Abstract
Background
Although accountable care organizations (ACOs) are rapidly being deployed in Medicare, little is known about how the model might affect high-risk, high cost groups such as cancer patients. The Physician Group Practice Demonstration, which ran from 2005 to 2010 in 10 physician groups, provides the best current evidence on the likely effectiveness of accountable care organizations for Medicare beneficiaries. Changes in cancer treatment and spending under this program may be indicative of cancer treatment under ACO payment reform.
Methods
Using Medicare fee-for-service claims data, regression analysis was used to estimate changes in payments for cancer patients using a difference-in-difference design comparing pre- (2001–2004) and post-intervention (2005–2009) trends in spending on cancer patients in PGPD participants to local control groups.
Results
Regression models indicate the Physician Group Practice Demonstration was associated with average Medicare spending reductions per cancer patient of $721 annually across participating sites, an annual 3.9% reduction in payments per patient. Savings derived entirely from reductions in acute care payments for inpatient stays. The Demonstration was also associated with a reduction in mortality among cancer patients. There was no significant change in the proportion of deaths occurring in the hospital. There were significant reductions in hospice use, hospital discharges and ICU days, but no reductions in cancer-specific procedures or chemotherapy. Estimates of all measures varied considerably across participating sites.
Conclusions
The Physician Group Practice Demonstration was associated with reductions in admissions for inpatient care among beneficiaries with prevalent cancer, with no adverse effect on mortality. Participants in the Physician Group Practice Demonstration did not change the trajectory of spending for cancer-specific treatments.
Implications
Inpatient care for beneficiaries with cancer may represent a significant source of potential savings for ACOs, but evidence from the Physician Group Practice Demonstration indicates that no changes were made to cancer treatments such as chemotherapy or surgical procedures.
Keywords: Accountable care organizations, Payment reform, Cancer, Medicare
1. Introduction
The economics of cancer care in the United States have garnered increasing attention as policymakers aim to curb spending growth while maintaining or improving quality of care. Average Medicare expenditures for beneficiaries with cancer are nearly four times higher than expenditures for those without, and cancer care comprises approximately 10% of all Medicare expenditures.1 In addition, spending on cancer care is expected to increase as the population ages and new, more expensive treatments are deployed to replace less expensive treatments. Despite the high cost of cancer care and growing attention to curbing cost growth, it has been difficult for payers or purchasers to slow cost growth. Payers are legally obligated to provide coverage for cancer treatment regardless of cost,2 and existing fee-for-service payment models incentivize physicians to use more expensive treatments and provide more treatment. In addition, a sense of urgency among both patients and providers may drive the use of additional treatments or technologies, even in the absence of evidence of effectiveness. As a result, cancer care currently is expensive and not often driven by value.
New health care payment reforms seek to shift reimbursement away from traditional fee-for-service toward approaches that encourage value-based care, by providing incentives for physicians and patients to improve care and lower costs. One of the most prominent reforms is accountable care organizations (ACOs), broadly directed at containing health care costs; under an ACO contract, a group of physicians are eligible to share in savings they create if they meet quality standards. In 2012, Medicare introduced ACOs through the Pioneer and Shared Savings Programs, and 252 organizations are now under these new contracts. In addition, many organizations have commercial payer ACO contracts and four states are now contracting with Medicaid ACOs.3 Despite this rapid adoption of the ACO model, little is known about how ACO payment reforms will affect cancer care, either on the cost or quality of the care delivered. Evidence on the effects of managed care and capitation on cancer care quality and cost is mixed.4 Based on this research, some hypothesize that under ACOs, cancer patients may experience worse care, particularly if physicians stint on effective or necessary care to cut costs.5,6 Although ACOs include a strong emphasis on pay for performance to prevent stinting, ACOs' quality measures are not focused on cancer care, and thus may not protect against stinting.7
The best evidence to date on how the accountable care organization model may affect cancer care comes from the Medicare Physician Group Practice Demonstration (PGPD). The Demonstration took place from 2005 to 2010 with 10 participating physician groups. Evidence from the Demonstration indicated that overall the program produced modest savings, with larger savings in the high-risk, high-cost group of beneficiaries eligible for both Medicare and Medicaid.8 Performance on quality measures included in the Demonstration – including cancer screening rates – improved over time and scored over 99% on average in the final year of the Demonstration.9 However, the effect of the Demonstration on cancer-specific spending and treatment patterns has not been studied to date.
