinical benefit of extended therapy. Therefore, all the aspects lead to some controversy in the anticoagulant treatment after a VTE [Albertsen et al. 2012]. Indeed, concerning the risk of VTE beyond discharge; some patients (i.e. surgery due to knee replacement and some cancer types) get extended primary prophylaxis, and in the case of secondary prophylaxis after a VTE event, treatment usually lasts at least 3 months and is often continued after discharge.
Overdosage
In the case of an overdose while on NOAC therapy, activated charcoal will decrease absorption of the anticoagulants if administered within 2–3 h of ingestion of the anticoagulant, but this strategy has only been tested in vitro [Huisman et al. 2012; Turpie et al. 2012; van Ryn et al. 2010]. Dialysis removes dabigatran but multiple dialysis sessions may be required due to its large distribution volume. Dabigatran is reported to bind only to plasma proteins (mainly thrombin) and the plasma volume is the volume of distribution. Of note, rivaroxaban and apixaban are not dialyzable [van Ryn et al. 2010; Wong et al. 2011]. A recently study has confirmed faster hemodialysis works; it takes a session of 4 h to eliminate 59.3% of dabigatran [Khadzhynov et al. 2013].
Effects of new oral anticoagulants in coagulation assays
In contrast to warfarin, none of the NOAC drugs require routine coagulation monitoring due to their more predictable pharmacological profiles (Table 1). In some situations it may be beneficial to measure the anticoagulation effect, for example during urgent surgery, severe bleeding, thrombosis despite treatment, overdose, bridging with other anticoagulants or in patients with a high risk of accumulation of these drugs (e.g. patients with renal failure) [Samama and Guinet, 2011; Turpie et al. 2012; van Ryn et al. 2010].
In patients taking dabigatran, the activated partial thromboplastin time (aPTT) increases with larger doses but not in a linear dose response manner [Stangier et al. 2007; van Ryn et al. 2010]. The prothrombin time (PT, and its derived measure, INR) is variably affected but has been shown to rise with therapeutic doses, and is not recommended [Stangier et al. 2007]. The thrombin time (TT) measures the direct activity of thrombin and is probably the most sensitive test for an anticoagulant effect of dabigatran and a normal TT usually excludes an anticoagulation effect due to dabigatran. The ecarin clotting time also directly measures the anticoagulant effect of direct thrombin inhibitors but is less sensitive than the TT. The HEMOCLOT test (Hyphen BioMed; France) measures the anticoagulant effect of dabigatran with a modified thrombin clotting time [van Ryn et al. 2010].
In the case of rivaroxaban or other direct FXa inhibitors, since FX is a part of the common coagulation pathway, these drugs should prolong the PT and aPTT [Turpie et al. 2012]. The prolongation degree depends on the reagent used, and recalibration of anti-Xa testing may not be necessary to determine the anticoagulation degree between the different anti-Xa [Ogata et al. 2010]. No effect was seen on the TT or fibrinogen activity assays for rivaroxaban [Mani et al. 2011]. Chromogenic anti-Xa assays can be standardized to measure rivaroxaban or apixaban, but this test usually is not routinely available [Lindhoff-Last et al. 2010].
The ‘usual’ coagulation laboratory tests do not allow measuring the anticoagulant intensity of NOACs, although a normal prothrombin time ratio usually excludes an anticoagulation effect due to rivaroxaban, and a normal activated partial thromboplastin time could exclude one due to dabigatran.
Periprocedural management of patients on chronic new oral anticoagulant therapy
The challenge in periprocedural management of patients receiving anticoagulant therapy focuses on the need to balance the risk of thromboembolism (in case of anticoagulation interruption) against the risk of bleeding during the procedure (in case of anticoagulation continuation). Traditionally, the use of periprocedural bridging therapy with heparin, either unfractionated heparin or low-molecular-weight heparin (LMWH), mitigates the risk of periprocedural thromboembolism by allowing for continued anticoagulation during temporary discontinuation of VKAs for an elective procedure or surgery [Spyropoulos, 2005].
However, a recent meta-analysis [Siegal et al. 2012], including 34 studies that assessed perioperative thromboembolism and bleeding events in patients undergoing elective surgical or invasive procedures, shows that heparin bridging conferred a fivefold increased risk of overall bleeding, whereas the risk of thromboembolic events was not significantly different between bridged and nonbridged patients. Even international guidelines usually provide recommendations with a low grade of evidence. If evidence is missing regarding the ‘old’ VKAs, even more gaps remain about NOACs with regards to periprocedural management [Healey et al. 2012].
