Abstract
Purpose
To explore the combination of clofarabine, cytarabine, and idarubicin (CIA) in patients with newly diagnosed acute myeloid leukemia (AML) </= 60 years.
Patients and Methods
Patients ≥18-60 years with AML and adequate organ functions were candidates. Induction therapy consisted of clofarabine (C) 22.5 mg/m2 IV daily (days 1-5), idarubicin (I) 6 mg/m2 IV daily (days 1-3), and cytarabine (A) 0.75 g/m2 IV daily (days 1-5). Patients in remission received up to 6 consolidation cycles (C 22.5 mg/m2 × 3, I 6 mg/m2 × 2, and A 0.75 g/m2 × 3).
Results
Fifty-seven patients were evaluable. The overall response rate was 79%. With a median follow up of 10.9 months (range, 1.6 - 23.1), the median overall survival (OS) was not reached, the median event-free survival (EFS) was 13.5 months, and the median relapse free survival was not reached. Most toxicities were ≤ grade 2. Four week mortality was 2%. In subgroup analysis, patients ≤ 40 years had better OS (P = 0.04) and EFS (P = 0.04) compared to patients > 40 years.
Compared to historical patients treated with IA combination, the OS and EFS were significantly higher (P = 0.005, 0.0001, respectively) for CIA treated patients. In multivariate analysis, CIA retained its superior impact on OS and EFS compared to IA.
Conclusion
CIA is an effective combination for patients </= 60 years with newly diagnosed AML. Patients ≤ 40 years had better OS and EFS. CIA achieved longer OS and EFS compared to IA alone.
Keywords: AML, frontline therapy, clofarabine, idarubicin, cytarabine
Introduction
For the last 4 decades, the combination of cytarabine and an anthracycline (“7+3”) has been the standard of care for patients with newly diagnosed acute myeloid leukemia (AML).1-3 Although the overall response rate (ORR) to this combination is 70-80%, only approximately 30 - 40% will live beyond 5 years and most patients will relapse and die from their disease or associated complications.4,5
Numerous modifications of this combination (using different anthracylines, adding a third agent such as cladribine or etoposide, extending the number of days of cytarabine, priming of leukemia blasts with hematopoietic growth factors, addition of modulators of multidrug resistance) failed to improve response rates and overall survival (OS).6-13 On the other hand, evidence has accumulated to suggest that dose intensification of cytarabine during induction and consolidation may result in higher complete remission rate (CR) and superior long term outcome mainly in patients ≤ 60 years old.14-18 Furthermore, higher doses of daunorubicin have also shown to increase response rate and long-term survival in a subset of these patients.5
Clofarabine is a second generation nucleoside analog which has shown significant activity in pediatric and adult acute leukemias.19,20 Although active in single agent trials of patients with AML, combinations of clofarabine with other agents have been more promising.18 A phase I trial of clofarabine in combination with either idarubicine (CI), or both cytarabine and idarubicine (CIA) in patients with primary refractory and first relapse AML has produced CR rates of 13% (3/23) and 48% (10/21) with median remission durations of 4.5 months and 15 months, respectively.21 In this phase I study, the maximum tolerated doses (MTD) for the CIA combination were defined as: clofarabine 22.5 mg/m2 intravenously (IV) daily × 5, idarubicin 6 mg/m2 IV daily × 3, and cytarabine 0.75 g/m2 IV daily × 5 days.21
Based on the suboptimal outcome of standard AML induction therapy and the encouraging activity of clofarabine in the context of the CIA regimen, we designed a phase II trial to investigate the efficacy and the safety of the CIA combination in patients ≤ 60 years with newly diagnosed AML.
