Abstract
Background
Previous studies have shown that frontostriatal networks, especially those involving dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) mediate cognitive functions some of which are abnormal in schizophrenia. This study examines white matter integrity of the tracts connecting DLPFC/VLPFC and striatum in patients with first-episode schizophrenia (FESZ), and their associations with cognitive and clinical correlates.
Methods
Diffusion tensor and structural magnetic resonance images were acquired on a 3T GE Echospeed system from 16 FESZ and 18 demographically comparable healthy controls. FreeSurfer software was used to parcellate regions of interest. Two-tensor tractography was applied to extract fibers connecting striatum with rostral middle frontal gyrus (rMFG) and inferior frontal gyrus (IFG), representing DLPFC and VLPFC respectively. DTI indices, including fractional anisotropy (FA), trace, axial diffusivity (AD) and radial diffusivity (RD), were used for group comparisons. Additionally, correlations were evaluated between these diffusion indices and the Wisconsin Card Sorting Task (WCST) and the Brief Psychiatric Rating Scale (BPRS).
Results
FA was significantly reduced in the left IFG-striatum tract, whereas trace and RD were significantly increased in rMFG-striatum and IFG-striatum tracts, bilaterally. The number of WCST categories completed correlated positively with FA of the right rMFG-striatum tract, and negatively with trace and RD of right rMFG-striatum and right IFG-striatum tracts in FESZ. The BPRS scores did not correlate with these indices.
Conclusions
These data suggest that white matter tract abnormalities between rMFG/IFG and striatum are present in FESZ and appear to be significantly associated with executive dysfunction but not with symptom severity.
Keywords: first-episode schizophrenia, corticostriatal pathway, diffusion tensor imaging, tractography, fractional anisotropy
1. Introduction
Positive and negative symptoms, along with cognitive impairments, represent three distinct clusters that characterize schizophrenia. Among them, cognitive deficits often precede the onset of other clinical symptoms (Reichenberg et al., 2006; Woodberry et al., 2008), and persist even after other symptoms have been effectively treated (e.g., Heinrichs, 2005). Thus, cognitive deficits are widely recognized as core features of schizophrenia (e.g., Barch, 2005), and have become a major focus in schizophrenia research (e.g., Keefe et al., 2007). Investigating these impairments in the early stages of schizophrenia is important because these patients are less affected by medication, substance abuse and/or aging, and thus findings here are less confounded and could yield substantial improvements in clinical treatment (e.g., Insel, 2010). An extensive meta-analysis of neurocognitive deficits in first episode schizophrenia (Mesholam-Gately et al., 2009) confirmed that deficits are virtually as severe at that point as in chronic phases (Heinrichs and Zakzanis, 1998). Thus, exploration of cognitive dysfunction in the early stages of disease, such as in first episode schizophrenia (FESZ), is a promising approach to understanding the mechanism of disease, especially when linked to brain measures.
Moreover, frontostriatal networks (Meyer-Lindenberg et al., 2009; Waltz et al., 2007), especially those involving striatal dopamine activity (Bach et al., 2008; Kegeles et al., 2010; Simpson et al., 2010) and functions of dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) (Manoach et al., 2000; Weinberger et al., 2001; Boettiger and D'Esposito, 2005), have been shown to mediate some cognitive functions that are abnormal in schizophrenia. The idea that both the VLPFC and DLPFC play a role in working memory has been reviewed by Lawrence et al. (1998). They note, for example, that a spatial span test activates ventrolateral, instead of the dosolateral prefrontal cortical regions whereas a spatial recognition test has the opposite activation pattern (Lawrence et al., 1998). The frontal cortex is the main driving force of the basal ganglia, and the main cortical inputs to the basal ganglia are to the striatum, which consists of the neostriatum (caudate and putamen) and ventral striatum (nucleus accumbens) (Alexander et al., 1986). Many investigators/anatomists divide the striatum into 3 functional/topographic zones, namely associative striatum (most of the head of caudate and rostral putamen), sensorimotor striatum (caudal and dorsolateral putamen and dorsolateral rim of caudate), and limbic striatum (ventral caudate and putamen), each of which receives input from different functionally related cortical areas (Selemon and Goldman-Rakic, 1985; Parent and Hazrati, 1995; Haber, 2003; Postuma and Dagher, 2006). In particular, associative striatum receives input from areas such as DLPFC and VLPFC that are involved in executive and other cognitive functions, sensorimotor striatum receives input from motor areas that are involved in execution and planning of motor actions, and limbic striatum receives input from areas such as orbital and medial prefrontal cortex that are involved in goal-directed behaviors and emotional processing. Based on these studies, it is hypothesized that the DLPFC and VLPFC project mostly to the associative striatum, and that these neural circuits are heavily involved in executive function.
Most structural studies that have focused on frontostriatal pathways are based on anatomical labeling and tracing studies performed in animal models, which only recently have begun to be confirmed in humans in vivo using diffusion tensor imaging (DTI) techniques (Lehericy et al., 2004a, 2004b). DTI is an imaging technique that can detect microstructural changes in white matter based on properties of water diffusion (Basser et al., 1994). With this technique, variables describing the anisotropy of diffusion such as fractional anisotropy (FA), mean diffusivity (MD), trace, axial diffusivity (AD) and radial diffusivity (RD), can be used as measures of white matter microstructure in vivo (Basser and Jones, 2002; Whitford et al., 2010). Such measures go beyond simple gray and white matter volumetric measures by providing information about the directionality of fiber tracts (Whitford et al., 2010).
Up to now, only a few studies have used DTI techniques to examine microstructural changes of the frontostriatal pathway in brain diseases (Casey et al., 2007; Haas et al., 2009; Liston et al., 2011). These studies report reduced structural connectivity, in terms of FA, in right frontostriatal projections in attention deficit hyperactivity disorder (ADHD) (Casey et al., 2007; Liston et al., 2011), and increased density of left ventral frontostriatal tracts in fragile X syndrome (Haas et al., 2009). There are currently no studies that have characterized frontostriatal pathways in FESZ. In order to understand further the role of frontostriatal pathways in schizophrenia, DTI is the technique of choice to quantify tracts connecting frontal and striatal regions.
A few methods to date have been used to analyze DTI white matter, including: (1) region of interest (ROI) analysis (see review in Kubicki et al., 2007), (2) voxel-based morphometry (VBM) (Prez-Iglesias et al., 2010; Radua et al., 2011), and (3) tract-based analysis (Maddah et al., 2008). As compared with ROI analysis, VBM and whole brain tract-based analysis are more objective because they do not assume prior knowledge regarding the existence of abnormalities. In addition, compared with VBM, tract-based analysis examines all voxels jointly instead of each voxel separately (Rathi et al., 2011). Accordingly, tract-based analysis is the most suitable method for obtaining objective and biologically meaningful information.