Thus, for the time being, the usual, although empirical, approach includes elective interruption of NOAC therapy before surgery, with timing of anticoagulant discontinuation depending on the half life of the anticoagulant, the patient’s renal function and the surgical risk of bleeding [Baumann Kreuziger et al. 2012].
Table 3 summarizes recommendations regarding timing of discontinuation in standard risk procedures. High-bleeding risk procedures including cardiac surgery, neurosurgery, abdominal surgery or procedures requiring spinal anesthesia may require 2–4 days off dabigatran in patients with normal renal function (elimination half life of dabigatran = 14–17 h) and 4 days off therapy with CLcr to 50 ml/min. In patients with moderately renal impairment, the half life of dabigatran is 16–18 h and the last dose to be given has to be 5 days before surgery [Spyropoulos and Douketis, 2012; Turpie et al. 2012; van Ryn et al. 2010].
Table 3.
Discontinuation guide for the main new oral anticoagulants, before standard and high-risk bleeding procedures.
| Renal function (CLcr ml/min ) | Dabigatran |
Rivaroxaban |
Apixaban |
|||
|---|---|---|---|---|---|---|
| Standard risk of bleeding | High risk of bleeding | Standard risk of bleeding | High risk of bleeding | Standard risk of bleeding | High risk of bleeding | |
| >50 | 24 h | 2–4 days | 24 h | 3 days | 24–36 h | 3 days |
| 30–50 | 48 h | 4 days | 48 h | 3 days | 48 h | 4 days |
| <30 | 2–5 days | >5 days | 3 days | 4 days | ||
Adapted from van Ryn et al. [2010], Spyropoulos and Douketis [2012] and Baumann Kreuziger et al. [2012].
CLcr, creatinine clearance.
Rivaroxaban and apixaban have a significantly shorter half life than dabigatran (8–9 h for rivaroxaban, 7–8 h for apixaban) and thus could be discontinued 24 h before surgery. In older patients the half life of rivaroxaban increases, so 48 h may be necessary to allow for proper elimination. A longer duration of interruption is probably required in patients with CKD focus in drug dependence on renal clearance (33% for rivaroxaban, 25% for apixaban). An increased risk of stroke has been reported after discontinuation of rivaroxaban and minimizing the duration without anticoagulation in high-risk patients is recommended. In older patients, higher levels of apixaban have been reported [Baumann Kreuziger et al. 2012; Spyropoulos and Douketis, 2012; Turpie et al. 2012; Watson et al. 2011]. Clinicians should consider discontinuing apixaban for 48 h or checking an anti-Xa level before surgery.
Timing of resumption of NOACs after surgery depends on bleeding risks and the dose used, as summarized in Table 4. These drugs fully anticoagulate the patient in 2–4 h. In clinical trials for VTE prophylaxis after orthopedic surgery, dabigatran was initiated at half a dose 1–4 h after surgery and a full dose given 12 h later [Baumann Kreuziger et al. 2012; Eriksson and Friedman, 2009]. Rivaroxaban was initiated 6–8 h after wound closure and apixaban was started 1–24 h postoperatively [Douketis, 2010; Turun et al. 2011]. For procedures with a low bleeding risk, full anticoagulation with apixaban could be restarted after 24 h, whereas resumption of anticoagulation after major surgery could be considered 48 h postoperatively [Douketis, 2010; Spyropoulos and Douketis, 2012].
Table 4.
Postoperative resumption of new oral anticoagulants: a suggested management approach.
| Drug | Low bleeding risk surgery | High bleeding risk surgery |
|---|---|---|
| Dabigatran | Resume on day after surgery | Resume 2–3 days after surgery |
| (24 h postoperative), | (48–72 h postoperative), | |
| 150 mg twice daily | 150 mg twice daily* | |
| Rivaroxaban | Resume on day after surgery | Resume 2–3 days after surgery |
| (24 h postoperative), | (48–72 h postoperative), | |
| 20 mg once daily | 20 mg once daily$ | |
| Apixaban | Resume on day after surgery | Resume 2–3 days after surgery |
| (24 h postoperative), | 5 mg twice daily | |
| (48–72 h postoperative), | 5 mg twice daily$ |
Adapted from Spyropoulos and Douketis [2012].