Patients and Methods
Study group
Eligible were adults ≥18-60 years with newly diagnosed AML according to the WHO classification. Patients must have been chemotherapy-naïve (no prior cytotoxic chemotherapy except for hydroxyurea), but could have received prior therapy with hypomethylating, targeted, or biological agents. Additional eligibility criteria included adequate renal (serum creatinine ≤ 1.0 mg/dL) hepatic (serum bilirubin ≤ 1.5 × upper limit of normal, serum transaminases [SGPT and/or SGOT]≤ 2.5 × ULN), cardiac (ejection fraction ≥ 45% by either echocardiography or MUGA [Multi Gated Acquisition] scan) and ECOG (Eastern cooperative Oncology Group) performance status of at least 2. Patients were excluded if they had any coexisting medical condition that in the judgment of the treating physician was likely to interfere with study procedures or results, or if they had any active uncontrolled infection. Patients with acute promyelocytic leukemia were also excluded.
The study was approved by the institutional review board (IRB) of the University of Texas M. D. Anderson Cancer Center and was conducted in accordance with the basic principles of the declaration of Helsinki. All patients signed informed consent to participate in the trail.
Treatment
Patients received induction chemotherapy with clofarabine 22.5 mg/m2 IV over approximately 1 hour daily for 5 days (days 1-5), idarubicin 6 mg/m2 IV over approximately 30 minutes daily for 3 days (days 1-3), and cytarabine 0.75 g/m2 IV over approximately 2 hours daily for 5 days (days 1-5). Patients, who have not achieved a complete remission following the induction course, could receive a second induction course. All patients ≥50 years were admitted to a laminar air flow where they spend an average of 28 days.
Patients who achieved remission (complete remission [CR] or complete remission without platelet recovery [CRp]) after their induction therapy were eligible to receive up to 6 cycles of consolidation therapy. Consolidation therapy consisted of clofarabine 22.5 mg/m2 IV daily for 3 days (days 1-3), idarubicin 6 mg/m2 IV daily for 2 days (days 1-2), and cytarabine 0.75 g/m2 IV daily for 3 days (days 1-3). Cycles were repeated every 4 to 6 weeks based on leukemia response and resolution of study drug-related toxicities. Interim assessment of the first 30 patients revealed low induction mortality and good tolerability; therefore, we increased the doses of both cytarabine and idarubicin to try and improve the outcome of the patients further. Hence, from patient 31 onward, induction doses were amended to clofarabine 20 mg/m2 IV (days 1-5), idarubicin 10 mg/m2 IV (days 1-3), and cytarabine 1 g/m2 IV (days 1-5) and the consolidation doses to clofarabine 15 mg/m2 IV (days 1-3), idarubicin 8 mg/m2 IV (days 1-2), and cytarabine 0.75 g/m2 IV (days 1-3).
Supportive measures for optimal medical care were provided throughout the study as determined by the treating physician and the patient's medical needs. Use of colony-stimulating factors was permitted, but not mandated. Prophylactic antibiotics, antifungals, and antiviral agents (eg, levofloxacin, itraconazole, valacyclovir) were administered to all patients.
The pretreatment evaluation included history and physical examination, complete blood count (CBC) with differential and platelet count, a complete chemistry survey, and marrow aspiration with cytogenetic and molecular markers. An echocardiogram or MUGA scan to evaluate the left ventricular ejection fraction was performed before therapy. Follow-up studies included CBC, differential, and platelet count at least weekly. Bone marrow aspirate and/or biopsy were repeated on day 21 of induction and every 1-2 weeks thereafter until remission or no-response was established.
Assessment of response
A CR was defined as disappearance of all clinical and/or radiologic evidence of disease with ≤ 5% bone marrow blasts, neutrophil count ≥ 1.0 × 109/L, and platelet count ≥ 100 × 109/L. A complete remission without platelet recovery (CRp) had identical marrow results and neutrophil recovery as for CR, but with platelets < 100 × 109/L. Partial remission consisted of a peripheral blood recovery as for CR, but with a decrease in marrow blasts of at least 50% compared with baseline before therapy and not more than 6% to 25% blasts in the marrow. All other responses were considered failures.
