Abstract
Reward facilitates performance and boosts cognitive performance across many tasks. At the same time, negative affective stimuli interfere with performance when they are not relevant to the task at hand. Yet, the investigation of how reward and negative stimuli impact perception and cognition has taken place in a manner that is largely independent of each other. How reward and negative emotion simultaneously contribute to behavioral performance is currently poorly understood. The aim of the present study was to investigate how the simultaneous manipulation of positive motivational processing (here manipulated via reward) and aversive processing (here manipulated via negative picture viewing) influence behavior during a perceptual task. We tested two competing hypothesis about the impact of reward on negative picture viewing. On the one hand, suggestions about the automaticity of emotional processing predict that negative picture interference would be relatively immune to reward. On the contrary, if affective visual processing is not obligatory as we have argued in the past, reward may counteract the deleterious effect of more potent negative pictures. We found that reward counteracted the effect of potent, negative distractors during a visual discrimination task. Thus, when sufficiently motivated, participants were able to reduce the deleterious impact of bodily mutilation stimuli.
Keywords: reward, emotion, aversive pictures, perception, automaticity
Introduction
Reward facilitates perceptual processing and boosts cognitive performance across a diverse set of tasks (Aarts et al., 2011; Pessoa, 2013; Pessoa & Engelmann, 2010). For instance, detection sensitivity increased as a function of absolute incentive magnitude during both exogenous (Engelmann & Pessoa, 2007) and endogenous (Engelmann et al., 2009) attentional tasks. At the same time, negative stimuli interfere with performance when they are not relevant to the task at hand (Pessoa, 2005; Vuilleumier, 2005). For instance, determining the orientation of a target visual stimulus was slower following negative pictures (Hartikainen et al., 2000) and the presence of a central unpleasant picture increased reaction times when participants discriminated the orientation of peripheral bars (Erthal et al., 2005).
Yet, the investigation of how reward and negative stimuli impact perception and cognition has proceeded in a manner that is largely independent of each other: how reward and negative emotion simultaneously contribute to behavioral performance is poorly understood (but see Hu et al., 2013). The aim of the present study was to investigate how the simultaneous manipulation of positive motivational processing (here manipulated via reward) and aversive processing (here manipulated via negative picture viewing) affect behavior during a perceptual task.
We have previously shown that viewing task irrelevant negative images slowed reaction times (RTs) relative to neutral ones when subjects performed a peripheral bar-orientation task (Erthal, et al., 2005) – a pattern that is observed when the difficulty of the main task is not high. In the current study, we sought to investigate the impact of performance based monetary rewards on interference caused by task-irrelevant negative pictures during the same bar-orientation task (Fig. 1). Because the processing of negative visual stimuli is prioritized, and indeed might take place in a fairly automatic fashion (Vuilleumier, 2005), their processing might be immune to positive motivational manipulations. On the contrary, if affective visual processing is not obligatory as we have argued in the past (Pessoa, 2005), offering an incentive to participants during the perception of negative images may counteract their impact. The present study (see Fig. 1) thus evaluated these two competing hypotheses to better understand the interactions between reward processing and negative emotion during perception.
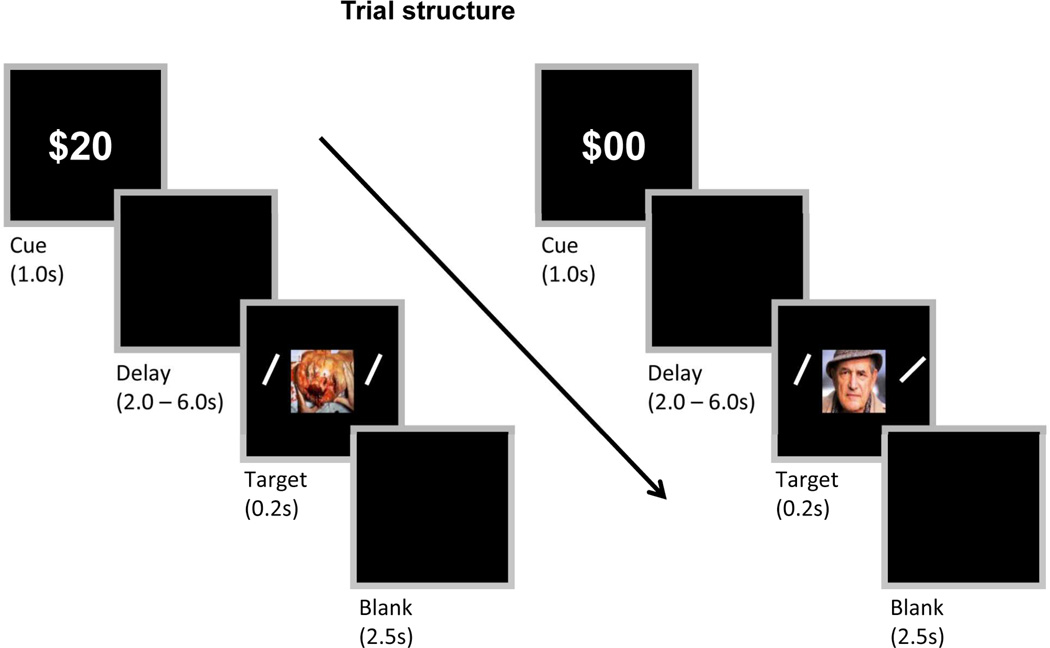
Figure 1.