In this paper, we examine how beneficiaries with cancer fared under the Physician Group Practice Demonstration. We compare beneficiaries at Demonstration sites before and during the Demonstration period as well as to local control groups receiving care from non-Demonstration providers. Specifically, we compare payments (overall, as well as broken into categories) and some patient experience outcomes (such as mortality and use of hospice). We test three specific hypotheses related to the care of cancer patients. First, we hypothesize the PGPD produced a reduction in acute care spending, based on a recent study that found across all Medicare beneficiaries, spending reductions under the Demonstration were concentrated in acute care.8 Second, we hypothesize that ACO payment reforms might reduce spending on imaging because much imaging use is discretionary. Finally, we hypothesize that under ACO incentives, cancer patients may be less likely to die in the hospital; this is often in line with patient preferences as well as less expensive. This analysis provides the first empirical evidence on how the shared savings ACO model may impact the cost and experience of care for cancer patients.
2. Material and methods
We use Medicare fee-for-service claims data from 2001 to 2009 to analyze changes in spending and quality for cancer beneficiaries assigned to PGPD participating physician group and local control groups. We use a difference-in-difference model to assess the impact of the Demonstration on participant sites compared over time to local controls.
2.1. Data
We use 2001–2009 Medicare fee-for-service claims data. For 2001–2005 we use a 20% sample; for 2006–2009 we use a 100% sample.
2.2. Cohorts
We identify beneficiaries assigned to 10 PGPD participating physician groups and local control groups using methodology matching the Demonstration.10 Beneficiaries are assigned to the group of physicians from whom they have the greatest allowed charges for ambulatory carevisits. Demonstration beneficiaries are those assigned to the PGPD sites. The control group consists of beneficiaries residing in the same counties as PGPD beneficiaries.a Our assignment algorithm and methods are detailed further in previously published work.8
We define our prevalent cancer cohort as beneficiaries with at least one inpatient claim with a cancer diagnosis or two physician visits at least a week apart with a specific cancer diagnosis based on Chronic Conditions Warehouse software. We group cancers into 32 diagnosis groups as described in Table 1, omitting skin cancer. The proportion of the full cohort with prevalent cancer differs by participating site, ranging from 8.3% to 15.8% with a mean of 12%. The proportion with cancer differed only slightly among the PGPD and non-PGPD practices. The proportion with cancer in the PGPD practices was 12.4% and the proportion in the controls was 10.8%. The higher proportion in the cases was largely due to higher prevalence in the two academic medical centers (Dartmouth-Hitchcock, 14.5% and University of Michigan, 15.8%).
Table 1.
Beneficiaries in the Physician Group Practice Demonstration descriptive characteristics for participating sites and local controls. Source: Medicare claims files, 2001-2005 (20% sample), 2006-2009 (100%).