Statistical Analysis
This is a prospective, single arm, open label, phase II trial to assess the efficacy of clofarabine, idarubicin, plus cytarabine (CIA) in chemotherapy-naïve AML patients ≤60 years. The primary endpoints of this study were overall response rate (CR+CRp) and event free survival (EFS). Secondary endpoints included safety assessment of this combination (based on guidelines as established by the National Cancer Institute Cancer Evaluation Program (NCI-CTEP) version 3).
Differences among variables were evaluated by the Chi Square and Mann Whitney U test for categorical and continuous variables among patent's group respectively. Time-to-event analyses were performed by the Kaplan-Meier method, and survival curves were compared with the 2-tailed log rank test. A two sides P value ≤ 0.05 was considered to be statistically significant. Univariate and multivariate Cox proportional hazards regression analysis was used to model the relationship between potential prognostic factors and survival (OS and EFS). Confidence interval (CI) estimation for the survival curves was based on the cumulative hazard function, using the Greenwood formula for standard error estimation. All statistical analyses were performed using STATA/SE version 12.1 statistical software (Stata Corp. LP, College Station, TX).
Results
Patient Characteristics
From April 2010 until February 2012, 59 patients were enrolled in the study. The patient's demographics and disease characteristics are summarized in Table 1. The median age was 48 years (range, 19 -60). The median WBC count at presentation was 3.2 > 109/L (range, 6-100.2). Forty patients (68%) had de novo AML, 18 (30%) had secondary AML {10 (17%) with MDS-related and 8 (13%) with therapy-related AML}, and 1 patient (2%) with mixed phenotypic leukemia. Out of the 59 patients, 39 (66%) had intermediate risk cytogenetics {21(36%) with diploid cytogenetics} and 20 (34%) were in the unfavorable cytogenetic group. Six patients (10%) had a FLT3- ITD mutation at presentation (two of them had a concomitant NPM1 mutation).
Table 1. Patient characteristics.
| Characteristics | No. | % |
|---|---|---|
| Patients | 59 | |
| Age, years | ||
| Median | 48 | |
| Range | 19-60 | |
| WBC X 109/L | ||
| Median | 3.2 | |
| Range | 6-100.2 | |
| Hemoglobin g/dl | ||
| Median | 9.3 | |
| Range | 7.3-14.6 | |
| Platelets 103/μL | ||
| Median | 49 | |
| Range | 6-270 | |
| Peripheral blood blast % | ||
| Median | 12 | |
| Range | 0-94 | |
| Bone marrow blast % | ||
| Median | 42 | |
| Range | 3-92 | |
| ECOG performance status ≥ 2 | 4 | 7 |
| AML history | ||
| de novo | 40 | 68 |
| MDS-related | 10 | 17 |
| Therapy-related | 8 | 13 |
| Mixed phenotype | 1 | 2 |
| Cytotogentic abnormalities* | ||
| Diploid | 21 | 36 |
| Intermediate | 14 | 25 |
| Unfavorable | 20 | 34 |
| Insufficient metaphases | 3 | 5 |
| Molecular abnormalities | ||
| FLT3-ITD | 6 | 10 |
| NPM1 | 8 | 14 |
| RAS | 4 | 7 |
| CEBPA | 4 | 7 |
Defined as per the MRC criteria26.
Abbreviations: AML: acute myeloid leukemia, FLT3-ITD: FMS-like tyrosine kinase-3 internal tandem duplication, NPM1: nucleophosmin 1, CEBPA: CCAAT/enhancer-binding protein alpha.