Experiment Design. On each trial, an initial cue indicated whether or not the trial involved reward. After a delay period, a negative or neutral task-irrelevant picture was presented centrally and two bars were presented peripherally (not drawn to scale). The participant’s task was to indicate whether or not the orientation of the bars matched. During the reward condition (left panel), participants were rewarded if performance was both fast and accurate.
METHODS
Subjects
Thirty-nine participants took part in the study and provided informed consent, as approved by the Institutional Review Board of Indiana University, Bloomington, IN. Subjects were free from psychiatric or neurological disease or related past history, as indicated via self-report. Data from two participants were excluded from the analysis as they did not complete the whole experiment. Thus the results reported in this study are based on thirty-seven participants (15 males; age range: 18–36 years old).
Stimuli and task
Each trial (Fig. 1) in the main runs started with the presentation (1000 ms) of a cue stimulus indicating the Reward condition (“$00”: no-reward; “$20”: reward). The cue was followed by a 2–6 s variable delay period. Then, a centrally positioned image (neutral or negative; 6.8° × 6.8°) was shown (200 ms) together with two oriented bars (0.3° × 2.7°) positioned peripherally (6.8° degrees to the left and right). The participant’s task was to indicate whether the two bars were of “same” or “different” orientation while ignoring the central stimulus. Forty-eight neutral and 48 negative images were employed from the International Affective Picture System (Lang et al., 1997) and a database of mutilation images developed by Mirtes Pereira from the Federal Flumimense University, Brazil. We attempted to match for picture complexity by carefully choosing images with a clear counterpart (e.g., a mutilated arm/hand and an intact arm/hand). The trial ended with a 2500-ms blank screen. Across the experiment, neutral and negative images were repeated once; they were shown once during the reward condition and once during the no-reward condition. Responses were made on the keyboard using index and middle fingers of the right hand and were counterbalanced across participants in terms of “same” and “different” responses. For the presentation of visual stimuli and recording of participant’s responses, Presentation software (Neurobehavioral Systems, Albany, CA, USA) was used.
Before the start of the main runs, two calibration runs were performed. The first one determined task difficulty; the subsequent one determined the RT threshold to be used during the reward condition (at the calibrated task difficulty level). In the first calibration run, the difficulty of the bar-orientation task was calibrated individually (using a separate stimulus set of neutral pictures) by varying bar orientation difference with staircasing such that accuracy was approximately 80% correct. The goal of this “intermediate” task difficulty was to leave spare attentional resources needed for the processing of negative pictures (and thus interfere with the task); at the same time, this level allowed reward to have an influence, namely, improve behavior. Subsequently, participants performed an RT calibration run that contained only neutral pictures (using a separate set of stimuli) and involved no reward. The median RT of this run was then used as the cut-off point that determined “fast” performance during the main runs. Specifically, the median RT constituted the threshold for the reward-neutral condition; for the reward-negative condition, 30 ms were added to this value. Participants were only told about the possibility of earning a bonus reward in this experiment after this calibration run. They were informed that they could earn 20 cents per trial during the reward condition if they were both fast and accurate. Participants performed a total of 6 experimental runs totaling 48 trials per condition. Trial order was balanced such that each trial type was preceded by every other trial type an equal number of times. The total dollar amount accrued was shown at the end of each run (not after each trial). Over the entire experiment, participants could earn an extra $20 based on their performance during reward trials. On average, participants won $12 of bonus reward (in addition to the base pay of $10).