Several fiber tracking algorithms are available to delineate white matter tracts automatically, such as streamline and stochastic tractography. The advantage of streamline is that it requires much less time and computer power than stochastic tractography. However, it is limited by a single tensor model, which does not take into account complex fiber configurations such as crossings and branchings (Tuch et al., 2002; Kubicki et al., 2011). An unscented Kalman filter (UKF) based two-tensor whole brain tractography algorithm created in our lab (Malcolm et al., 2010a, 2010b), can solve the fiber crossing problem (Rathi et al., 2011), and is thus optimal for studying complex connections such as the frontostriatal tracts.
In addition to white matter tracts, in order to understand fully structural changes of the frontostriatal pathway, gray matter ROIs that the tracts connect cannot be ignored. Accordingly, ROIs from structural imaging can be used to represent the anatomical locations of DLPFC and VLPFC, which are terms from functional imaging studies. FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) is able to parcellate the human brain into standard gyral-based neuroanatomical regions, and the automated procedure is both anatomically valid and highly reliable (Desikan et al., 2006). Previous studies have suggested that the FreeSurfer parcellation called rostral middle frontal gyrus (rMFG) is most likely to represent the DLPFC (Kikinis et al., 2010), and inferior frontal gyrus (IFG), which consists of pars opercularis, pars triangularis and pars orbitalis parcellations in FreeSurfer (Desikan et al., 2006), is most likely to represent the VLPFC (Rygula et al., 2010). The striatum consists of the combined FreeSurfer parcellations of caudate, putamen and nucleus accumbens. Based on these studies, FreeSurfer parcellations can be used to obtain gray matter ROIs to represent DLPFC, VLPFC and striatum, respectively.
In this study, structural changes in DLPFC/VLPFC-striatum tracts in FESZ were examined using FreeSurfer generated parcellations as ROIs and a two-tensor tractography algorithm. We hypothesized that there would be abnormal diffusion indices in white matter pathways connecting DLPFC and VLPFC with the striatum in FESZ. In addition, we hypothesized that psychopathology and executive dysfunction in FESZ subjects would correlate with abnormalities in diffusion indices.
2. Methods
2.1. Subjects
Sixteen first episode schizophrenia patients (FESZ) were recruited by referrals from clinicians or through local hospitals. Eighteen healthy control subjects (HC) were recruited through newspaper and website advertisements. All participant recruitment was done as part of the Boston CIDAR Center (www.bostoncidar.org). DSM-IV diagnoses were based on interviews with the Structured Clinical Interview for DSM-IV-TR (SCID), Research Version (First et al., 2002) and information from patient medical records. All FESZ participants met DSM-IV-TR criteria for either schizophrenia, schizoaffective disorder or schizophreniform disorder. Controls were drawn from the same geographic base as the FESZ group with comparable age, gender, race and ethnicity, and parental socioeconomic status (PSES). No controls met criteria for any current major DSM-IV-TR Axis I disorders, or any history of psychosis, Major Depression (recurrent), Bipolar disorder, Obsessive Compulsive Disorder, Post Traumatic Stress Disorder, or developmental disorders. Controls were also excluded for any history of psychiatric hospitalizations, prodromal symptoms, schizotypal or other Cluster A personality disorders, first degree relatives with psychosis, or any current or past use of antipsychotics (other past psychotropic medication use was acceptable, but the subjects must have been off medicine for at least 6 months before participating in the study, except for prn medications like sleeping medications or anxiolytic agents, such as beta-blockers for performance anxiety, tremors, etc.). Exclusion criteria for all subjects were: sensory-motor handicaps, neurological disorders, medical illnesses that significantly impair neurocognitive function, diagnosis of mental retardation, education less than 5th grade if under 18 or less than 9th grade if 18 or above, not fluent in English, DSM-IV substance abuse in the past month, DSM-IV substance dependence, excluding nicotine, in the past 3 months, current suicidality, no history of ECT within the past five years for patients and no history of ECT ever for controls, or study participation by another family member.
Table 1 provides the demographic and clinical details for all subjects. All participants were right-handed. Premorbid intellectual abilities were estimated using the reading subtest from the Wide Range Achievements Test-4 (WRAT-4) (Wilkinson and Robertson, 2006). PSES was evaluated using the Hollingshead (Hollingshead, 1975) two-factor index. All patients were scanned at Brigham and Women's Hospital. The study was approved by the local IRB committees at Harvard Medical School, Beth Israel Deaconess Medical Center, Massachusetts General Hospital, Brigham and Women's Hospital and at the Veteran Affairs Boston Healthcare System, Brockton campus. All study participants (or legal guardians for those under 18) gave written informed consent prior to study participation, and subjects received payment for participation.
Table 1. Demographic and cognitive information for first-episode schizophrenia patients (FESZ) and healthy control subjects (HC).
| FESZ (n=16) | HC (n=18) | Independent sample t-test | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean±SD | Mean±SD | df | t | p | |||
| Age at scan(years) | 21.13±4.69 | 22.83±3.48 | 32 | 1.22 | 0.23 | ||
|
| |||||||
| Gender (Male/Female) | 12/4 | 12/6 | - | ||||
|
| |||||||
| Education(years) | 13.07±2.89 | 13.94±1.59 | 31 | 1.11 | 0.28 | ||
|
| |||||||
| PSESa | 2.44±1.50 | 1.89±0.76 | 32 | -1.37 | 0.20 | ||
|
| |||||||
| Estimated Premorbid Intellectb | 113.88±18.61 | 110.38±19.92 | 22 | -0.43 | 0.67 | ||
|
| |||||||
| Ethnicity (H/N/U)c | 3/13/0 | 1/15/2 | - | ||||
|
| |||||||
| Race | American Indian or Alaskan Native | ||||||
|
|
|||||||
| Asian East Southwest | 1 | ||||||
|
|
|||||||
| Asian, Western | 1 | ||||||
|
|
|||||||
| Native Hawaiian or Other Pacific Islander | |||||||
|
|
|||||||
| Black or African American | 4 | ||||||
|
|
|||||||
| White | 12 | 12 | |||||
|
|
|||||||
| Multi-Racial | |||||||
|
|
|||||||
| Unknown | 1 | 2 | |||||
|
|
|||||||
| Other | 1 | ||||||
|
|
|||||||
| Medication duration (months)d (n=10) | 5.19±6.51 | ||||||
|
| |||||||
| Medication dose (mg) e (n=10) | 349.58±368.15 | ||||||
|
| |||||||
| WCSTf | categories completed | 3.23±1.69 | 5.14±1.35 | 25 | 3.26 | 0.003 | |
|
|
|||||||
| (13FESZ; 14HC) | perseverative responses | 8.08±6.12 | 6.57±2.65 | 25 | -0.84 | 0.408 | |
|
| |||||||
| BPRSg | (n=15) thinking disturbance | 6.33±2.87 | - | - | - | - | |
|
|
|||||||
| hostile-suspiciousness | 5.20±2.31 | - | - | - | - | ||
|
|
|||||||
| withdrawal/retardation | 5.93±2.91 | - | - | - | - | ||
|
|
|||||||
| anxious-depression | 7.60±3.22 | - | - | - | - | ||
|
|
|||||||
| total | 42.20±12.43 | - | - | - | - | ||
PSES: parental socioeconomic status
Premorbid intellect was estimated with the reading subtest from the WRAT-4
Ethnicity: H=Hispanic or Latino; N=Not Hispanic or Latino; U=Unknown
Antipsychotic medication duration of all patients
Antipsychotic medication dose of all patients equivalent to chloropromazine at the time of scan.