Response and Outcome
Fifty seven patients were included in the final analysis (2 patients were in-evaluable; one was withdrawn from the study on day 8 due to insurance problems and the other completed 5 days of induction chemotherapy and was then lost to follow up). The response rate and characteristics of responders are summarized in Table 2. Forty-two patients (74%) achieved CR and 3 (5%) CRp for an overall response rate (ORR) of 79%. Of the patients who did not achieve CR/CRp after one induction cycle, 10 patients (18%) received a second induction cycle. Four of these patients (40%) achieved CR and 2(20%) CRp. One patient died following re-induction (developed Steven Johnson syndrome). Patients received a median of 2 cycles (range 1-8 cycles) including induction, re-induction (where applicable) and consolidation. Of the 45 patients who achieved CR/CRp after induction, 24 (42%) patients proceeded with an allogeneic stem cell transplant (ASCT) in first CR.
Table 2. Response to treatment.
| Response | No. of patients (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Original Dose | Amended Dose | All Patients | ||||
|
| ||||||
| No. | % | No. | % | No. | % | |
| CR | 19 | 66 | 23 | 77 | 42 | 74 |
| CRp | 1 | 3 | 2 | 7 | 3 | 5 |
| ORR | 20 | 69 | 25 | 84 | 45 | 79 |
Abbreviations: CR: complete remission, CRp: complete remission without platelet recovery, ORR: overall response rate.
With a median follow up of 10.9 months (range, 1.6 - 23.1), the median OS for the entire group was not reached (Figure 1A), the median EFS was 13.5 months (Figure 1B), and the median RFS was not reached (Figure 1C). The patients who underwent ASCT had similar OS, EFS, and RFS compared to patients who received chemotherapy alone (P = 0.25, 0.78, 0.91, respectively). (Figures included in supplemental data). There were no significant differences in terms of response rate, OS, EFS, and RFS between the two dose schedules.
Figure 1. Overall survival, event free survival, and relapse free survival in CIA patients.
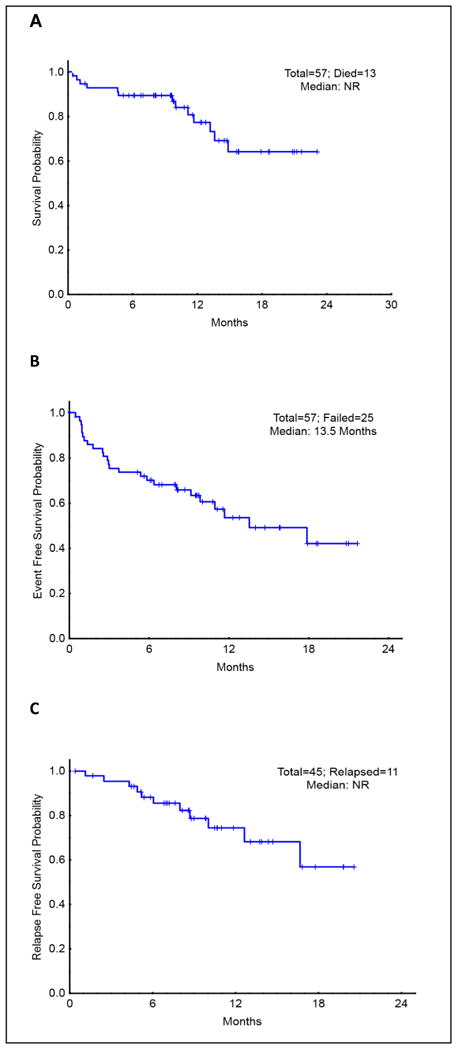
Subgroup analysis revealed a trend toward higher OS (HR 0.12, 95%CI, 0.02-0.90, P = 0.04) and EFS (HR 0.12, 95%CI, 0.02-0.93, P = 0.04) among patients ≤ 40 years old who were treated with CIA compared to patients > 40 years old (remission rate was similar 81% vs. 78 %, P = 0.92, respectively).
Adverse events and early mortality
The treatment regimen was tolerated well with no unexpected toxicities. Adverse events (AEs) are summarized in Table 3. Most AEs were ≤ grade 2 including: nausea (45%), rash (39 %), diarrhea (25%), elevated transaminases (23%), and elevated bilirubin (12%). Toxicities > grade 2 included elevated bilirubin (4%), hypokalemia (2 %), and seizure (2%).
