Abstract
OBJECTIVE
The dawn phenomenon is a transient rise in blood glucose between 4 and 6 AM that is attributed to the pulsatile release of pituitary growth hormone (GH). In pregnancy, GH is suppressed by placental GH. Hence, we hypothesize that there is no evidence for the dawn phenomenon in late pregnancy in healthy women.
STUDY DESIGN
Twenty glucose-tolerant women with singleton gestations between 28 weeks and 36 weeks 6 days’ gestation were recruited. The women were admitted overnight to the Clinical Research Unit and had continuous glucose monitoring. Insulin and GH were measured at 2-hour intervals from 8 PM to 8 AM. GH was grouped into times 1A (8–10 PM), 2A (12–2 AM), and 3A (4–8 AM) for changes over time. Further analysis was performed with time 1B (8 PM to 2 AM) and 2B (4–8 AM). Insulin was measured between 4 and 8 AM.
RESULTS
Plasma glucose decreased over time (P < .001). There were no significant changes in GH among times 1A, 2A, and 3A (P = .45) or times 1B and 2B (P = .12). Insulin concentrations increased after meals, but there were no changes from 4 AM (8.5 ± 1.4 μU/mL) through 8 AM (8.6 ± 1.1 μU/mL; P = .98).
CONCLUSION
Glucose and insulin concentrations show no increase from 4–8 AM; although there is variability in GH, there is no evidence for the dawn phenomenon in late pregnancy in healthy women.
Keywords: dawn phenomenon, growth hormone, pregnancy
The dawn phenomenon is defined as a transient rise in blood glucose concentration that occurs overnight between 4 and 6 AM. This transient hyperglycemia has been attributed to the pulsatile release of growth hormone (GH) overnight. GH is released by the anterior pituitary in a pulsatile and diurnal fashion and is central in the balance between metabolic and catabolic states.1 Pituitary GH functions include stimulation of linear growth, lipolysis, protein synthesis, and antagonism of insulin. There are significant alterations in GH metabolism during pregnancy. GH is inhibited by somatostatin, insulin-like growth factor-I, hyperglycemia, and leptin in the normal patient.1
In the pregnant patient, however, GH is inhibited by placental GH,2–4 which is synthesized by the syncytiotrophoblasts of the placenta and is released directly into maternal circulation. Placental GH secretion is modified by Glut1, the major glucose transporter in the placenta, in response to maternal blood glucose levels.5 The inhibition of GH during pregnancy dominates other stimulatory factors, including estrogen. As placental GH concentrations increase, the GH concentrations decrease. By 15–20 weeks’ gestation, placental GH is the dominant GH, with the GH virtually undetectable. 2–4 This dominance continues until parturition, after which 75% of the placental GH can be cleared as soon as 30 minutes after delivery.3
In the treatment of people with diabetes mellitus, insulin management must take into account the hyperglycemic effects of the dawn phenomenon. Management of diabetes mellitus is particularly important in the pregnant patient, for whom normalization of glucose is the priority. Because placental GH functionally replaces GH during pregnancy, we hypothesize that there is no dawn phenomenon during late pregnancy. Hence, the primary aim of this study was to document the relationship between blood glucose, insulin, and placental GH levels during pregnancy.
Materials and Methods
Twenty healthy glucose-tolerant women in the third trimester of pregnancy (28 weeks to 36 weeks 6 days gestation) were recruited prospectively. All participants signed a consent form that was approved by the Institutional Review Board at MetroHealth Medical Center/Case Western Reserve University. The protocol was reviewed and approved by the MetroHealth Scientific Review Committee of the Clinical Research Unit of the Case Western Reserve University Clinical and Translational Science Collaborative. Women with a normal 1-hr 50-g glucose challenge test and a nonanomalous singleton gestation were eligible for participation. Women were excluded from participation if their pregnancy was complicated by intrauterine growth restriction, preterm labor, premature rupture of membranes, abnormal placentation, hypertension, preexisting diabetes mellitus, gestational diabetes mellitus, autoimmune disorders, illicit drug use, or chronic steroid use. Each woman completed 2 visits at the Clinical Research Unit. The first visit included body composition estimates with the use of air displacement plethysmography (Bod Pod; COSMED, Rome, Italy). The women also met with the Clinical Research Unit research nutritionist to review and discuss components of their current diet.