WCST: the Wisconsin Card Sorting Test
BPRS: the Brief Psychiatric Rating Scale
2.2. Image acquisition
Diffusion weighted images (DWI) were acquired on a 3 Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI) using an echo planar imaging (EPI) DWI sequence. The following scan parameters were used: TR 17000 ms, TE 78 ms, FOV 24 cm, 144×144 matrix, 1.7 mm slice thickness. A double echo option was used to reduce eddy-current related distortions. To reduce impact of EPI spatial distortions, an 8 Channel coil was used that allowed parallel imaging, using ASSET (Array Spatial Sensitivity Encoding Techniques, GE), with a Sensitivity encoding (SENSE)-factor (speed-up) of 2. Acquisitions used 51 gradient directions with b=900s/mm2 and 8 baseline scans with b=0. All scans had 85 axial slices parallel to the anterior commissure to posterior commissure (AC–PC) line and covered the whole brain. The original GE sequence was modified to increase spatial resolution and to minimize further image artifacts. The data was pre-processed in order to reduce noise effects (Aja-Fernandez et al., 2008). Also, an affine registration of the DWI to the baseline image, using FSL's linear image registration tool (FLIRT) was employed in order to remove artifacts due to eddy currents and head motion (Rathi et al., 2011). Diffusion images were estimated from the DWI using a Least-Squares method.
2.3. ROI acquisition
The FreeSurfer software package (version 4.0.2) was used to parcellate T1-weighted Spoiled Gradient Echo (SPGR) images into cortical and subcortical gray and white matter regions for each subject (Fischl et al., 2002). A T2 image of the same subject in the same coordinate space as the SPGR image was then registered to the baseline DWI using the FSL's nonlinear image registration tool (FNIRT) algorithm in the FSL software (Smith et al., 2004). This diffeomorphism was applied to the label map obtained from T1 segmentation to obtain a label map in the coordinate space of the diffusion images. Thus, a correspondence was obtained across all the subjects by representing each ROI with the same label. We used combined parcellations of caudate, putamen, and nucleus accumbens for the striatum gray matter ROI. We used pars opercularis, pars triangularis and pars orbitalis for the IFG gray matter ROI, which likely represents VLPFC, and we used rMFG as the third separate gray matter ROI, which likely represents DLPFC, based on previous studies (Desikan et al., 2006; Kikinis et al., 2010). Then the volume of each ROI for each subject was calculated from the FreeSurfer software automatically.
2.4. Two-tensor tractography
The UKF based two-tensor tractography algorithm was used to trace fiber paths throughout the brain (Malcolm et al., 2010a). Seeding was done in all the voxels where single tensor FA was greater than 0.18. Each voxel was randomly seeded 10 times and each fiber tract was traced from seed to termination, with the termination criteria FA<0.15 for the primary tensor component, which is most consistent with the tracking direction. In order to ensure that the connections were distinct and that the fiber tracts did not overlap, only those fibers that had their end points on the surface of the desired ROIs were retained. Fiber extraction was done for each subject for further analysis. This two-tensor tractography method has been processed in this sample previously using a whole brain analysis (Rathi et al., 2011). In comparison with other tractography methods, this method greatly improves the angular resolution at fiber crossings and branching and thus allows for recovering tracts that pass through such regions of the brain (Malcolm et al., 2010a). Specifically, two- tensor tractography was initially applied in order to obtain fibers of the whole brain. The resulting whole brain tractography, in turn, allows for the extraction of specific fiber tracts that connect any specific ROIs of choice. In this study, we separately extracted fibers connecting 2 distinct frontal ROIs, the rMFG and the IFG, with the striatum.
In the neuroimaging research field, several diffusion indices have been used, namely FA, that could capture the 3 dimensional character of the diffusion tensor (Basser and Jones, 2002). In our study, four indices were computed at each point along the fiber tract for all selected fiber bundles. These indices included FA, the asphericity of diffusion, purported to measure white matter integrity (Basser and Jones, 2002; Kubicki et al., 2007), trace, the sum of the 3 directional diffusion, purported to measure white matter microstructures (Lee et al., 2009; Oh et al., 2009), and AD and RD, the principal and perpendicular direction of the diffusion ellipsoid, purported to measure axonal and myelin integrity respectively (Song et al., 2003; Whitford et al., 2010).
2.5. Neuropsychological and clinical measures
Executive function was measured using the computerized Wisconsin Card Sorting Test (WCST) (Heaton, 1981; Nestor et al., 1993), which was administered by a research assistant supervised by a trained psychologist. The WCST is one well established measure of executive function and we chose in this initial study to reduce the numbers of executive function variables to minimize Type I error, and we have studied its relationship to brain structure in chronic schizophrenia previously (Seidman et al., 1994). In this study, we used the WCST indices “categories completed” and “perseverative responses”. Clinical symptoms were measured using the Brief Psychiatric Rating Scale (BPRS) (Overall and Klett, 1972). We calculated BPRS total score and the four individual factors including “thinking disturbance”, “hostile-suspiciousness”, “withdrawal/retardation” and “anxious-depression”.