Data analysis
In the past, we have investigated interference effects of the type investigated in the present study in terms of RT data (Erthal, et al., 2005). So we mainly focused on RT data, but additional analyses of accuracy data were also conducted. For the RT analysis, error trials and trials with an RT exceeding three standard deviations from the condition-specific mean (0.9% of the trials) were excluded in each participant. For each participant, mean RT and accuracy rate data were determined as a function of Reward (reward, no-reward) and Stimulus type (neutral, negative) and repeated-measures ANOVAs were conducted. We used an alpha-level of 0.05 for all statistical tests.
RESULTS
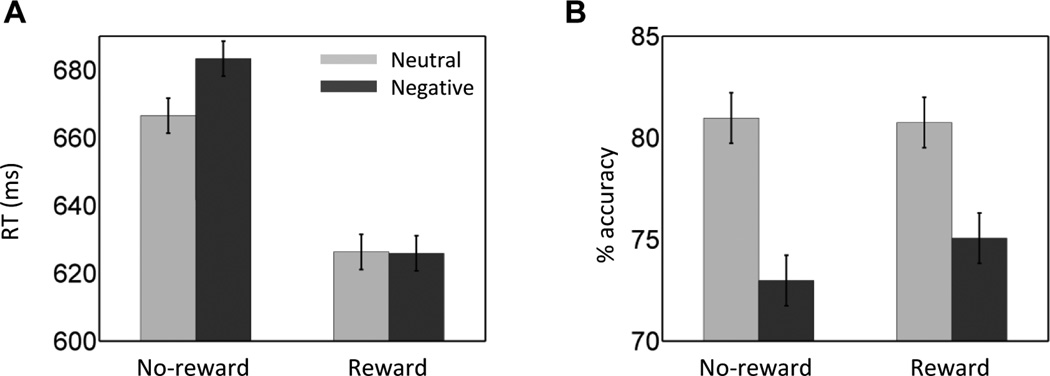
RT data (Fig. 2A) were evaluated according to a 2 Reward (no-reward, reward) × 2 Stimulus (neutral, negative) repeated-measures ANOVA. The main effect of Reward was significant (F(1,36) = 52.32, p < .001, ηp2 = 0.59). Mean RT was faster during the reward (626 msec) compared to the no-reward condition (675 msec), demonstrating the effectiveness of the reward manipulation. The main effect of Stimulus was significant (F(1,36) = 6.17, p = .018, ηp2 = 0.15), such that RTs during the negative picture condition (655 msec) were slower compared to the neutral condition (646 msec). Critically, a significant Reward by Stimulus interaction was detected (F(1, 36) = 7.79, p = .008, ηp2 = 0.18). For completeness, we performed separate pairwise tests: a significant negative-picture interference was present during the no-reward condition (t(36) = 3.72, p < .001, Cohen’s d = 0.61), but was eliminated during the reward condition (t(36) = −0.09, p = .930, Cohen’s d = 0.01).
Figure 2.
Results. (A) During no-reward trials, negative images slowed responses relative to neutral ones. This difference was eliminated during the reward condition. (B) During both no-reward and reward trials, negative images decreased accuracy relative to neutral ones. Error bars denote the standard within-subject error term for interaction effects (Loftus & Masson, 1994).
Given that we observed a main effect of Reward such that overall RT was faster during the reward condition, it is conceivable that the reduced interference during the reward condition could be due to overall faster RTs (faster RTs would leave less “room” for interference). Therefore, we calculated a ratio-based index of interference (negative RT/neutral RT) separately for the reward and no-reward conditions. A comparison of the two via a paired t test revealed a significant difference (t(36) = 2.83, p = .008, Cohen’s d = 0.62), with smaller interference during the reward condition. This analysis demonstrates that the reduction of interference with reward was not simply due to overall faster RTs.
We also evaluated accuracy data (Fig. 2B) according to a 2 Reward (no-reward, reward) × 2 Stimulus (neutral, negative) repeated-measures ANOVA. The main effect of Stimulus was significant (F(1,36) = 46.32, p < .001, ηp2 = 0.56), such that accuracy rate was smaller during negative (74%) compared to neutral (81%) condition. Both the main effect of Reward (F(1,36) = 0.941, p = .339, ηp2 = 0.02) and Reward × Stimulus interaction were not significant (F(1,36) = 2.748, p = .106, ηp2 = 0.07).