Standard dosages shown, consider dose reduction in cases of renal function impairment (creatinine clearance < 50 ml/min).
For patients at high risk for thromboembolism, consider administering a reduced dose of dabigatran (e.g. 110–150 mg once daily) on the evening after surgery and on the following day (first postoperative day) after surgery.
Consider a reduced dose (i.e. rivaroxaban 10 mg once a day or apixaban 2.5 mg twice a day) in patients at high risk of thromboembolism.
Management of bleeding events with new oral anticoagulants
For all available anticoagulants, bleeding is the most important adverse event in any type of patient [van Ryn et al. 2010]. Comorbidities, comedications, age and history of bleeding are the main risk determinants. Indeed, the number of bleeding events is rising due to the ageing of the population and the increasing need for interventional treatment [Miesbach and Seifried, 2012]. The shorter half life of the NOACs might facilitate the management of bleeding events during scheduled interventions or emergency situations.
Bleeding rates with NOACs are generally equal to or less than bleeding rates with warfarin [Eikelboom et al. 2011; Lip et al. 2011]. However, a recent meta-analysis [Miller et al. 2012], comparing dabigatran, rivaroxaban and apixaban, suggests that NOACs lower the risk for intracranial bleeding, with nonconclusive data regarding overall risk of bleeding. In contrast, they suggest an increased risk for gastrointestinal bleeding associated with the new agents, probably associated with their local absorption. The problem regarding what sort of bleeding is more life threatening depends upon specific patient characteristics.
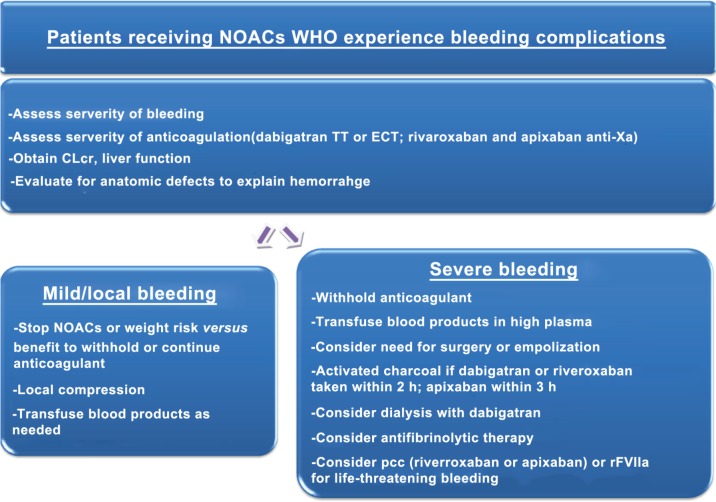
As antidotes are not available, algorithms for managing hemorrhage in patients taking NOACs have been developed (Figure 1) [Baumann Kreuziger et al. 2012]. In general, NOACs should be discontinued and initial evaluation should include hemodynamic stability assessment, intensity of anticoagulation (degree of coagulation impairment reached by the treatment), severity of bleeding and anatomic etiology of the hemorrhage.
Figure 1.
Management guideline for bleeding while taking dabigatran, rivaroxaban or apixaban. Adapted from Baumann Kreuziger et al. [2012].
CLcr, creatinine clearance; ECT, ecarin clotting time; NOAC, new oral anticoagulant; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII; TT, thrombin time.
Life-threatening bleeding requires the most urgent response. Baseline clotting times, complete blood count, CLcr and liver function tests should be obtained. Standard treatment, for example, surgical hemostasis and blood volume replacement, is recommended and consideration may be given to the use of fresh whole blood or fresh frozen plasma [Baumann Kreuziger et al. 2012; Huisman et al. 2012; Miesbach and Seifried, 2012; Turpie et al. 2012; van Ryn et al. 2010].
Assessment for anatomic etiology of the hemorrhage should be sought with the use of local control measures if possible. In the case of severe bleeding, additional haemostatic agents should be considered (Figure 1). Antifibrinolytic medication provides clot stabilization if fibrin is able to form, but such agents have been ineffective in reducing bleeding times with the direct thrombin inhibitors and should not be used for patients taking dabigatran [van Ryn et al. 2010]. In a recent study, tranexamic acid decreased postoperative blood loss in patients treated with rivaroxaban [Clave et al. 2012].
