Abstract
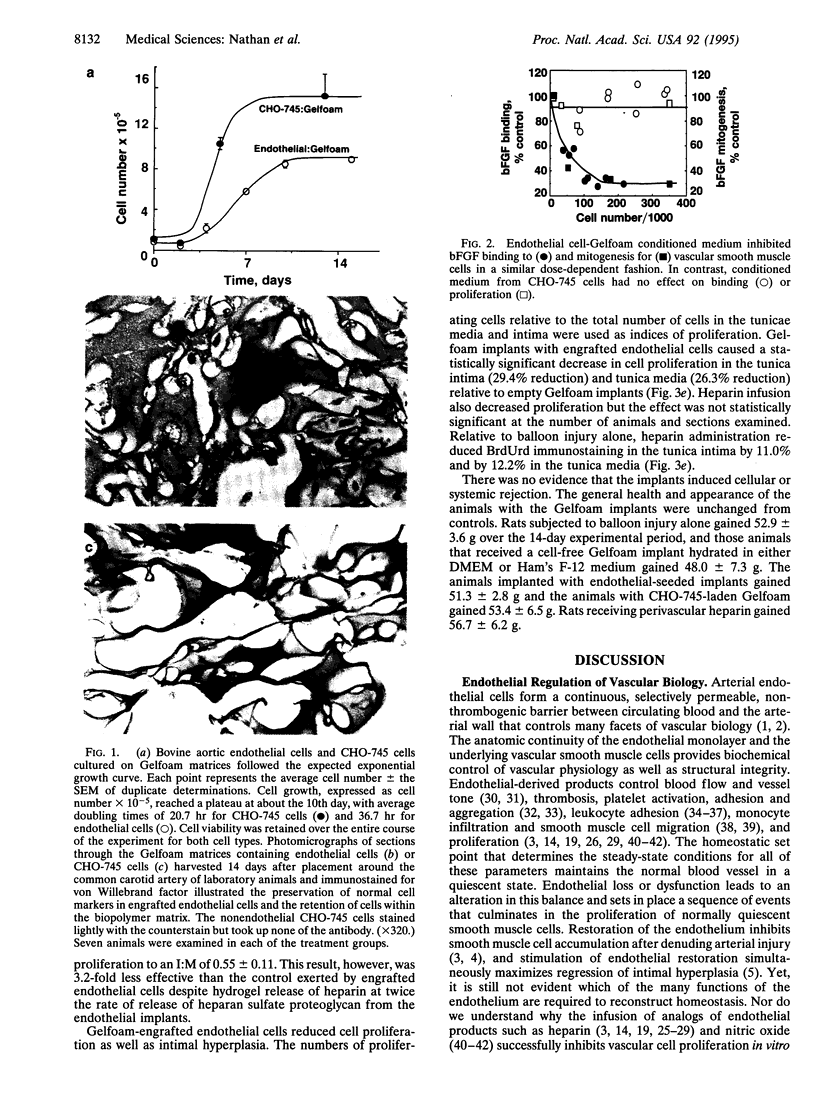
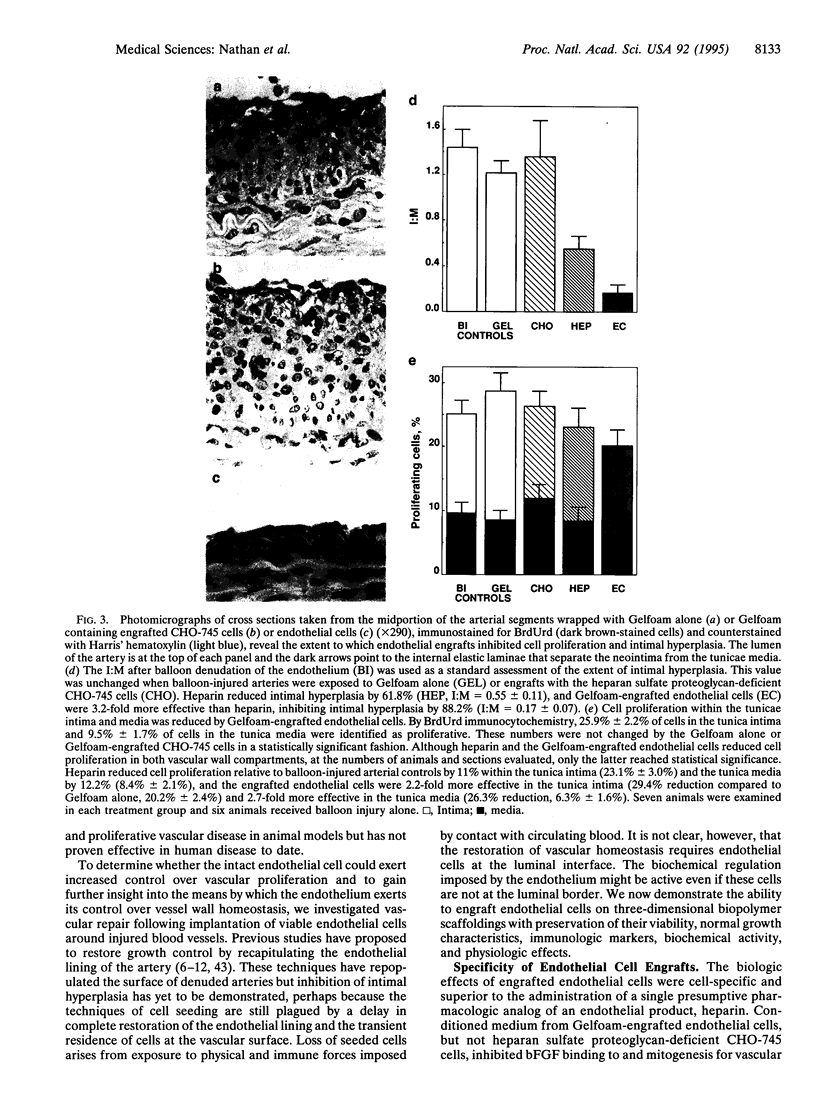
Molecular biomaterial engineering permits in vivo transplantation of cells and tissues, offering the promise of restoration of physiologic control rather than pharmacologic dosing with isolated compounds. We engrafted endothelial cells on Gelfoam biopolymeric matrices with retention of viability, normal growth kinetics, immunoreactivity, and biochemical activity. The production of heparan sulfate proteoglycan and inhibition of basic fibroblast growth factor binding and activity by engrafted cells were indistinguishable from endothelial cells grown in culture. Perivascular implantation of Gelfoam-endothelial cell scaffolds around balloon-denuded rat carotid arteries reduced intimal hyperplasia 88.1%, far better than the isolated administration of heparin, the most effective endothelial mimic compound. In concert with a reduction in intimal area, cell proliferation was reduced by > 90%. To our knowledge, there have been no previous reports of extravascular cell implants controlling vasculoproliferative disease. Tissue engineered cells offer the potential for potent methods of vascular growth regulation and insight into the complex autocrine-paracrine control mechanisms within the blood vessel wall.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autio I., Malo-Ranta U., Kallioniemi O. P., Nikkari T. Cultured bovine aortic endothelial cells secrete factor(s) chemotactic for aortic smooth muscle cells. Artery. 1989;16(2):72–83. [PubMed] [Google Scholar]
- Bjornsson T. D., Dryjski M., Tluczek J., Mennie R., Ronan J., Mellin T. N., Thomas K. A. Acidic fibroblast growth factor promotes vascular repair. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8651–8655. doi: 10.1073/pnas.88.19.8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Centra M., Ratych R. E., Cao G. L., Li J., Williams E., Taylor R. M., Rosen G. M. Culture of bovine pulmonary artery endothelial cells on Gelfoam blocks. FASEB J. 1992 Sep;6(12):3117–3121. doi: 10.1096/fasebj.6.12.1521742. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985 Jun;52(6):611–616. [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Conte M. S., Birinyi L. K., Miyata T., Fallon J. T., Gold H. K., Whittemore A. D., Mulligan R. C. Efficient repopulation of denuded rabbit arteries with autologous genetically modified endothelial cells. Circulation. 1994 May;89(5):2161–2169. doi: 10.1161/01.cir.89.5.2161. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Davies M. G., Hagen P. O. The vascular endothelium. A new horizon. Ann Surg. 1993 Nov;218(5):593–609. doi: 10.1097/00000658-199321850-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichek D. A., Neville R. F., Zwiebel J. A., Freeman S. M., Leon M. B., Anderson W. F. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation. 1989 Nov;80(5):1347–1353. doi: 10.1161/01.cir.80.5.1347. [DOI] [PubMed] [Google Scholar]
- Doherty D. E., Haslett C., Tonnesen M. G., Henson P. M. Human monocyte adherence: a primary effect of chemotactic factors on the monocyte to stimulate adherence to human endothelium. J Immunol. 1987 Mar 15;138(6):1762–1771. [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Karnovsky M. J. Contrasting effects of the intermittent and continuous administration of heparin in experimental restenosis. Circulation. 1994 Feb;89(2):770–776. doi: 10.1161/01.cir.89.2.770. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Karnovsky M. J. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1513–1517. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Smith L. T., Karnovsky M. J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992 Feb;89(2):465–473. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Pukac L. A., Karnovsky M. J. Protamine and protamine-insulins exacerbate the vascular response to injury. J Clin Invest. 1993 May;91(5):2308–2313. doi: 10.1172/JCI116460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D. Animal cell mutants defective in heparan sulfate polymerization. Adv Exp Med Biol. 1992;313:97–106. doi: 10.1007/978-1-4899-2444-5_10. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Flugelman M. Y., Jaklitsch M. T., Newman K. D., Casscells W., Bratthauer G. L., Dichek D. A. Low level in vivo gene transfer into the arterial wall through a perforated balloon catheter. Circulation. 1992 Mar;85(3):1110–1117. doi: 10.1161/01.cir.85.3.1110. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gee A. P., Minta J. O. Migration of porcine endothelial and smooth muscle cells in response to platelet-associated factors. Am J Hematol. 1984 Jul;17(1):29–38. doi: 10.1002/ajh.2830170105. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Jarrell B. E., Williams S. K. Microvessel derived endothelial cell isolation, adherence, and monolayer formation for vascular grafts. J Vasc Surg. 1991 May;13(5):733–734. doi: 10.1016/0741-5214(91)90366-3. [DOI] [PubMed] [Google Scholar]