DISCUSSION
We investigated interactions between motivation and aversive processing during perception. We observed that reward cues counteracted the effect of potent, negative distractors during a visual discrimination task.
Emotional visual stimuli that are task irrelevant elicit robust interference with the main task (Erthal, et al., 2005; Hartikainen, et al., 2000; MacNamara & Hajcak, 2009) – this is especially the case when the stimuli are more potent, like high-arousal unpleasant images. We evaluated whether reward would counteract the deleterious effect of task-irrelevant aversive pictures during a perceptual task. Surprisingly, reward completely eliminated RT differences between the negative and neutral conditions. Whereas the impact of aversive pictures is often described as fairly automatic (Vuilleumier, 2005), the findings of current study demonstrate that, when sufficiently motivated, participants are able to reduce the deleterious impact of aversive stimuli (see also below).
Previous studies have shown that motivation enhances attention when participants are explicitly informed of the possibility of reward (Engelmann, et al., 2009; Engelmann & Pessoa, 2007; Hubner & Schlosser, 2010; Krebs, Boehler, Roberts, et al., 2011) – for implicit effects, see also refs. (Anderson et al., 2011; Della Libera & Chelazzi, 2009; Kiss et al., 2009; Krebs, Boehler, Egner, et al., 2011; Kristjansson et al., 2010). In a recent study, we showed that motivation is capable of influencing distractor processing by up-regulating attention when reward is at stake (Padmala & Pessoa, 2011). As in the current study, participants were informed of the possibility of reward by a cue stimulus that preceded the target phase during which a Stroop-like interference stimulus was displayed. We proposed that, because reward enhanced attention, the influence of task-irrelevant (neutral) distractors was reduced leading to decreased RT interference and facilitation effects. It is thus possible that, during reward trials in the current study, participants were better able to filter out the aversive stimulus in visual cortex. Alternatively, reward may have allowed better processing at more “central” stages (for further discussion, see (Hubner & Schlosser, 2010)).
This discussion brings up a shortcoming of the present study. Because the instructions during the reward condition emphasized “fast and accurate” while the same was not done during the control condition, it is conceivable that reward was not necessary for the observed effect. Although this possibility cannot be countered without an additional study, similar designs in our lab have allowed us to make stronger ties with reward processing. For instance, in the response interference study above, reward-related brain regions (including dorsal and ventral striatum) were engaged by cues signaling reward, and the strength of their functional interactions with attention-related regions was linearly related to individual differences in reward sensitivity (Padmala & Pessoa, 2011). We thus believe that the present behavioral effects likewise involved reward circuits in the brain.
The reduction of aversive stimuli interference during reward in the current study is also consistent with another recent finding from our lab (Hu, et al., 2013). Unlike in the present study, reward was manipulated in a reactive fashion, that is, information about reward was not provided in advance via a cue stimulus; instead one of the stimulus types (either a foreground building or a house picture) was associated with reward. Behaviorally, reduced interference was observed from a stimulus background previously paired with shock. The results from the current study complement these results by showing that reward manipulated in a proactive fashion -- where it is signaled by a cue that allows preparation for thoughts and actions -- also reduces the interference from negative stimuli.
More broadly, the present study is also connected to understanding the role of “goal relevance”. Vogt and colleagues have characterized several properties of goal-based attention. Vogt and colleagues (2012) examined whether goal-relevant information evokes an attentional bias when it competes with threatening stimuli. In three experiments, subjects performed a dot-probe task, which evaluates mechanisms of spatial attention, combined with a separate task that induced a temporary goal. Their attention was oriented to goal-relevant pictures even when the stimulus was simultaneously displayed with threatening pictures. This was observed even in a group of high-anxious subjects, and in the presence of a more powerful threat (a colored patch signaling the presentation of an aversive noise).