Potential nonspecific haemostatic reversal agents for new oral anticoagulants
Potential agents for reversing the anticoagulation effect of NOACs have been proposed. The fact remains that there are no clinical trial data for these agents [Levi et al. 2011; Pengo et al. 2011; van Ryn et al. 2010] compared with the effects on ex vivo tests [Marlu et al. 2012]. The potential reversal agents for each NOAC are summarized in Table 5.
Table 5.
Actions to evaluate and nonspecific reversal agents to use with the new oral anticoagulants.
| Drug | Therapeutic options |
|---|---|
| Dabigatran | Stop treatment with OACs |
| Haemodialysis | |
| PCC (25 U/kg, repeat if needed) | |
| rFVIIa (90 µg/kg) | |
| Rivaroxaban | Stop treatment with OACs |
| PCC (25 U/kg, repeat if needed) | |
| FEIBA (50 IE/kg, max 200 IE/day) | |
| rFVIIa (90 µg/kg) | |
| Apixaban | Stop treatment with OACs |
| PCC (25 U/kg, repeat if needed) | |
| FEIBA (50 IE/kg, max 200 IE/day) | |
| rFVIIa (90 µg/kg) | |
| Edoxaban | Stop treatment with OACs |
| PCC (25 U/kg, repeat if needed) | |
| FEIBA (50 IE/kg, max 200 IE/day) | |
| rFVIIa (90 µg/kg) |
Adapted from Miesbach and Seifried [2012].
The therapeutic options are not validated by large-scale trials.
OAC, oral anticoagulant; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII.
Reversal of the anticoagulation effects of new oral anticoagulants by prothrombin complex concentrates and FEIBA
Prothrombin complex concentrates (PCCs) are used for the treatment of VKA-induced bleeding. Of note, PCCs can be divided into ‘four-factor-concentrates’ containing adequate amounts of vitamin K dependent FII, FVII, FIX and FX and ‘three-factor concentrates’ containing significantly lower amounts of FVII [Miesbach and Seifried, 2012]. Clinical studies have been published with human data in healthy volunteers showing the reversibility of the anticoagulant activity of rivaroxaban using PCCs [Eerenberg et al. 2011].
FVIII inhibitor bypassing activity (FEIBA; Baxter, USA) is an activated PCC that can also be used to reverse VKAs and recombinant activated FVII (rFVIIa) has been reported as an efficient reversal drug, at least based on changes in blood tests among nonbleeding patients under VKA and NOAC treatment [Levi et al. 2010; Wojcik et al. 2009]. However, FEIBA has been associated with major thrombotic risk compared with nonactivated PCCs [Weitz et al. 2012].
The anticoagulant effect of rivaroxaban was completely reversed in all subjects immediately after bolus infusion of 50 IU/kg PCC (Cofact; Sanquin, Netherlands). However, the effect of PCCs has yet to be confirmed in patients with bleeding events who are treated with these anticoagulants. A recent case report suggests there is an efficient combination of PCC and fresh frozen plasma to reverse dabigatran related to bleeding events [Dumkow et al. 2012].
Another study by Marlu and colleagues in healthy men also showed that PCCs and FEIBA were able to reverse the anticoagulant effect of dabigatran and rivaroxaban. FEIBA showed the most efficient action, given that this complex contains FVII, mainly in the activated form, and FII, FIX and FX, mainly in nonactivated forms, and combines the effect of both rFVIIa and PCC [Marlu et al. 2012]. In addition, several animal studies confirm the efficacy of PCCs as reversal agents of the anticoagulant effect of FXa inhibitors, such as rivaroxavan [Godier et al. 2012] or edoxaban [Fukuda et al. 2012]. The same PCC effect has been described for dabigatran, at least in animal studies [Zhou et al. 2011].
Reversal of the anticoagulation effects of new oral anticoagulants by recombinant activated FVII
The safety and usefulness of fresh frozen plasma (FFP) or PCC still needs to be established because the drug may also block newly administered coagulation factors. rFVIIa was reported to reduce bleeding time in animal models treated with dabigatran and more recently its use to manage dabigatran-associated post-surgery bleeding has been described [Warkentin et al. 2012]. However, in this case report, the combined use of rFVIIa with hemodialysis makes it difficult to assess the real efficacy of each specific intervention. We should also keep in mind the potential high risk for arterial thrombosis of rFVIIa [Pengo et al. 2011].