2.6. Statistical Analysis
Demographic data between FESZ and HC were compared using independent sample t-tests. To compare the differences for ROI volumes, a three-factor repeated measures ANOVA was applied, with group as a ‘between-subjects’ factor, and side (left, right) and ROI (rMFG, IFG, striatum) as the two ‘within-subjects’ factors. Group differences for the diffusion indices were compared using three-factor repeated measures ANOVA, with group as ‘between-subjects’ factor, side (left, right) and tract (rMFG-striatum, IFG-striatum) as the two ‘within-subjects’ factors Post hoc two-factor ANOVA was applied for each ROI and tract separately, with group as “between-subjects” factor, and side as “within-subjects” factor. If the two-factor repeated measures ANOVA identified an overall between-group difference, or group by side interaction, in a ROI volume or diffusion index, then independent sample t-tests were applied with the significance level set at 0.05.
After group comparison, Spearman's rho correlations were conducted, so as not to be unduly influenced by outliers, in FESZ and HC groups, separately, to investigate the relationship between “categories completed” and “perseverative responses” from WCST and our measures of white matter integrity. Correlations were only performed with diffusion measures that demonstrated significant group differences. Spearman's rho correlations were also conducted in the FESZ group to investigate the relationship between DTI measures and symptom severity of each of the BPRS four factor scores, and of the total score.
3. Results
3.1. Representative ROIs and tracts
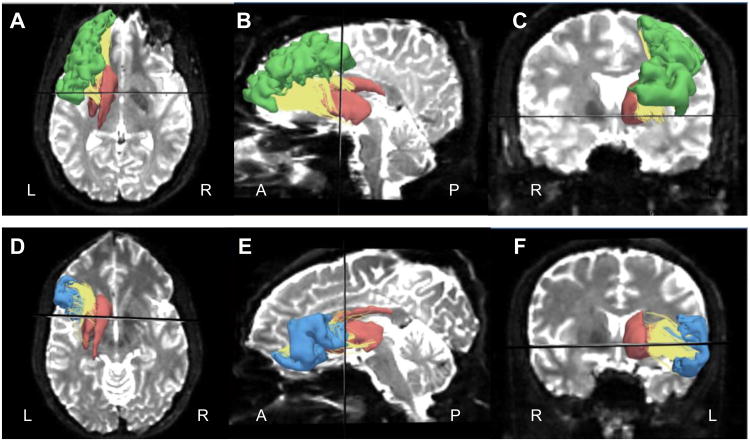
Figure 1 shows the representative ROIs: rMFG, IFG, striatum as well as the connecting tracts between those ROIs, such as rMFG-striatum and IFG-striatum of one subject on the left hemisphere. In order to exclude the confounding differences caused by the size of ROIs from which the tractography was seeded, the ROI volumes were analyzed by three-factor repeated measures ANOVA. No significant overall between-group differences (F(1,32)=0.063, p=0.804), group by ROI interactions (F(2,31)=0.491, p=0.617), nor group by ROI by side interactions (F(2,31)=1.395, p=0.263) were detected. Post hoc two-factor ANOVA for each ROI volume did not show group differences (for rMFG, F(1,32)=0.013, p=0.910; for IFG, F(1,32)=0.482, p=0.493; for striatum, F(1,32)=0.103, p=0.750). Careful review of the tracts revealed that, in all subjects, most of the tracts ended in the precommissural striatum, which was defined as being localized anterior to the coronal slice that contains the anterior commissure (AC). This observation is represented in the axial slice seen in Figure 1A and 1D. The midline sagittal slice showed that a small part of the rMFG-striatum tracts ended in the lateral postcommissural caudate (Figure 1B), while a small part of the IFG-striatum tracts ended in the lateral postcommissural putamen (Figure 1E). The AC localized coronal slice showed that most of rMFG-striatum and IFG-striatum tracts ended in the dorsal striatum that was above the AC-PC plane (Figure 1C and 1F), while a small part of the IFG-striatum tracts ended in the ventral striatum (Figure 1F).
Figure 1.
Tracts (yellow) and ROIs including rMFG (green), IFG (blue) and striatum (red) overlaid on a diffusion image. A-C show rMFG-striatum tracts and related ROIs in axial, sagittal and coronal slices respectively. D-F show IFG-striatum tracts and related ROIs in axial, sagittal and coronal slices respectively. The black line represents a plane, which includes the anterior commissure. L: left, R: right, A: anterior, P: posterior.
3.2. Group differences of diffusion indices
In order to test whether or not the diffusion indices differed between groups, a three-factor repeated measures ANOVA was performed. The results showed that there were significant overall group differences for FA (F(1,32)=6.612, p=0.015), trace (F(1,32)=7.951, p=0.008), and RD (F(1,32)=8.746, p=0.006), but not for AD (F(1,32)=2.844, p=0.101). Furthermore, a post hoc two-factor ANOVA for the rMFG-striatum tract showed a significant group difference for trace (F(1,32)=7.944, p=0.008) and RD (F(1,32)=8.736, p=0.006) and approached a significant group difference for FA (F(1,32)=3.793, p=0.060). A post hoc two-factor ANOVA for the IFG-striatum tract similarly showed a significant group difference for trace (F(1,32)=13.892, p=0.001) and RD (F(1,32)=14.579, p=0.001) and, again, approached a significant group difference for FA (F(1,32)=3.499, p=0.071).