The second visit entailed an overnight admission to the Clinical Research Unit. Women were admitted at 6 PM. Weight, blood pressure, and fetal heart tones were recorded. The Continuous Glucose Monitoring System (CGMS) iPro sensor (Medtronic Inc, Northridge, CA) and an intravenous catheter were placed on admission. The CGMS obtained glucose values every 5 minutes over a 12-hour period for a total of 145 values per subject. The iPro sensor was placed in the suprailiac region and was calibrated with serum glucose measurements that were obtained at each scheduled blood draw on each subject. Eighty-eight percent of values were obtained and available for analysis. Peripheral intravenous access was obtained for repeated blood draws overnight to obtain samples without awakening the women. Women received a standard 2000–2200 calorie/day diet for dinner (75–80 g carbohydrates) and a bedtime snack (30 g carbohydrates) then received nothing by mouth overnight. GH and insulin values were obtained every 2 hours overnight for a total of 7 values. A fasting lipid profile and hemoglobin A1c level were also obtained the next morning. A fasting lipid panel was obtained in addition to the HbA1c, body mass index, and percent body fat to obtain a representation of the metabolic state of our study population and to confirm that there were no underlying disturbances in lipid metabolism.
There are currently no assays specific for placental GH available for research purposes in the United States. Plasma human GH was assayed by enzyme-linked immunosorbent assay (Quantikine Human Growth Hormone Immunoassay DGH00; R&D Systems, Inc, Minneapolis, MN). The antibody does not differentiate between pituitary and placental GH.
Glucose values for all the women were analyzed with time series analysis. The median blood glucose, GH, and insulin levels were analyzed with Kruskal-Wallis 1-way analysis of variance based on ranks between different epochs overnight. We also used repeated measures analysis of variance, 1-way analysis of variance, regression analysis, and time series analysis to study the changes over time. Considering a paired sample size of 20 patients, a coefficient of variation of 0.04, and a correlation between measurements of 0.30, we were able to detect a 20% increase from baseline at the .05 significance level with 90% power. Analyses were performed with SPSS software (version 20; SPSS Inc, Chicago, IL) and Statistix software (version 9; Analytical Software, Tallahassee, FL).
Results
Cohort characteristics are depicted in Table 1. Most of the women were African American, multiparous, and evenly represented by normal, overweight, and obese prepregnancy body mass index measurements. The mean percentage of body fat was 38%. Metabolic data are presented in Table 2. The mean 1-hr 50-g glucose challenge test was 102.2 mg/dL; the hemoglobin A1c level was 5.5%. Fasting lipid profiles were consistent with late gestation. The mean glucose value during the 12 hour acquisition was 85.4 ± 0.49 mg/dL.
TABLE 1.
Cohort characteristics
| Characteristic | Measure |
|---|---|
| Age, ya | 27.2 ± 4.7 |
| Gestational age at first visit, wk/da | 30/4 ± 12 d |
| Parity >1, n (%) | 15 (75) |
| Ethnicity, n (%) | |
| White | 7 (35.0) |
| African American | 11 (55.0) |
| Hispanic | 1 (5.0) |
| Other | 1 (5.0) |
| Prepregnancy body mass index, n (%) | |
| Normal | 6 (30) |
| Overweight | 6 (30) |
| Obese | 8 (40) |
| Body mass index at first visit, kg/m2a | 31.1 ± 5.2 |
| Weight gain at first visit, kga | 7.0 ± 7.1 |
| Body fat, %a | 37.8 ± 5.8 |
Data are presented as mean ± SD.
TABLE 2.