In a rabbit experimental model using rivaroxaban, Godier and colleagues demonstrated that neither rFVIIa nor PCC fully reversed the bleeding induced by rivaroxaban overdose [Godier et al. 2012]. PCC and rFVIIa corrected several laboratory parameters but were ineffective in reducing rivaroxaban-induced bleeding. These data appear to contrast with the data obtained by Marlu and colleagues for PCC in healthy volunteers [Marlu et al. 2012]. That study also showed an anticoagulant effect with a higher dose of rFVIIa, but this also raises the issue of safety, as rFVIIa increases the amount of thrombin generated. Thus, the risk of hemorrhage needs to be weighed against the risk of using any of these procoagulant agents and patients must be monitored closely.
Future antidotes
The search for specific antidotes is currently the main research field. Recent reports show data about proposed molecules such as aDabi-Fab, a humanized antibody fragment to bind dabigatran [Schiele et al. 2013], and r-Antidote (PRT064445), an inactive recombinant protein that lacks the membrane-binding γ-carboxyglutamic acid domain of native FXa (which not only reverses FXa inhibitors but also LMWHs and fondaparinux) [Lu et al. 2013].
Conclusion and perspectives
Compared with traditional therapies with VKAs, the NOACs offer greater patient compliance owing to easier management and oral administration and, therefore, improved thromboprophylaxis and treatment. However, the safety of NOACs should be focused on the management of patients receiving anticoagulant therapy with renal impairment as well as those who need surgery. Indeed patients at high risk of bleeding with traditional anticoagulation do not have a lower risk of bleeding with NOACs.
As a perspective in the use of NOACs, a new clinical score has recently being developed to assess the probability of good TTR based on several common clinical characteristics, called the SAMe-TT2R2 score [Apostolakis et al. 2013]. This score includes female Sex, Age (<60 years), Medical history (at least two of the following: hypertension, diabetes, coronary artery disease/myocardial infarction, peripheral arterial disease, congestive heart failure, previous stroke, pulmonary disease, hepatic or renal disease), Treatment (interacting drugs, e.g. amiodarone for rhythm control) (all one point), as well as current Tobacco use (two points) and Race (nonwhite people; two points). This simple score (SAMe-TT2R2) can help predict poor INR control and aid decision making by identifying patients with AF who would do well on VKAs (SAMe-TT2R2 score = 0–1), or conversely, those (i.e. SAMe-TT2R2 score ≥ 2) who require additional interventions to achieve acceptable anticoagulation control. In that sense, patients with a poor predicted TTR (i.e. SAMe-TT2R2 score ≥ 2) could be candidates who are more suitable for NOACs.
In the case of severe hemorrhage, needing rapid reversal of anticoagulant therapy, in the absence of specific antidotes, alternatives such as one of the nonspecific haemostatic reversal agents must be considered. However, clinical evaluation in bleeding situations and a meticulous risk–benefit appraisal of NOACs are needed when using these procoagulant agents.
Due to the partial or predominant renal metabolism of NOACs, it is always necessary to evaluate renal function as part of the holistic management of patients receiving anticoagulant therapy [Kirchhof et al. 2012].
Footnotes
Funding: J.A. Vílchez holds a research grant ‘Río Hortega’ from the Instituto de Salud Carlos III. P. Gallego holds a grant from the Spanish Foundation Alfonso Martin Escudero.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Juan Antonio Vílchez, University of Birmingham Centre for Cardiovascular Sciences, City Hospital, Birmingham, UK and Department of Cardiology and Department of Clinical Analysis, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain.
Pilar Gallego, University of Birmingham Centre for Cardiovascular Sciences, City Hospital, Birmingham, UK and Department of Hematology and Clinical Oncology, Hospital Universitario Morales Meseguer, Murcia, Spain.