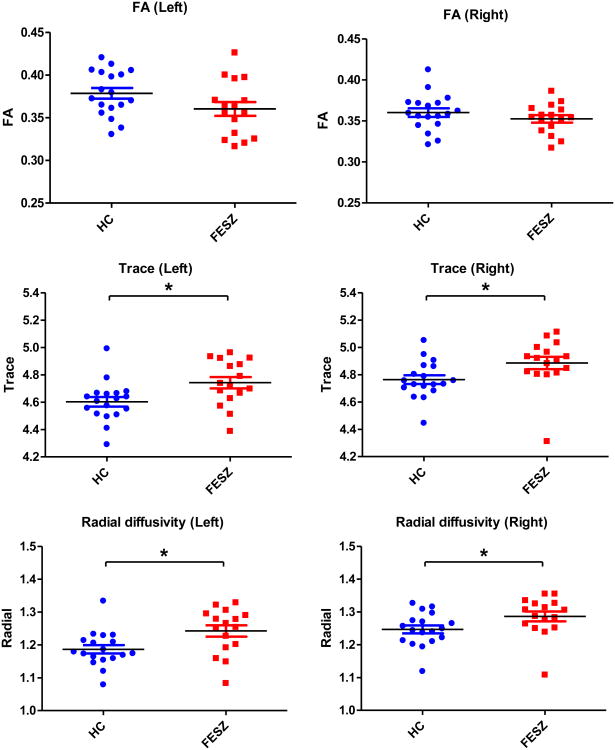
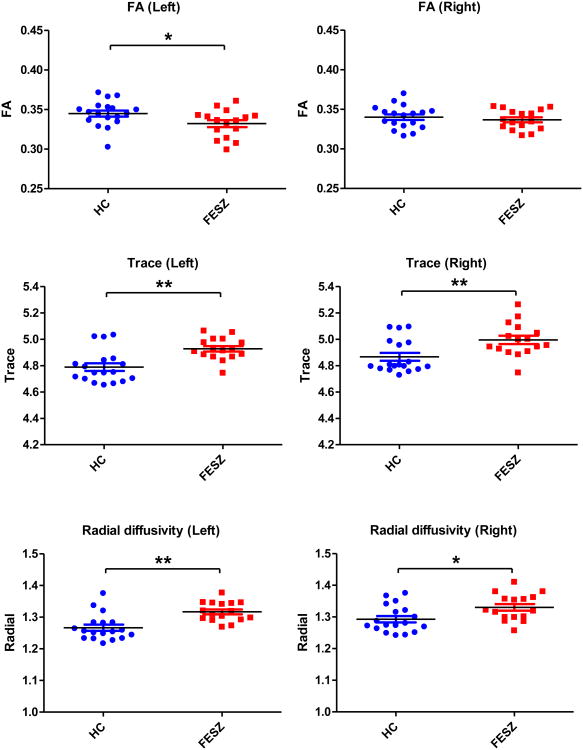
Figures 2 and 3 show the follow-up independent sample t-tests results of FA, trace and RD for the individual tracts. For the rMFG-striatum tracts, there were significant trace increases bilaterally (left: t(32)=-2.586, p=0.014; right: t(32)=-2.243, p=0.032) and significant RD increases bilaterally (left: t(32)=-2.649, p=0.012; right: t(32)=-2.087, p=0.045) in FESZ (Figure 2). For the IFG-striatum tracts, there were significant FA reductions in the left side (t(32)=2.199, p=0.035), significant trace increases bilaterally (left: t(32)=-3.781, p=0.001; right: t(32)=-2.965, p=0.006) and significant RD increases bilaterally (left: t(32)=-3.974, p<0.001; right: t(32)=-2.566, p=0.015) (Figure 3).
Figure 2.
Group differences of diffusion indices of the rMFG-striatum tracts. The black bars represent mean values. The blue and red bars represent mean±sem. The asterisks represent significant group differences, * 0.01<p<0.05. HC: healthy controls, and FESZ: first-pisode schizophrenia patients. Trace and radial diffusivity are expressed in 10-3 mm2/sec.
Figure 3.
Group differences of diffusion indices of the IFG-striatum tracts. The black bars represent mean values. The blue and red bars represent mean±sem. The asterisks represent significant group differences, * 0.01<p<0.05, ** p<0.01. HC: healthy controls, and FESZ: first-episode schizophrenia patients. Trace and radial diffusivity are expressed in 10-3 mm2/sec.
3.3. Cognitive and clinical correlations of diffusion indices in FESZ group
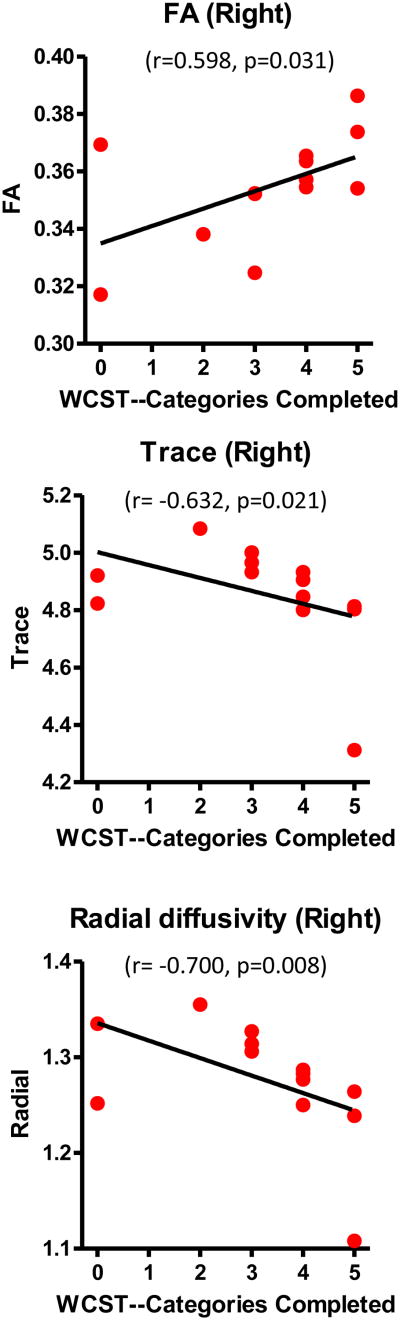
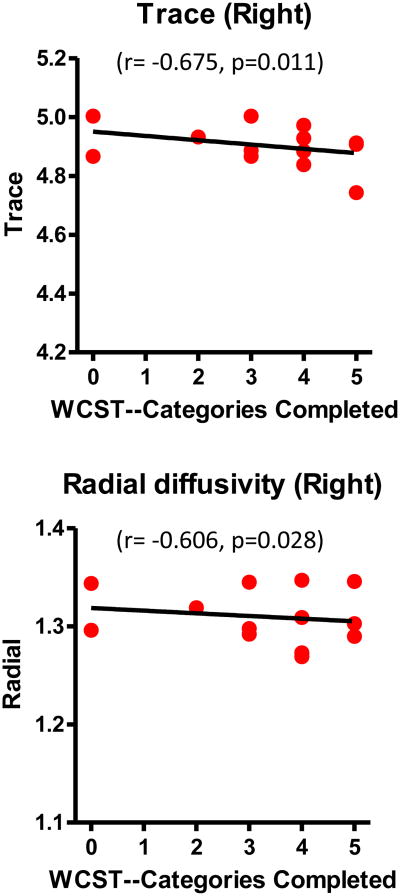
In FESZ, Spearman's rho correlations showed that the “categories completed” score in the WCST was significantly positively correlated with FA (r=0.598, n=13, p=0.031), and significantly negatively correlated with trace (r=-0.632, n=13, p=0.021) and RD (r=-0.700, n=13, p=0.008) in the right rMFG-striatum tract (Figure 4). It was also significantly negatively correlated with trace (r=-0.675, n=13, p=0.011) and RD (r=-0.606, n=13, p=0.028) in the right IFG-striatum tract (Figure 5). There were no significant correlations between “perseverative responses” and DTI measures in FESZ. No significant WCST correlations were found in the HC group. Moreover, the BPRS total score and four individual factor scores in the FESZ group did not show significant correlations with any of the diffusion indices.