Baseline metabolic analyses
| Variable | Mean ± SD |
|---|---|
| Hemoglobin A1c, % | 5.5 ± 0.6 |
| Cholesterol, mg/dL | 238.0 ± 50.2 |
| Triglycerides, mg/dL | 165.0 ± 58.0 |
| High-density lipoprotein, mg/dL | 59.6 ± 12.8 |
| Low-density lipoprotein, mg/dL | 154.6 ± 42.6 |
| Very low-density lipoprotein, mg/dL | 26.4 ± 9.3 |
| Glucose, mg/dL | 85.4 ± 0.49 |
| 1-hour glucose tolerance test, mg/dL | 102.2 ± 18.4 |
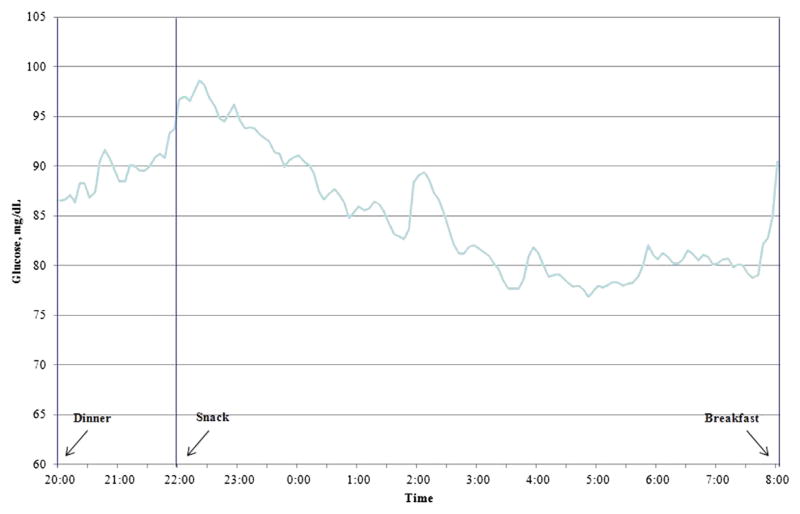
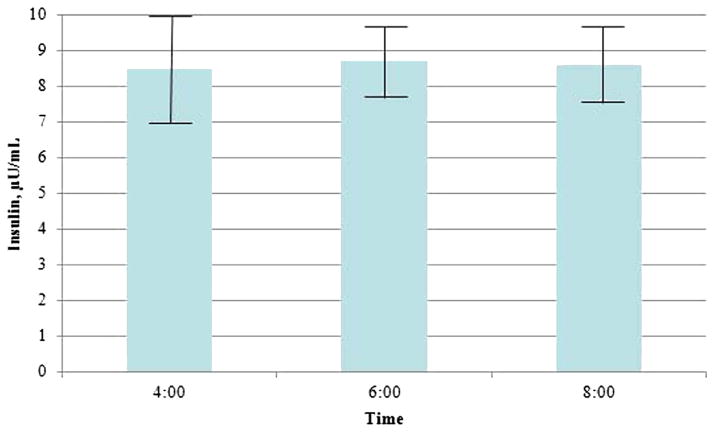
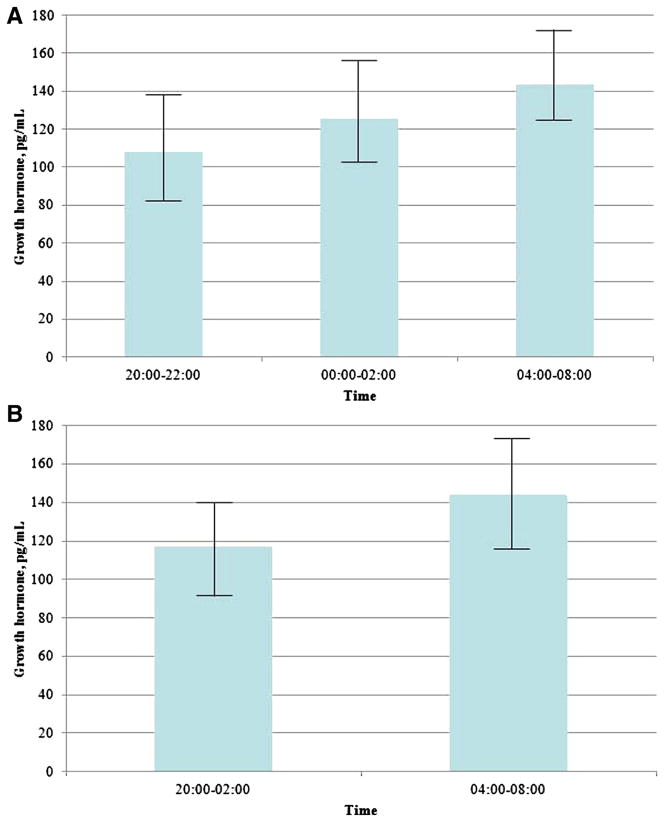
Longitudinal variations in plasma glucose that was obtained from the CGMS are shown in Figure 1. A time series analysis identified the auto regressive integrated moving average time series model (1,0,1) to be the best fit model for forecasting plasma glucose level during the study period. Auto regressive integrated moving average (1,0,1) means auto regressive and moving average of order 1 without differencing. 1 Mean glucose values decreased over time (P < .001). Insulin levels peaked by 10 PM then gradually decrease through 8 AM. There were no significant differences in insulin levels over time with 8.5 ± 1.4, 8.7 ± 1.0, and 8.6 ± 1.1 μU/mL, respectively, at 4, 6, and 8 AM (P = .84; Figure 2). GH levels were compared among 3 time periods (1A, 1B, and 1C; Figure 3). There were no significant differences (108 ± 25, 125.8 ± 23.9, and 143.9 ± 30.6 pg/mL at 8–10 PM, 12–2 AM, and 4–6 AM, respectively; P = .45). Similar results were noted when made the comparisons over the 2 time periods 8 PM to 2 AM and 4–8 AM (116.9 ± 23.7 and 143.9 ± 30.6 pg/mL, respectively; P =.74). These results were confirmed with the use of the repeated measures analysis of variance and 1-way analysis of variance.