| Beneficiaries with cancer |
||||
|---|---|---|---|---|
| Participants |
Control |
|||
| Pre (2001-2004) | Post (2005-2009) | Pre (2001-2004) | Post (2005-2009) | |
| N | 18,483 | 104,766 | 137,563 | 727,969 |
| Demographics | ||||
| Mean age | 74.4 | 74.1 | 74.9 | 74.7 |
| % Female | 48.8 | 50.1 | 48.0 | 48.0 |
| % Medicaid | 8.5 | 9.9 | 8.4 | 9.2 |
| % Black | 1.4 | 1.8 | 3.2 | 3.6 |
| % < Age 65 | 6.2 | 8.2 | 5.6 | 7.2 |
| % Disabled and ≥Age 65a | 6.2 | 6.8 | 7.1 | 7.2 |
| % Blacks below FPL in zipcode | 18.6 | 18.4 | 22.3 | 21.5 |
| % Non-Black below FPL in zipcode | 8.1 | 8.0 | 8.3 | 8.2 |
| % Black high-income in zipcode | 11.5 | 13.3 | 11.5 | 14.2 |
| % Non-Black high-income in zipcode | 10.8 | 12.0 | 8.4 | 10.1 |
| Risk adjustment | ||||
| Mean HCC | 1.83 | 2.01 | 1.82 | 1.98 |
| Mean comorbidity count (of 10 below) | 1.13 | 1.15 | 1.17 | 1.20 |
| % Metastatic cancerb | 14.3 | 14.1 | 13.1 | 12.4 |
| % Chronic pulmonary disease | 17.7 | 16.9 | 18.6 | 18.4 |
| % Coronary artery disease | 20.9 | 19.4 | 22.4 | 22.0 |
| % Congestive heart failure | 11.8 | 10.3 | 12.7 | 11.2 |
| % Peripheral vascular disease | 8.0 | 8.5 | 8.7 | 9.3 |
| % Severe chronic liver disease | 0.5 | 0.6 | 0.5 | 0.7 |
| % Diabetes with end organ damage | 1.9 | 2.0 | 1.8 | 1.8 |
| % Chronic renal failure | 3.3 | 6.4 | 3.7 | 6.5 |
| % Dementia | 4.7 | 4.7 | 5.0 | 5.5 |
| % Diabetes (without end organ damage) | 17.5 | 20.4 | 19.7 | 21.4 |
| Low-variation indicators (per 1000) | ||||
| Hip fracture | 10.69 | 9.79 | 11.41 | 10.30 |
| Stroke | 11.13 | 10.09 | 12.22 | 10.80 |
| Acute myocardial infarction | 14.88 | 12.10 | 15.09 | 11.76 |
| Cancer typeb | ||||
| Cancer of prostate | 26.2 | 24.1 | 28.3 | 27.4 |
| Cancer of breast | 22.4 | 23.0 | 22.0 | 22.5 |
| Cancer of colon | 9.8 | 9.3 | 11.0 | 9.8 |
| Cancer of bronchus, lung | 8.9 | 9.2 | 8.8 | 9.1 |
| Cancer of bladder | 6.5 | 6.8 | 8.0 | 8.0 |
| Leukemias | 4.3 | 4.7 | 3.4 | 3.9 |
| Melanomas of skin | 4.1 | 4.7 | 2.9 | 3.6 |
| Cancer of head and neck | 3.9 | 3.5 | 3.0 | 3.1 |
| Cancer of rectum and anus | 3.8 | 3.4 | 4.2 | 3.4 |
| Cancer of uterus | 3.1 | 2.9 | 2.5 | 2.4 |
| Cancer of kidney and renal pelvis | 2.4 | 2.6 | 2.6 | 2.8 |
| Cancer of ovary | 1.9 | 2.0 | 1.6 | 1.7 |
| Multiple myeloma | 1.5 | 1.8 | 1.4 | 1.5 |
| Cancer of cervix | 1.2 | 0.8 | 1.2 | 0.9 |
| Cancer of esophagus | 1.1 | 1.0 | 0.9 | 0.9 |
| Cancer of pancreas | 1.1 | 1.3 | 1.0 | 1.1 |
| Cancer of stomach | 1.0 | 0.8 | 1.0 | 0.9 |
| Cancer of bone and connective tissue | 1.0 | 1.1 | 0.8 | 0.9 |
| Cancer of brain and nervous system | 1.0 | 1.1 | 0.8 | 1.0 |
| Cancer of other GI organs, peritoneum | 0.9 | 1.2 | 1.2 | 1.1 |
| Cancer of other female genital organs | 0.9 | 0.8 | 0.7 | 0.6 |
| Non-Hodgkins lymphoma | 0.9 | 0.8 | 0.7 | 0.6 |
| Cancer of thyroid | 0.8 | 1.2 | 0.9 | 1.1 |
| Hodgkins disease | 0.6 | 0.6 | 0.4 | 0.4 |
| Cancer of liver and intrahepatic bile duct | 0.5 | 0.7 | 0.7 | 0.7 |
| Cancer, other respiratory and intrathoracic | 0.4 | 0.3 | 0.3 | 0.3 |
| Cancer of other urinary organs | 0.3 | 0.3 | 0.4 | 0.4 |
| Cancer of other male genital organs | 0.2 | 0.1 | 0.1 | 0.1 |
| Cancer of testis | 0.1 | 0.2 | 0.1 | 0.2 |
| Annual spending | ||||
| Mean per capita payments | $18,594 | $20,066 | $18,100 | $19,921 |
| Mean payments—acute care | $8116 | $7736 | $7520 | $7617 |