Figure 4.
Correlations between diffusion indices of rMFG-striatum tracts and WCST scores in first-episode schizophrenia patients. The black line represents the best-linear-fit for the data. Although Spearman correlations were used for testing statistical significance, regression lines have been plotted for the convenience of the reader. Trace and radial diffusivity are expressed in 10-3 mm2/sec.
Figure 5.
Correlations between diffusion indices of IFG-striatum tracts and WCST scores in first-episode schizophrenia patients. The black line represents the best-linear-fit for the data. Although Spearman correlations were used for testing statistical significance, regression lines have been plotted for the convenience of the reader. Trace and radial diffusivity are expressed in 10-3 mm2/sec.
4. Discussion
While most imaging studies have focused on individual regions of the frontostriatal pathway, DTI was employed in this study to investigate tracts that comprise the frontostriatal pathway. To our knowledge, this is the first study using DTI techniques to examine white matter microstructural changes of the frontostriatal pathway in FESZ. Overall, we showed disruption of white matter in the rMFG-striatum and IFG-striatum tracts. Specifically, we showed a reduction of FA in the left hemisphere of the IFG-striatum tract together with bilateral increases of trace and RD in both tracts in FESZ. Interestingly, there were no group differences in gray matter volumes using Freesurfer, of rMFG, IFG, or striatum. We further showed that the diffusion indices in the right but not the left rMFG-striatum and IFG-striatum tracts were correlated with categories completed in the WCST, but not with BPRS scores. These results indicate that there are white matter structural changes in the frontostriatal pathway in FESZ, which are correlated with executive dysfunction, but not with psychiatric symptoms.
Visual inspection of rMFG/IFG and striatum tracts confirms the hypothesis that most of the tracts project to the associative striatum, namely the head of the caudate and rostral putamen. This is in agreement with other anatomic studies that have been performed primarily in animals (Selemon and Goldman-Rakic, 1985; Parent and Hazrati, 1995; Haber, 2003; Postuma and Dagher, 2006). Of note, Kegeles et al. (2010) recently used structural magnetic resonance imaging (MRI) in human subjects to divide the striatum into 5 ROIs, including the ventral striatum, the precommissural dorsal caudate and putamen, and the postcommissural caudate and putamen. They classified the precommissural dorsal caudate and putamen, and postcommissural caudate as associative striatum, and they reported increased synaptic dopamine function in schizophrenia using positron emission tomography (PET). Our finding is anatomically consistent with their definition, which also indicates that the dopamine activity in the associative striatum might be related to the white matter integrity of the rMFG/IFG-striatum pathway in FESZ.
FA is an index of the asphericity of diffusion. It is high in areas of high structural coherence, such as white matter, lower in gray matter, and close to zero in cerebrospinal fluid. Thus, FA reduction in white matter, though a non-specific measure, is generally thought to reflect a reduction in white matter integrity (Kanaan et al., 2005; Kubicki et al., 2007). MD is the mean extent of the 3 directional diffusion, which measures the average diffusion of water molecules within tissues, whereas trace is the sum of the 3 directional diffusion. Thus an increase in trace or MD in white matter generally indicates a disruption of white matter microstructures (Lee et al., 2009; Oh et al., 2009). Although it is not possible to determine with certainty the structural meaning of diffusion measures, our results of reduced FA and increased trace in FESZ patients are consistent with the loss of axon fibers, myelin pathology with an associated loss of fiber coherence, decrease in packing density, or an increase in extracellular fluid (Peters et al., 2010; Levitt et al., 2012). Furthermore, our finding of increased RD but no change in AD may point to a deficit in myelin integrity without axonal damage to frontostriatal pathways in FESZ as AD, which is the principal direction of the diffusion ellipsoid, has been shown to assess axonal integrity while RD, which is the perpendicular direction, has been shown to assess myelin integrity (Song et al., 2003; Whitford et al., 2010). These findings also concur with previous neuropathologic investigations in schizophrenia that have reported reduced density and numbers of oligodendrocytes, the myelinating cells, in white matter in schizophrenia (Uranova et al., 2004; Tkachev et al., 2007). Furthermore, prominent dysregulation of myelin-associated gene expression and marked abnormalities in the ultrastructure of myelin sheaths have been identified in schizophrenia (e.g., Haroutunian and Davis, 2007).
Besides the structural analysis of this frontostriatal pathway, we further investigated correlations between white matter and executive function and symptom severity. Our results showed a correlation between white matter integrity of the rMFG/IFG-striatum pathway and executive function measured by the WCST in FESZ, but not in the HC group, and no significant correlations with symptom severity as measured by BPRS. These findings suggest that the frontostriatal pathway plays an important role in executive dysfunction. The disrupted white matter integrity, especially myelin integrity, was related to worse executive function, and this correlation was seen only in the right frontostriatal tracts. Previous studies have reported that prefrontal cortex (Seidman et al., 1994; Manoach et al., 2000; Weinberger et al., 2001; Boettiger and D'Esposito, 2005) and striatal dopamine activity (Bach et al., 2008; Kegeles et al., 2010; Simpson et al., 2010) mediate executive function, which are abnormal in schizophrenia. Our study is consistent with these studies, and provides evidence that executive function is not only related to the activity of individual frontal or striatal regions, but is also related to the networks or pathways that connect these regions. Our data support that the associative striatum is an important location where convergent abnormal inputs may adversely affect it in schizophnrenia. In schizophrenia, Kegeles et al (2010), using PET have shown that there is an increase in dopamine neurotranmission/activity in the striatum, but in particular in the associative striatum. In our paper, we showed white matter abnormalities in FESZ in frontostriatal pathways connecting the DLPFC and VLPFC to the striatum which on visual inspection predominantly targeted precommisural putamen and caudate, which represent a good portion of the associative striatum (Kegeles et al., 2010). Thus, we believe these two findings are convergent. That is, schizophrenia patients appear both to have increased dopamine activity in the associative striatum and to receive abnormal white matter input from the cortex to the associative striatum.