Gregory Y.H. Lip, University of Birmingham Centre for Cardiovascular Sciences, City Hospital, Dudley Road, Birmingham B18 7QH, UK
References
- Albertsen I., Larsen T., Rasmussen L., Overvad T., Lip G. (2012) Prevention of venous thromboembolism with new oral anticoagulants versus standard pharmacological treatment in acute medically ill patients: a systematic review and meta-analysis. Drugs 72: 1755–1764 [DOI] [PubMed] [Google Scholar]
- Apostolakis S., Sullivan R., Olshansky B., Lip G. (2013) Factors affecting quality of anticoagulation control amongst atrial fibrillation patients on warfarin: the SAMe-TT2R2 (sex female, age less than 60, medical history, treatment strategy [rhythm control], tobacco use [doubled], race [doubled] score. Chest 9 May (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Baumann Kreuziger L., Morton C., Dries D. (2012) New anticoagulants: a concise review. J Trauma Acute Care Surg 73: 983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller H., Prins M., Lensin A., Decousus H., Jacobson B., Minar E., et al. (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366: 1287–1297 [DOI] [PubMed] [Google Scholar]
- Clave A., Fazilleau F., Dumser D., Lacroix J. (2012) Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a case–control study in 70 patients. Orthop Traumatol Surg Res 98: 484–490 [DOI] [PubMed] [Google Scholar]
- Cohen A., Spiro T., Buller H., Haskell L., Hu D., Hull R., et al. (2013) Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 368: 513–523 [DOI] [PubMed] [Google Scholar]
- Connolly S., Eikelboom J., Joyner C., Diener H., Hart R., Golitsyn S., et al. (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364: 806–817 [DOI] [PubMed] [Google Scholar]
- Connolly S., Ezekowitz M., Yusuf S., Eikelboom J., Oldgren J., Parekh A., et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151 [DOI] [PubMed] [Google Scholar]
- De Caterina R. (2009) The current role of anticoagulants in cardiovascular medicine. J Cardiovasc Med (Hagerstown) 10: 595–604 [DOI] [PubMed] [Google Scholar]
- Douketis J. (2010) Pharmacologic properties of the new oral anticoagulants: a clinician-oriented review with a focus on perioperative management. Curr Pharm Des 16: 3436–3441 [DOI] [PubMed] [Google Scholar]
- Dumkow L., Voss J., Peters M., Jennings D. (2012) Reversal of dabigatran-induced bleeding with a prothrombin complex concentrate and fresh frozen plasma. Am J Health Syst Pharm 69: 1646–1650 [DOI] [PubMed] [Google Scholar]
- Eerenberg E., Kamphuisen P., Sijpkens M., Meijers J., Buller H., Levi M. (2011) Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 124: 1573–1579 [DOI] [PubMed] [Google Scholar]
- Eikelboom J., Wallentin L., Connolly S., Ezekowitz M., Healey J., Oldgren J., et al. (2011) Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 123: 2363–2372 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Friedman R. (2009) Dabigatran etexilate: pivotal trials for venous thromboembolism prophylaxis after hip or knee arthroplasty. Clin Appl Thromb Hemost 15(Suppl. 1): 25S–31S [DOI] [PubMed] [Google Scholar]
- Eriksson B., Quinlan D., Weitz J. (2009) Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet 48: 1–22 [DOI] [PubMed] [Google Scholar]
- Fukuda T., Honda Y., Kamisato C., Morishima Y., Shibano T. (2012) Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost 107: 253–259 [DOI] [PubMed] [Google Scholar]
- Gallego P., Roldan V., Lip G. (2013) Conventional and new oral anticoagulants in the treatment of chest disease and its complications. Am J Respir Crit Care Med 188: 413–421 [DOI] [PubMed] [Google Scholar]
- Geerts W., Bergqvist D., Pineo G., Heit J., Samama C., Lassen M., et al. (2008) Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 133: 381S-453S [DOI] [PubMed] [Google Scholar]
- Go A., Fang M., Udaltsova N., Chang Y., Pomernacki N., Borowsky L., et al. (2009) Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 119: 1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godier A., Miclot A., Le B., Durand M., Fischer A., Emmerich J., et al. (2012) Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology 116: 94–102 [DOI] [PubMed] [Google Scholar]
- Granger C., Alexander J., McMurray J., Lopes R., Hylek E., Hanna M., et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981–992 [DOI] [PubMed] [Google Scholar]
- Healey J., Eikelboom J., Douketis J., Wallentin L., Oldgren J., Yang S., et al. (2012) Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 126: 343–348 [DOI] [PubMed] [Google Scholar]