| Mean payments—procedures | $2301 | $2709 | $2365 | $2754 |
| Mean payments—cancer specific | $1708 | $1572 | $1716 | $1441 |
| Mean payments—chemotherapy | $1161 | $822 | $1164 | $715 |
| Mean payments—cancer procedures | $631 | $832 | $639 | $800 |
| Mean payments—Evaluation & management | $1482 | $1626 | $1475 | $1602 |
| Mean payments—Double medical equipment | $1211 | $1774 | $889 | $1281 |
| Mean payments—Skilled nursing facility | $987 | $1186 | $1109 | $1360 |
| Mean payments—imaging | $854 | $1138 | $812 | $1102 |
| Mean payments—home health | $574 | $625 | $571 | $675 |
| Mean payments—tests | $557 | $782 | $541 | $757 |
| Mean payments—long-term care | $533 | $688 | $614 | $697 |
| Outcomes | ||||
| % Diedb | 11.6 | 10.3 | 11.3 | 10.5 |
| % Death in hospitalb | 3.0 | 2.6 | 3.4 | 2.9 |
| % Hospice useb | 7.1% | 6.8% | 6.2% | 6.5% |
| Mean Hospice daysb Mean Hospice days among hospice usersb Mean ICU days |
3.16 44.38 0.60 |
2.83 41.54 0.61 |
2.73 43.73 0.73 |
2.87 44.02 0.81` |
| % who use acute care (%) | 42.1 | 39.4 | 43.0 | 40.8 |
| Mean hospital discharges | 0.857 | 0.806 | 0.852 | 0.832 |
| Mean days in hospital | 4.35 | 3.96 | 4.58 | 4.21 |
Notes: Cases and controls are weighted by person-years. Controls are weighted such that the sum of the weights equals the number of cases by county and cancer type. Spending is inflated to 2009 US dollars using the gross domestic product deflator. Proportion in a high income group is defined by race at the 85th percentile.
Disability is defined using original reason for entitlement. is weighted using only county weights (not follow up time which incorporates deaths).
Variable
2.3. Covariates
We use patient characteristics to adjust for differences between PGPD participants and local controls. All models adjust for age, gender, race, and interactions between these variables. We adjust for disability and race-specific income at the ZIP code level for both poverty (proportion under the federal poverty line) and high income (proportion over the 85th percentile of income distribution).11 We utilize a low-variation condition rate approach to risk adjustment by including the proportion with an acute myocardial infarction, hip fracture, or stroke in each year.8 In addition, we adjust for cancer type based on diagnoses category and an indicator for metastatic cancer.
2.4. Outcomes
Our primary outcome measure is total annual Medicare payments per beneficiary summed across all services, presented in constant 2009 dollars.12,13 We next divide payments into subcategories based on the Berenson-Eggers Type of Service (BETOS) classification system and describe the distribution of payments across these groups before and after implementation of the Demonstration. We model payments for acute care (payments to hospitals for inpatient care), procedures, tests, imaging, and hospice. We also create an estimate of cancer-specific spending using physician and outpatient facility billing. This spending includes cancer-specific procedures, including radiation and payments for chemotherapy. While Medicare chose to cap payments at $100,000 per beneficiary per year in the Demonstration for the purpose of calculating bonuses, we chose not to cap payments for the 1.6% of our sample who exceeded this threshold.