Our structural findings also support a functional imaging study that has shown reduced activity in the prefrontal cortex in schizophrenia is associated with increased presynaptic striatal dopaminergic function (Meyer-Lindenberg et al., 2002). Another functional imaging study in subjects at clinical high risk for schizophrenia (Fusar-Poli et al., 2011) has also found significant correlations between prefrontal activity and striatal dopamine function. The results of the two studies were in different prefrontal subregions and different sides, which is likely due to the different cognitive function tasks that were used and to differences in subject samples. For example, the first study (Meyer-Lindenberg et al., 2002) used the WCST task in chronic schizophrenia patients, and showed significant negative correlations of striatal dopamine level with right DLPFC (relevant to the rMFG in our study), while the second study (Fusar-Poli et al., 2011) used a verbal fluency task in persons at clinical high risk for psychosis, and showed positive correlations of striatal dopamine level with left IFG. The precise localization of executive function within the prefrontal cortex has proven to be controversial (Duncan and Owen, 2000). As explained in the second study, both reduced activation of DLPFC and a compensatory activation of the VLPFC contribute to the core functional abnormalities underlying neurocognitive impairments in schizophrenia (Tan et al., 2007). Our study supports the above hypothesis and indicates that the white matter integrity of both DLPFC-striatum and VLPFC-striatum tracts are involved in executive function in FESZ. However, the former tracts might be more associated with executive function while the latter tracts, VLPFC-striatum tracts, do not show significant correlations between FA and any index of the WCST.
To underscore the importance of white matter in contrast to gray matter, we also measured gray matter volumes of these frontal regions and striatum. That no volume change was found in our study may indicate that change in white matter integrity could occur in the absence of gray matter pathology. Previous studies have not reached an agreement regarding the presence of gray matter volume changes in rMFG, IFG and striatum in FESZ (see reviews in Ellison-Wright et al., 2008; Levitt et al., 2010), which probably is due to different ways of obtaining ROIs and analyzing group differences. Ellison-Wright et al. (2008) reviewed studies that used VBM analysis for volume comparisons and found in FESZ volume reduction in bilateral IFG, while no volume difference in MFG or striatum. Levitt et al. (2010) reviewed studies that used ROI manual tracing methodology for volume comparisons and found that in chronic schizophrenia 25% of studies reported volume reduction in MFG and 66.7% of studies reported volume reduction in IFG, while no studies compared volume change in MFG or IFG in FESZ. Meanwhile, 100% of 3 studies reported no volume change in the caudate in FESZ. Our study, which used FreeSurfer, an automatic method to obtain ROIs, found no volume change for any of the gray matter ROIs. Additional studies are clearly needed in order further to explore the issue of gray matter volume changes in FESZ.
Potential limitations of the paper include the following. First, although the patients in our study were FESZ, some of them had already received medication at the time of scanning, which could potentially influence structural changes. Second, we compared the tracts connecting prefrontal regions with the whole striatum, instead of looking at the tracts projecting to specific subregions of the striatum such as precommissural and postcommissural caudate and putamen. Thus, our finding of topographic specificity, though consistent with animal studies, remains non-quantitative. Third, we used automatic methods to obtain gray matter ROIs, which is still limited in accuracy as compared with manual tracing. Fourth, although two-tensor tractography is advantageous in extracting crossing fibers, some of the fibers might be false positive results. Fifth, our cognitive correlations should be considered preliminary and exploratory as we selected only one measure of cognitive function and multiple comparisons were performed, raising the possibility of a Type 1 error. We note, however, that the findings were in the predicted directions. Moreover, despite the fact that the number of computed correlations increased the risk of a Type I error, there are fewer studies investigating the brain behavior correlations for white matter than gray matter in schizophrenia and, thus, we believe it remains helpful to search for possible useful clinical correlations which subsequent studies can then either replicate or not. Sixth, the absence of our finding correlations with symptoms should be considered preliminary, since more comprehensive symptom scales such as PANSS and/or SAPS/SANS were not used in this study. Additionally, we note that follow-up 2-factor ANOVAs when we analyzed the rMFG-striatum tract and the IFG-striatum tracts, separately, were confirmatory of our 3-factor repeated measures ANOVA analyses for both trace and RA and largely confirmatory of our FA measures with a main effect for group in both tracts approaching significance. We, thus, believe, follow-up t-test performed comparing groups on all 3 measures are justified. Finally, the sample size was small and thus we were unable to test the potential effect of neuroleptic medications on our findings. Therefore, further studies are needed, with larger samples, to explore both medicated and unmedicated subjects, to focus on subregions of the striatum, and to improve automatic methods for assessing ROIs, in order to increase our understanding of structural changes in frontostriatal pathways in schizophrenia.
In conclusion, we found disrupted white matter integrity, especially myelin integrity, in the tracts connecting striatum with rMFG and IFG in FESZ, and these disruptions were associated with one measure of impaired executive function. This indicates that abnormalities in the structure and/or function of the frontostriatal networks, instead of the frontal and striatal regions alone, are the pathophysiological features of schizophrenia, which will shed light on the etiology and early diagnosis of schizophrenia. Future studies need to focus on the frontostriatal pathway in different stages of the development of illness, as well as to investigate different factors that affect this pathway, such as dopamine activity related genes and proteins, namely COMT (Tunbridge et al., 2006), GABA and glutamate function (Carlsson et al., 2001; Meisenzahl et al., 2007), and other brain regions that are closely related to this pathway, such as the thalamus (Csernansky and Cronenwett, 2008; Hoffman et al., 2011). Furthermore, future studies also should examine specific tract connections between the cortex and the striatum such as tracts projecting from multiple cortical ROIs to specific functional subregions of the striatum.
Acknowledgments
This study was supported, in part, by grants from P50 MH 080272 (RWM, MES, LJS, RMG), RO1 MH 50747 (MES), VA MERITs (JJL, MES, RWM), RO1 MH 40799 (RWM), the Commonwealth Research Center of the Massachusetts Department of Mental Health, SCDMH82101008006 (LJS, RMG), and NARSAD Young Investigator Award (ZK). This study was also supported by Clinical Translational Science Award UL1RR025758 and General Clinical Research Center Grant M01RR01032 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We thank Psychiatry Neuroimaging Laboratory Software Engineer, Ryan Eckbo, M.Sc., Research Assistants, including Padmapriya Srinivasan, M.S., Kathryn Hawley, B.A., Mai-Anh Vu, B.A., Thomas Ballinger, B.A., Tali Swisher, B.A. and Paula Pelavin, B.A., for their help and support. We also thank the clinical and data management staff from the Boston CIDAR study, including: Matcheri Keshavan, M.D., Joanne Wojcik, Ph.D, APRN, Ann Cousins, Ph.D., APRN, Michelle Friedman-Yakoobian, Ph.D., Kristen Woodberry, Ph.D., Corin Pilo, LMHC, Andréa Gnong Granato, MSW, Lauren Gibson, Ed.M., Sarah Hornbach, B.A., Julia Schutt, B.A., Kristy Klein, Ph.D., Maria Hiraldo, Ph.D., Grace Francis, Ph.D., Rachael Serur, B.S., Grace Min, Ed.M., Alison Thomas, B.A., and Molly Franz, B.A..