- Huisman M., Lip G., Diener H., Brueckmann M., van Ryn J., Clemens A. (2012) Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost 107: 838–847 [DOI] [PubMed] [Google Scholar]
- Jover E., Roldan V., Gallego P., Hernandez-Romero D., Valdes M., Vicente V., et al. (2012) Predictive value of the CHA2DS2-VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl Ed) 65: 627–633 [DOI] [PubMed] [Google Scholar]
- Kearon C., Kahn S., Agnelli G., Goldhaber S., Raskob G., Comerota A. (2008) Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 133: 454S-545S [DOI] [PubMed] [Google Scholar]
- Khadzhynov D., Wagner F., Formella S., Wiegert E., Moschetti V., Slowinski T., et al. (2013) Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 109: 596–605 [DOI] [PubMed] [Google Scholar]
- Kirchhof P., Lip G., Van Gelder I., Bax J., Hylek E., Kaab S., et al. (2012) Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options – a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 14: 8–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komocsi A., Vorobcsuk A., Kehl D., Aradi D. (2012) Use of new-generation oral anticoagulant agents in patients receiving antiplatelet therapy after an acute coronary syndrome: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 172: 1537–1545 [DOI] [PubMed] [Google Scholar]
- Kyrle P., Eichinger S. (2012) Clinical scores to predict recurrence risk of venous thromboembolism. Thromb Haemost 108: 1061–1064 [DOI] [PubMed] [Google Scholar]
- Larsen T., Rasmussen L., Skjoth F., Due K., Callreus T., Rosenzweig M., et al. (2013) Efficacy and safety of dabigatran etexilate and warfarin in ‘real world’ patients with atrial fibrillation: A prospective nationwide cohort study. J Am Coll Cardiol 61: 2264–2273 [DOI] [PubMed] [Google Scholar]
- Levi M., Eerenberg E., Kamphuisen P. (2011) Bleeding risk and reversal strategies for old and new anticoagulants and antiplatelet agents. J Thromb Haemost 9: 1705–1712 [DOI] [PubMed] [Google Scholar]
- Levi M., Eerenberg E., Lowenberg E., Kamphuisen P. (2010) Bleeding in patients using new anticoagulants or antiplatelet agents: risk factors and management. Neth J Med 68: 68–76 [PubMed] [Google Scholar]
- Lindhoff-Last E., Samama M., Ortel T., Weitz J., Spiro T. (2010) Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit 32: 673–679 [DOI] [PubMed] [Google Scholar]
- Lip G., Andreotti F., Fauchier L., Huber K., Hylek E., Knight E., et al. (2011) Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] Working Group on Thrombosis. Thromb Haemost 106: 997–1011 [DOI] [PubMed] [Google Scholar]
- Lip G., Larsen T., Skjoth F., Rasmussen L. (2012) Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 60: 738–746 [DOI] [PubMed] [Google Scholar]
- Lu G., Deguzman F., Hollenbach S., Karbarz M., Abe K., Lee G., et al. (2013) A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 19: 446–451 [DOI] [PubMed] [Google Scholar]
- Mani H., Hesse C., Stratmann G., Lindhoff-Last E. (2011) Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost 106: 156–164 [DOI] [PubMed] [Google Scholar]
- Marlu R., Hodaj E., Paris A., Albaladejo P., Crackowski J., Pernod G. (2012) Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 108: 217–224 [DOI] [PubMed] [Google Scholar]
- Miesbach W., Seifried E. (2012) New direct oral anticoagulants–current therapeutic options and treatment recommendations for bleeding complications. Thromb Haemost 108: 625–632 [DOI] [PubMed] [Google Scholar]
- Miller C., Grandi S., Shimony A., Filion K., Eisenberg M. (2012) Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 110: 453–460 [DOI] [PubMed] [Google Scholar]
- Ogata K., Mendell-Harary J., Tachibana M., Masumoto H., Oguma T., Kojima M., et al. (2010) Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol 50: 743–753 [DOI] [PubMed] [Google Scholar]
- Oldgren J., Wallentin L., Alexander J., James S., Jonelid B., Steg G., et al. (2013) New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J 34: 1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Mahaffey K., Garg J., Pan G., Singer D., Hacke W., et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891 [DOI] [PubMed] [Google Scholar]
- Pavord S., Myers B. (2011) Bleeding and thrombotic complications of kidney disease. Blood Rev 25: 271–278 [DOI] [PubMed] [Google Scholar]