FIGURE 1. Plasma glucose concentrations over time.
The arrows depict the time at which the women received dinner, a snack, or breakfast. Times series analysis, P > .001.
FIGURE 2. Plasma insulin concentrations over time.
Plasma insulin levels at times 4, 6, and 8 AM. Kruskal-Wallis, P = .84. The bars indicate standard error.
FIGURE 3. Plasma growth hormone concentrations over time.
A, Plasma growth hormone levels at times 8–10 PM, 12–2 AM, and 4–8 AM. Kruskal-Wallis, P=.45. B, Plasma growth hormone levels at 8 PM to 2 AM and 4–8 AM. Mann-Whitney U, P = .74. The bars indicate standard error.
Comment
We examined the interactions between glucose, insulin, and GH in response to specified dietary intake in women during the third trimester of pregnancy. There were no significant increases in glucose, insulin, or placental GH that correlated to the dawn phenomenon. Previous studies have documented the existence of the dawn phenomenon in non-pregnant individuals with normal glucose tolerance6–8 and the suppression of pituitary-derived GH in pregnancy.2–4,9–11 To date, however, there have been no studies to support or refute the existence of the dawn phenomenon during pregnancy. Our study clearly documents the lack of any surge in glucose levels, insulin release, and GH secretion that could be attributed to the dawn phenomenon.
Diurnal variations in GH secretion with peak secretion occurring within stages III–IV of sleep is well-documented.1 This diurnal secretion has been proposed as the cause of the dawn phenomenon in both normal patients and patients with diabetes mellitus. The dawn phenomenon constitutes a transitory increase in plasma glucose that is typically seen at approximately 4 AM. Several studies have proposed that the antagonistic effects of GH on insulin resistance are the cause of the dawn phenomenon.5 In contrast to the pulsatile release of pituitary GH, placental GH lacks diurnal variation9,12 and is released in a continuous fashion throughout pregnancy.2–4,9–13
A strength of our study is that this is the first prospective study in pregnancy to document the relationship between glucose, insulin, and placental GH over time. Although other studies have documented the effect of pregnancy on each of those elements separately, we observed the relationship of all 3 elements to each other over the same time period. Our cohort was also evaluated within the Clinical Research Unit, which provided a controlled and consistent environment to each subject. The use of the CGMS sensor, >2500 glucose values were obtained within a 12-hour time period for analysis. In addition, the use of time series analysis for these glucose values limited the impact of any isolated missing variables because they were distributed throughout various time points.
A limitation of our study was that the kit that was used to measure placental GH was unavailable in the United States. The Quantikine Human Growth Hormone Immunoassay was an acceptable alternative because it quantifies both placental and pituitary GHs. Several studies have documented the normal changes in GH secretion during pregnancy (specifically, the inhibition of GH to undetectable levels and the steady-state secretion of placental GH after 15–20 weeks’ gestation).9,10,13,14 As such, we accepted that this immunoassay would quantify placental GH appropriately in our patients. Furthermore, although we made every effort to obtain blood draws without awakening the women, not all the women were able to sleep throughout the night. This limitation was not expected to result in significant impedance because of the secretory pattern of placental GH, which lacks the circadian release that is seen with pituitary GH. Although GH levels appeared to increase overnight, there were substantial overlap between time periods and no significant difference. This study was powered to determine at least a 20% difference in levels; thus, we would not be powered adequately to detect a difference of <20%. This cutoff was selected as a level at which a clinical impact may be appreciated. Moreover, our patients were predominantly African American from an urban population with a prepregnancy body mass index category of overweight or obese, which may limit the application of our findings to a specific subgroup of the population. Additional studies are warranted to determine whether these characteristics play a greater impact than placental GH.
Controlling glucose in pregnant women with diabetes mellitus must take into account the intricate hormonal changes of pregnancy. Placental GH is the dominant GH in pregnancy, and its secretion is modified in response to maternal glucose levels. The results of our study document the diurnal relationship among glucose, insulin, and placental GH levels during the third trimester in normal glucose-tolerant pregnancies. Our data highlight the absence of the dawn phenomenon in normal pregnancy. Women with preexisting diabetes mellitus exhibit either no (type 1) or impaired (type 2 and gestational diabetes mellitus) beta-cell function and decreased insulin sensitivity compared with normal glucose-tolerant women and, as such, the glycemic response to placental GH secretion may vary. Further studies that will include pregnant women with evidence of glucose intolerance (such as type 1 and 2 diabetes mellitus) are warranted. These data provided further information regarding factors that affect glucosemetabolism during pregnancy and a framework to assist in the management of glucose intolerance and insulin therapy during pregnancy.