- Ley K., Cerrito M., Arfors K. E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991 May;260(5 Pt 2):H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- McNamara D. B., Bedi B., Aurora H., Tena L., Ignarro L. J., Kadowitz P. J., Akers D. L. L-arginine inhibits balloon catheter-induced intimal hyperplasia. Biochem Biophys Res Commun. 1993 May 28;193(1):291–296. doi: 10.1006/bbrc.1993.1622. [DOI] [PubMed] [Google Scholar]
- Messina L. M., Podrazik R. M., Whitehill T. A., Ekhterae D., Brothers T. E., Wilson J. M., Burkel W. E., Stanley J. C. Adhesion and incorporation of lacZ-transduced endothelial cells into the intact capillary wall in the rat. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12018–12022. doi: 10.1073/pnas.89.24.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Boyce F. M., Stanley J. C., Nabel G. J. Recombinant gene expression in vivo within endothelial cells of the arterial wall. Science. 1989 Jun 16;244(4910):1342–1344. doi: 10.1126/science.2499928. [DOI] [PubMed] [Google Scholar]
- Nugent M. A., Karnovsky M. J., Edelman E. R. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993 Dec;73(6):1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Yeaman C. A quantitative solid-phase assay for identifying radiolabeled glycosaminoglycans in crude cell extracts. Anal Biochem. 1989 Jun;179(2):361–365. doi: 10.1016/0003-2697(89)90145-0. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22 (Suppl 4):S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Stemerman M. B., Benditt E. P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- Sheppard B. L., French J. E. Platelet adhesion in the rabbit abdominal aorta following the removal of the endothelium: a scanning and transmission electron microscopical study. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):427–432. doi: 10.1098/rspb.1971.0006. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Birinyi L. K., Salomon R. N., Libby P., Callow A. D., Mulligan R. C. Implantation of vascular grafts lined with genetically modified endothelial cells. Science. 1989 Jun 16;244(4910):1344–1346. doi: 10.1126/science.2734614. [DOI] [PubMed] [Google Scholar]
- Zwiebel J. A., Freeman S. M., Kantoff P. W., Cornetta K., Ryan U. S., Anderson W. F. High-level recombinant gene expression in rabbit endothelial cells transduced by retroviral vectors. Science. 1989 Jan 13;243(4888):220–222. doi: 10.1126/science.2911735. [DOI] [PubMed] [Google Scholar]