- Pengo V., Crippa L., Falanga A., Finazzi G., Marongiu F., Palareti G., et al. (2011) Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost 106: 868–876 [DOI] [PubMed] [Google Scholar]
- Perez A., Eraso L., Merli G. (2013) Implications of new anticoagulants in primary practice. Int J Clin Pract 67: 139–156 [DOI] [PubMed] [Google Scholar]
- Pisters R., Lane D., Nieuwlaat R., de Vos C., Crijns H., Lip G. (2010) A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138: 1093–1100 [DOI] [PubMed] [Google Scholar]
- Poulsen B., Grove E., Husted S. (2012) New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs 72: 1739–1753 [DOI] [PubMed] [Google Scholar]
- ROCKET AF. Study Investigators (2010) Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 159: 340–347 [DOI] [PubMed] [Google Scholar]
- Samama M., Guinet C. (2011) Laboratory assessment of new anticoagulants. Clin Chem Lab Med 49: 761–772 [DOI] [PubMed] [Google Scholar]
- Schiele F., van Ryn J., Canada K., Newsome C., Sepulveda E., Park J., et al. (2013) A specific antidote for dabigatran: functional and structural characterization. Blood 121: 3554–3562 [DOI] [PubMed] [Google Scholar]
- Siegal D., Yudin J., Kaatz S., Douketis J., Lim W., Spyropoulos A. (2012) Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 126: 1630–1639 [DOI] [PubMed] [Google Scholar]
- Soliman E., Prineas R., Go A., Xie D., Lash J., Rahman M., et al. (2010) Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 159: 1102–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos A. (2005) Bridging of oral anticoagulation therapy for invasive procedures. Curr Hematol Rep 4: 405–413 [PubMed] [Google Scholar]
- Spyropoulos A., Douketis J. (2012) How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 120: 2954–2962 [DOI] [PubMed] [Google Scholar]
- Stangier J., Rathgen K., Stahle H., Gansser D., Roth W. (2007) The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 64: 292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsu L., Dager W. (2013) Safety of new oral anticoagulants with dual antiplatelet therapy in patients with acute coronary syndromes. Ann Pharmacother 47: 573–577 [DOI] [PubMed] [Google Scholar]
- Turpie A., Kreutz R., Llau J., Norrving B., Haas S. (2012) Management consensus guidance for the use of rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost 108: 876–886 [DOI] [PubMed] [Google Scholar]
- Turun S., Banghua L., Yuan Y., Zhenhui L., Ying N., Jin C. (2011) A systematic review of rivaroxaban versus enoxaparin in the prevention of venous thromboembolism after hip or knee replacement. Thromb Res 127: 525–534 [DOI] [PubMed] [Google Scholar]
- Van de Werf F., Brueckmann M., Connolly S., Friedman J., Granger C., Hartter S., et al. (2012) A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: the randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J 163: 931–937 [DOI] [PubMed] [Google Scholar]
- van Ryn J., Stangier J., Haertter S., Liesenfeld K., Wienen W., Feuring M., et al. (2010) Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 103: 1116–1127 [DOI] [PubMed] [Google Scholar]
- Wallentin L., Lopes R., Hanna M., Thomas L., Hellkamp A., Nepal S., et al. (2013) Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 127: 2166–2176 [DOI] [PubMed] [Google Scholar]
- Wallentin L., Yusuf S., Ezekowitz M., Alings M., Flather M., Franzosi M., et al. (2010) Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 376: 975–983 [DOI] [PubMed] [Google Scholar]
- Warkentin T., Margetts P., Connolly S., Lamy A., Ricci C., Eikelboom J. (2012) Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 119: 2172–2174 [DOI] [PubMed] [Google Scholar]
- Watson J., Whiteside G., Perry C. (2011) Apixaban: first global approval. Drugs 71: 2079–2089 [DOI] [PubMed] [Google Scholar]
- Weitz J., Quinlan D., Eikelboom J. (2012) Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation 126: 2428–2432 [DOI] [PubMed] [Google Scholar]
- Wojcik C., Schymik M., Cure E. (2009) Activated prothrombin complex concentrate factor VIII inhibitor bypassing activity (FEIBA) for the reversal of warfarin-induced coagulopathy. Int J Emerg Med 2: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P., Pinto D., Zhang D. (2011) Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 31: 478–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Schwarting S., Illanes S., Liesz A., Middelhoff M., Zorn M., et al. (2011) Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 42: 3594–3599 [DOI] [PubMed] [Google Scholar]