As reviewed elsewhere (Pessoa, 2013), goal-relevant items acquire properties similar to those observed for stimuli that involuntarily capture attention, such as abrupt onsets and emotional stimuli. In this regard, endogenous attentional processing may act like “automatic” processing. In particular, goal-relevant items are powerful enough to win the competition against simultaneously presented emotion-laden items, illustrating that emotional stimuli do not have a unique, “special” status. How do goal-relevant stimuli acquire their competitive advantage? One idea is that templates prime visual mechanisms to facilitate detection (Desimone & Duncan, 1995; Grossberg, 1980), as implemented in several computational models of visual search (Grossberg et al., 1994; Wolfe, 1994). Finally, although the literature on goal-relevance and attention has developed independently of the literature on motivation and attention, they are obviously closely related. For one thing, motivation is an effective way to induce goals, as when subjects are told they will be rewarded for correct performance in trials involving a specific stimulus (e.g., Kristjansson, Sigurjonsdottir, and Driver 2010).
In summary, the present findings thus underscore the need to go beyond studies that only focus on the effects of reward or aversive processing on perception. Solving perceptual problems involves the joint consideration of positive and negative signals whose forces shape perception.
Acknowledgements
Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589). We thank Andrew Bauer for help with the data collection.
References
- Aarts E, van Holstein M, Cools R. Striatal Dopamine and the Interface between Motivation and Cognition. Frontiers in Psychology. 2011;2:163. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences USA. 2011;108(25):10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychological Science. 2009;20(6):778–784. doi: 10.1111/j.1467-9280.2009.02360.x. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju EC, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009;3(4) doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Pessoa L. Motivation sharpens exogenous spatial attention. Emotion. 2007;7(3):668–674. doi: 10.1037/1528-3542.7.3.668. [DOI] [PubMed] [Google Scholar]
- Erthal FS, de Oliveira L, Mocaiber I, Pereira MG, Machado-Pinheiro W, Volchan E, Pessoa L. Load-dependent modulation of affective picture processing. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(4):388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Grossberg S. How does a brain build a cognitive code? Psychological Review. 1980;87(1):1–51. doi: 10.1007/978-94-009-7758-7_1. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Mingolla E, Ross WD. A neural theory of attentive visual search: interactions of boundary, surface, spatial, and object representations. Psychological Review. 1994;101(3):470–489. doi: 10.1037/0033-295x.101.3.470. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia. 2000;38(12):1576–1580. doi: 10.1016/s0028-3932(00)00072-5. [DOI] [PubMed] [Google Scholar]
- Hu K, Padmala S, Pessoa L. Interactions between reward and threat during visual processing. Neuropsychologia. 2013;51(9):1763–1772. doi: 10.1016/j.neuropsychologia.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner R, Schlosser J. Monetary reward increases attentional effort in the flanker task. Psychonomic Bulletin & Review. 2010;17(6):821–826. doi: 10.3758/PBR.17.6.821. [DOI] [PubMed] [Google Scholar]
- Kiss M, Driver J, Eimer M. Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychological Science. 2009;20(2):245–251. doi: 10.1111/j.1467-9280.2009.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Egner T, Woldorff MG. The neural underpinnings of how reward associations can both guide and misguide attention. Journal of Neuroscience. 2011;31(26):9752–9759. doi: 10.1523/JNEUROSCI.0732-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Roberts KC, Song AW, Woldorff MG. The Involvement of the Dopaminergic Midbrain and Cortico-Striatal-Thalamic Circuits in the Integration of Reward Prospect and Attentional Task Demands. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson A, Sigurjonsdottir O, Driver J. Fortune and reversals of fortune in visual search: Reward contingencies for pop-out targets affect search efficiency and target repetition effects. Attention, Perception & Psychophysics. 2010;72(5):1229–1236. doi: 10.3758/APP.72.5.1229. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert B. International affective picture system (IAPS): Instruction Manual and Affective ratings: NIMH Center for the Study of Emotion and Attention. 1997 [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1(4):476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47(13):2975–2980. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward Reduces Conflict by Enhancing Attentional Control and Biasing Visual Cortical Processing. Journal of Cognitive Neuroscience. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology. 2005;15(2):188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pessoa L. The Cognitive-Emotional Brain: From Interactions to Integration. Cambridge: MIT Press; 2013. [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Frontiers in Neuroscience. 2010;4 doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, De Houwer J, Crombez G, Van Damme S. Competing for attentional priority: Temporary goals versus threats. Emotion. 2012 doi: 10.1037/a0027204. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0: A revised model of visual search. Psychonomic Bulletin & Review. 1994;1(2):202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]