Table 3. Toxicities.
| Adverse Event | Grade 1 to 2 |
Grade 3 to 4 |
||
|---|---|---|---|---|
|
| ||||
| No. | % | No. | % | |
| Nausea | 26 | 47 | ||
| Rash | 22 | 39 | ||
| Diarrhea | 14 | 25 | ||
| AST and ALT elevations | 13 | 23 | ||
| Hyperbilirubinemia | 7 | 12 | 2 | 4 |
| Constipation | 5 | 9 | ||
| Mucositis/stomatitis | 5 | 9 | 1 | 2 |
| Hand-foot syndrome | 4 | 7 | ||
| Hypokalemia | 3 | 5 | 1 | 2 |
| Vomiting | 3 | 5 | ||
| Creatinine elevations | 2 | 4 | ||
| Seizure | 1 | 2 | 1 | 2 |
Abbreviations: AST: aspartate aminotransferase, ALT: alanine aminotransferase
Myelosuppression was ubiquitous but prolonged myelosuppression > 42 days was infrequent. Neutropenic fever was common; however, most of the patients were treated successfully with intravenous or oral antibiotics. One (2%) patient died during the early induction period (≤28 days, from septic shock and respiratory failure) and a total of 2 (4%) patients within the first 8 weeks. There were no significant differences in toxicities between the two dose schedules.
CIA Vs IA
We compared the CIA-treated patients in the current study to a historical cohort of patients who received the combination of idarubicin plus cytarabine (IA; idarubicin 12 mg/m2 IV daily on days 1-3 plus cytarabine 1.5 g/m2 IV daily on days1-4; the consolidation consisted of idarubicin 8 mg/m2 IV daily on days1-2 and cytarabine 0.75 g/m2 IV daily on days 1-3 given every 4-6 weeks for up to 6 cycles). One hundred twenty-two patients treated with IA at our institution between December 2006 and February 2012 were analyzed. There were no differences in the patient's