Acknowledgments
Supported by the Clinical and Translational Science Collaborative of Cleveland and grant number UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research.
Footnotes
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The authors report no conflict of interest.
Presented in abstract form at the 72nd annual meeting of the American Diabetes Association, Philadelphia, PA, June 8-12, 2012.
References
- 1.Eisenbarth GS, Polonsky KS, Buse JB. Type 1 diabetes mellitus. In: Wilson JD, Foster DW, editors. Williams textbook of endocrinology. 8. Philadelphia: Saunders; 1992. [Google Scholar]
- 2.Lacroix MC, Guibourdenche J, Frendo JL, Muller F, Evain-Brion D. Human placental growth hormone: a review. Placenta. 2002;23(suppl A):S87–94. doi: 10.1053/plac.2002.0811. [DOI] [PubMed] [Google Scholar]
- 3.Lonberg U, Damm P, Andersson A, et al. Increase in maternal placental growth hormone during pregnancy and disappearance during parturition in normal and growth hormone-deficient pregnancies. Am J Obstet Gynecol. 2003;188:247–51. doi: 10.1067/mob.2003.82. [DOI] [PubMed] [Google Scholar]
- 4.Burney RO, Mooney SB, Giudice LC. [Accessed Nov. 6, 2009];Endocrinology in pregnancy. Available at: Endotext.com.
- 5.Perriello G, De Feo P, Torlone E, et al. Nocturnal spikes of growth hormone secretion cause the dawn phenomenon in Type 1 (insulin-dependent) diabetes mellitus by decreasing hepatic (and extrahepatic) sensitivity to insulin in the absence of insulin waning. Diabetologia. 1990;33:52–9. doi: 10.1007/BF00586461. [DOI] [PubMed] [Google Scholar]
- 6.Bolli GB, de Feo P, de Cosmo S, et al. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. 1984;33:1150–3. doi: 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- 7.Arslanian S, Ohki Y, Becker DJ, Drash AL. The dawn phenomenon: comparison between normal and insulin-dependent diabetic adolescents. Pediatr Res. 1992;31:203–6. doi: 10.1203/00006450-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt MI, Lin QX, Gwynne JT, Jacobs S. Fasting early morning rise in peripheral insulin: evidence of the dawn phenomenon in non-diabetes. Diabetes Care. 1984;7:32–5. doi: 10.2337/diacare.7.1.32. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson L, Frankenne F, Edén S, Hennen G, Von Schoultz B. Growth hormone 24-h serum profiles during pregnancy: lack of pulsatility for the secretion of the placental variant. BJOG. 1989;96:949–53. doi: 10.1111/j.1471-0528.1989.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson L. Growth hormone in human pregnancy: maternal 24-hour serum profiles and experimental effects of continuous growth hormone secretion. Acta Obstet Gynecol Scan Suppl. 1989;147:1–38. doi: 10.3109/00016348709156496. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson L, Edén S, Fröhlander N, Bengtsson B, von Shoultz B. Continuous 24- hour secretion of growth hormone during late pregnancy: a regulator of maternal metabolic adjustment? Acta Obstet Gynecol Scan. 1988;67:543–7. doi: 10.3109/00016348809029867. [DOI] [PubMed] [Google Scholar]
- 12.Alsat E, Guibourdenche J, Luton D, Frankenne F, Evain-Brion D. Human placental growth hormone. Am J Obstet Gynecol. 1997;177:1526–34. doi: 10.1016/s0002-9378(97)70103-0. [DOI] [PubMed] [Google Scholar]
- 13.Frankenne F, Closset J, Gomez F, Scippo ML, Smal J, Hennen G. The physiology of growth hormones (GHs) in pregnant women and partial characterization of the placental GH variant. J Clin Endocrinol Metab. 1988;66:1171–80. doi: 10.1210/jcem-66-6-1171. [DOI] [PubMed] [Google Scholar]
- 14.Mirlesse V, Frankenne F, Alsat E, Poncelet M, Hennen G, Evain-Brion D. Placental growth hormone levels in normal pregnancy and in pregnancies with intrauterine growth retardation. Pediatr Res. 1993;34:439–42. doi: 10.1203/00006450-199310000-00011. [DOI] [PubMed] [Google Scholar]