Role of funding source: The funding source had no role in study design; the collection, analysis nor interpretation of data; writing of the paper; nor in the decision for publication.
Footnotes
Conflict of interest: All authors declare that they have no competing financial interests.
Contributors: Meina Quan, Sang-Hyuk Lee, Marek Kubicki, Martha E. Shenton and James J. Levitt designed the study and wrote the protocol. Meina Quan also wrote the first draft of the manuscript. Zora Kikinis advised on the neuroanatomy of frontal regions. Yogesh Rathi advised on the tractography method. Larry J. Seidman, Raquelle I. Mesholam-Gately, Jill M. Goldstein, Robert W. McCarley and Martha E. Shenton managed the recruitment and collected clinical information of participants. Martha E. Shenton and James J. Levitt supervised the statistical analyses and edited multiple iterations of the manuscript. All authors contributed to and have improved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aja-Fernandez S, Niethammer M, Kubicki M, Shenton M, Westin C. Restoration of DWI data using a Rician LMMSE estimator. IEEE Trans Med Imaging. 2008;27:1389–1403. doi: 10.1109/TMI.2008.920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci USA. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, D'Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Cronenwett WJ. Neural networks in schizophrenia. Am J Psychiatry. 2008;165:937–939. doi: 10.1176/appi.ajp.2008.08050700. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Montgomery AJ, Grasby PM, McGuire P. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, Piven J, Reiss AL. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Davis KL. Introduction to the Special Section: Myelin and oligodendrocyte abnormalities in schizophrenia. Int J Neuropsychopharmacol. 2007;10:499–502. doi: 10.1017/S1461145706007449. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test Manual. Psychological Assessment Resources; Odessa, FL: 1981. [Google Scholar]
- Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA CATIE Investigators. Neurocognitive Working Group Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Fallon JH, Niznikiewicz M, Nestor P, Davidson C, Bobrow L, Pelavin PE, Fischl B, Yendiki A, McCarley RW, Kikinis R, Kubicki M, Shenton ME. Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res. 2010;123:153–159. doi: 10.1016/j.schres.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Alvarado JL, Westin CF, Tate DF, Markant D, Terry DP, Whitford TJ, De Siebenthal J, Bouix S, McCarley RW, Kikinis R, Shenton ME. Stochastic tractography study of Inferior Frontal Gyrus anatomical connectivity in schizophrenia. Neuroimage. 2011;55:1657–1664. doi: 10.1016/j.neuroimage.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: insights from Huntington's disease. Trends Cogn Sci. 1998;2:379–388. doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- Lee K, Yoshida T, Kubicki M, Bouix S, Westin CF, Kindlmann G, Niznikiewicz M, Cohen A, McCarley RW, Shenton ME. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr Res. 2009;108:33–40. doi: 10.1016/j.schres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K, Kim DS. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 2004a;14:1302–1309. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004b;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Alvarado JL, Nestor PG, Rosow L, Pelavin PE, McCarley RW, Kubicki M, Shenton ME. Fractional anisotropy and radial diffusivity: Diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr Res. 2012;136:55–62. doi: 10.1016/j.schres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Bobrow L, Lucia D, Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Curr Top Behav Neurosci. 2010;4:243–281. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- Liston C, Cohen MM, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Maddah M, Kubicki M, Wells W, Westin CF, Shenton M, Grimson W. Findings in schizophrenia by tract-oriented DT-MRI analysis. Med Image Comput Comput Assist Interv. 2008;11:917–924. doi: 10.1007/978-3-540-85988-8_109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm JG, Michailovich O, Bouix S, Westin CF, Shenton ME, Rathi Y. A filtered approach to neural tractography using the Watson directional function. Med Image Anal. 2010a;14:58–69. doi: 10.1016/j.media.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm JG, Shenton ME, Rathi Y. Filtered multitensor tractography. IEEE Trans Med Imaging. 2010b;29:1664–1675. doi: 10.1109/TMI.2010.2048121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Schmitt GJ, Scheuerecker J, Moller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19:337–345. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately R, Giuliano AJ, Faraone SV, Goff KP, Seidman LJ. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O'Donnell B, Kimble M, Kikinis R, Jolesz FA. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry. 1993;150:1849–1855. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, Westin CF, Shenton ME. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Klett CJ. Applied multivariate analysis. New York: McGraw-Hill; 1972. [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44:993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Prez-Iglesias R, Tordesillas-Gutirrez D, Barker GJ, McGuire PK, Roiz-Santiañez R, Mata I, de Lucas EM, Quintana F, Vazquez-Barquero JL, Crespo-Facorro B. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage. 2010;49:199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–1550. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- Rathi Y, Kubicki M, Bouix S, Westin CF, Goldstein J, Seidman L, Mesholam-Gately R, McCarley RW, Shenton ME. Statistical analysis of fiber bundles using multi-tensor tractography: application to first-episode schizophrenia. Magn Reson Imaging. 2011;29:507–515. doi: 10.1016/j.mri.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Caspi A, Knobler HY, Lubin G, Harvey PD, Rabinowitz J, Davidson M. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol. 2006;28:193–207. doi: 10.1080/13803390500360372. [DOI] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Yurgelun–Todd D, Kremen WS, Woods BT, Goldstein JM, Faraone SV, Tsuang MT. Relationship of prefrontal and temporal lobe MRI measures and neuropsychological performance in chronic schizophrenia. Biol Psychiatry. 1994;35:235–246. doi: 10.1016/0006-3223(94)91254-8. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17:i171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Huffaker SJ, Ryan M, Bahn S. Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia. Int J Neuropsychopharmacol. 2007;10:557–563. doi: 10.1017/S1461145706007334. [DOI] [PubMed] [Google Scholar]
- Tuch D, Reese T, Wiegella M, Makris N, Belliveau J, Wedeen V. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglia density in the prefrontal cortex in schizophrenia and mood disorder: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;55:597–610. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 professional manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Woodberry K, Giulianom AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]