clinical and prognostic characteristics between the CIA and IA groups except for age (patients treated with IA were older, P = 0.02). (Data not reported). CIA treated patients had significantly superior OS (P = 0.005) and EFS (P < 0.001) compared to IA (Figure 2).
Figure 2. Overall survival and event free survival in CIA vs. IA treated patients.
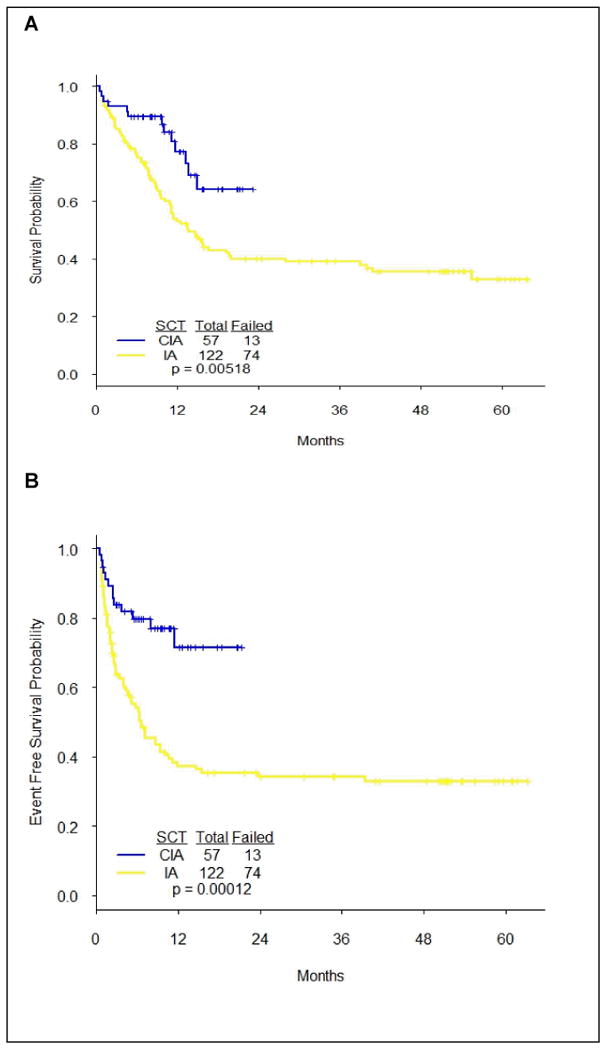
To further assess the effect of age on outcome, we conducted a multivariate analysis including all the prognostic markers. Comparing patients of age ≤40 to those age>40, the impact of age on OS (HR = 0.41, 95% CI = 0.22-0.76, P=0.005) and EFS (HR = 0.46, 95% CI = 0.25-0.85, P=0.014) was statistically significant. After controlling for age, cytogenetics and other important clinical factors (see supplemental data Tables 1 and 2), patients treated by CIA had significantly better OS (HR = 0.53, 95% CI = 0.29-0.97, P=0.039) and EFS (HR = 0.40, 95% CI = 0.22-0.73, P=0.003) than those treated by IA (Supplemental data).
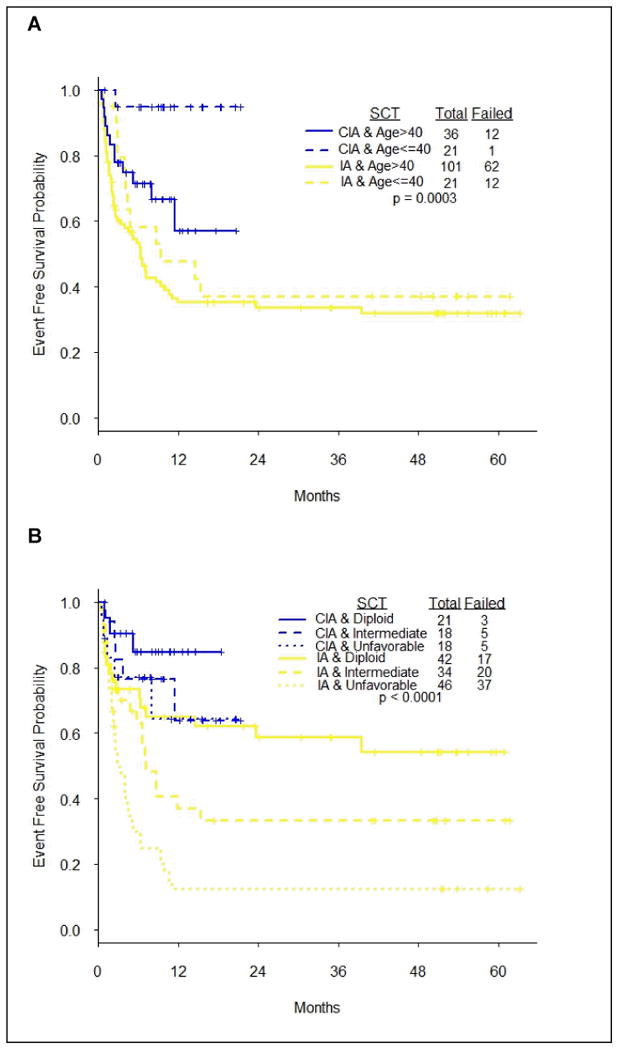
In subgroup analysis, patients ≤ 40 years old who were treated with CIA had significantly superior OS (HR 0.12, 95% CI, 0.02-0.90, P = 0.04) and EFS (HR 0.12, 95% CI, 0.02-0.93, P = 0.04) compared patients > 40 years. Furthermore, patients ≤ 40 years old who treated with CIA had better OS and EFS rates compared to identical age group of patients who received IA (Figure 3). In addition, patients with unfavorable karyotype who treated with CIA achieved significantly longer OS (P< 0.0001) and EFS (P< 0.0001) compared to similar patients group treated with I A (Figure 3).
Figure 3. Subgroup analysis by age and cytogenetics in CIA vs. IA treated patients.
Discussion
Despite the advances over the last 3 decades in the treatment of younger patients with acute myeloid leukemia, outcome remains unsatisfactory and hence new treatment approaches need to be explored.
In this pilot study, we combined IA with clofarabine (CIA) based on previous clinical and laboratory experiences with this combination in patients with AML. Cytarabine requires intracellular phosphorylation to the triphosphate compound (ara-CTP) to become biologically active.22 Plunkett et al. demonstrated that accumulation of ara-CTP by human leukemia cells in vivo is saturated at cytarabine plasma concentrations achieved by intermediate doses of cytarabine (dose range of 1 to 2 g/m2/day) obviating the need to high dose of cytarabine regimens.23 In addition, clofarabine is a potent ribonucleotide reductase (RnR) inhibitor which can modulate ara-CTP accumulation in leukemic cells.22 The combination of clofarabine with cytarabine can therefore lead to increased retention of ara-CTP in leukemic cells so that the antileukemic activity of clofarabine is complemented by a biochemical synergy between these agents.24 Furthermore, Anthracyclines are most commonly combined with nucleoside analogs in AML therapy. Although the optimal choice of anthracyclines during induction remains conflicting, idarubicin has been widely adopted as part of the current induction regimen. Additionally, the combination of cytarabine and anthracyclines synergizes its antileukemic activity by inhibiting DNA repair damage and potentiate the antileukemic activity of cytarabine.25
Purine nucleoside analogs such as fludarabine and cladribine have been investigated in addition to cytarabine and anthracyclines in the treatment of patients with AML. In a phase III multicenter trial of fludarabine combined with cytarabine and idrubicine (FLAI) compared to idarubicin, cytarabine and etoposide (ICE) in newly diagnosed patients with AML < 60 years of age, treatment with FLAI was associated with superior CR rate (75% vs 51%, P =0.01) and lower toxicity. In another large randomized study by the Polish group, cladribine 5 mg/m2/day was added to daunorubicin 60 mg/m2 for 3 days and cytarabine 200 mg/m2 for 7 days and compared to cytarabine and daunorubicin.. The addition of cladribine resulted in a higher CR rate and better OS benefit (P = 0.05)9.
In our study, the ORR was 79% (including 74% CR and 5% CRp rates) in subgroup of patients with only intermediate and unfavorable cytogenetic profile. Only 10 patients required a re-induction with 60 % of them responded thereafter. Significant number of the patients (42%) proceeded with allogeneic stem cell transplant after achieving CR. Although the protocol was amended to optimize the efficacy of the commination, the amended dose schedule had similar response rate and toxicity profile to the original dose. In subgroup analysis, patients ≤ 40 years had significantly better EFS and OS compared to patients > 40 years. Although the remission rate of patients treated with CIA combination was not statistically significant compared to patients treated with IA, the OS and EFS were superior. Furthermore, in subgroup analysis, patients ≤ 40 years old and patients with unfavorable cytogentics had better OS and EFS when they were treated with CIA compared to IA.
Although the results are promising, the study has some limitations including a small sample size, single institution experience and the comparison to historical based regimen, therefore the results should be considered preliminary.
In conclusion, CIA combination is safe and active in newly diagnosed patients with AML ≤ 60 years and the results are encouraging. A randomized clinical trial with larger number of patients to compare this combination to standard induction therapy is warranted.
Supplemental data
Figure 1: Outcome (OS, EFS, RFS) in CIA patients with or without transplant
Table 1. Multivariate Cox's regression for OS
Table 2. Multivariate Cox's regression for EFS
Footnotes
The study was presented in part at the 54th annual meeting of the American Society of Hematology, San Diego, 2011
References
- 1.Yates JW, Wallace HJ, Jr, Ellison RR, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485–8. [PubMed] [Google Scholar]
- 2.Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58:1203–12. [PubMed] [Google Scholar]
- 3.O'Donnell MR, Abboud CN, Altman J, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9:280–317. doi: 10.6004/jnccn.2011.0027. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–59. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelli F, Vignetti M, Suciu S, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol. 2009;27:5397–403. doi: 10.1200/JCO.2008.20.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28:586–95. doi: 10.1200/JCO.2009.22.9088. [DOI] [PubMed] [Google Scholar]
- 8.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22:4290–301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 9.Holowiecki J, Grosicki S, Robak T, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia. 2004;18:989–97. doi: 10.1038/sj.leu.2403336. [DOI] [PubMed] [Google Scholar]
- 10.Ohtake S, Miyawaki S, Fujita H, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117:2358–65. doi: 10.1182/blood-2010-03-273243. [DOI] [PubMed] [Google Scholar]
- 11.Lowenberg B, van Putten W, Theobald M, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349:743–52. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 12.Thomas X, Raffoux E, Renneville A, et al. Which AML subsets benefit from leukemic cell priming during chemotherapy? Long-term analysis of the ALFA-9802 GM-CSF study. Cancer. 2010;116:1725–32. doi: 10.1002/cncr.24943. [DOI] [PubMed] [Google Scholar]
- 13.List AF, Kopecky KJ, Willman CL, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98:3212–20. doi: 10.1182/blood.v98.12.3212. [DOI] [PubMed] [Google Scholar]
- 14.Dillman RO, Davis RB, Green MR, et al. A comparative study of two different doses of cytarabine for acute myeloid leukemia: a phase III trial of Cancer and Leukemia Group B. Blood. 1991;78:2520–6. [PubMed] [Google Scholar]
- 15.Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer. 2006;107:116–24. doi: 10.1002/cncr.21543. [DOI] [PubMed] [Google Scholar]
- 16.Ravandi F, Cortes J, Faderl S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116:5818–23. doi: 10.1182/blood-2010-07-296392. quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 18.Bloomfield CD, Lawrence D, Byrd JC, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–9. [PubMed] [Google Scholar]
- 19.Kantarjian HM, Jeha S, Gandhi V, et al. Clofarabine: past, present, and future. Leuk Lymphoma. 2007;48:1922–30. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–73. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Faderl S, Ferrajoli A, Wierda W, et al. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer. 2008;113:2090–6. doi: 10.1002/cncr.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie C, Plunkett W. Metabolism and actions of 2-chloro-9-(2-deoxy-2-fluoro-beta-D- arabinofuranosyl)-adenine in human lymphoblastoid cells. Cancer Res. 1995;55:2847–52. [PubMed] [Google Scholar]
- 23.Plunkett W, Liliemark JO, Estey E, et al. Saturation of ara-CTP accumulation during high-dose ara-C therapy: pharmacologic rationale for intermediate-dose ara-C. Semin Oncol. 1987;14:159–66. [PubMed] [Google Scholar]
- 24.Cooper T, Ayres M, Nowak B, et al. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother Pharmacol. 2005;55:361–8. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- 25.Szmigielska-Kaplon A, Ciesielska E, Szmigiero L, et al. Anthracyclines potentiate activity against murine leukemias L1210 and P388 in vivo and in vitro. Eur J Haematol. 2002;68:370–5. doi: 10.1034/j.1600-0609.2002.01598.x. [DOI] [PubMed] [Google Scholar]
- 26.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: Outcome (OS, EFS, RFS) in CIA patients with or without transplant
Table 1. Multivariate Cox's regression for OS
Table 2. Multivariate Cox's regression for EFS