Other outcome measures include referral to hospice, hospice days, days in the intensive care unit (ICU), days in the hospital, the hospital visit rate, mortality, and death in the hospital.
2.5. Analysis
We describe demographic, clinical, and spending patterns for the participant and control cohorts before and after the PGPD intervention and then use ordinary least squares regression to estimate the effect of participating in the PGPD using a difference-in-difference design. This model accounts for local trends in health care over time by focusing on the difference between changes in the intervention group at the time of policy implementation and changes in the control group at the time of policy implementation. We control for available demographic and clinical variables (patient age, gender, race, % in poverty in the beneficiary's ZIP code, % high-income in the beneficiary's ZIP code, Medicaid eligibility status, disability, cancer type, and the low-variation condition rate in a given site, in a given year, specific to participants or controls), as well as annual indicators for each local area (90 indicators for each of the 10 areas for 9 years) to control for local and time-specific factors unrelated to the PGPD that could affect payments. The level of observation is beneficiary-year.
This research design controls for fixed differences between the local controls and participant groups, as well as biases from comparisons over time in the treatment group that could reflect broader trends in health care spending or Medicare beneficiary population health. The coefficient of interest is estimated for each PGPD group and measures the impact of being in the PGPD (relative to controls) after the intervention begins (2005–2009). We cluster standard errors to adjust for correlation of observations within practice groups (defined by area and PGP participation status) and within beneficiaries over time, using Huber–White–Sandwich estimators.14
We estimate the cumulative impact of the PGPD as the weighted average of the 10 independent site-specific treatment effects, weighting estimates by the relative population share of each region. Our significance test for the overall cost savings in cancer patients from the PGPD assumes that the treatment effects in each region are independent. If spillover effects occurred whereby one PGPD learned from the experiences of others how to reduce expenditures, our confidence intervals would be biased downward.
3. Results
3.1. Descriptive characteristics of cohort
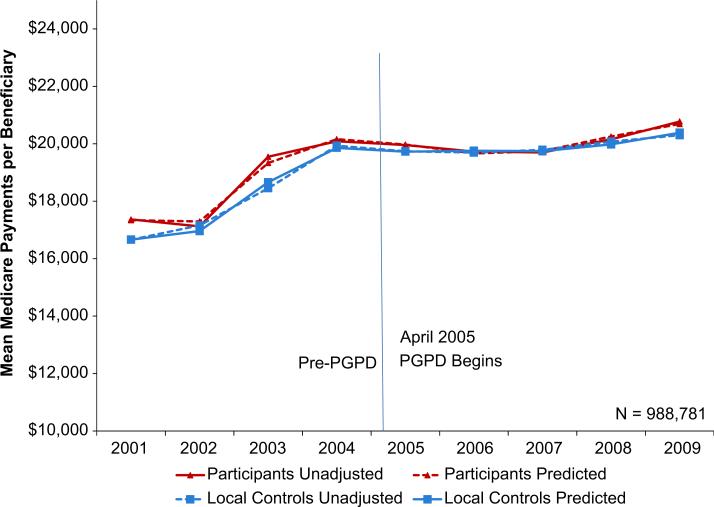
Eleven percent of beneficiaries assigned to PGPD participants or local controls have a cancer diagnosis, leaving us with 988,781 person-years in our analytic sample. Medicare payments for bene-ficiaries with cancer in our study were $19,750 annually on average over 2001–2009 compared to about $8500 in the overall Medicare population. Fig. 1 illustrates a number of important results. First, it shows that the control group is a valid comparison, as trends in the participants and controls are similar in the pre-PGPD period. Second, it shows that the PGPD did appear to slightly slow cost growth (or “bend” the cost curve) for cancer patients in the initial years of the Demonstration. Though spending growth slowed considerably for both participants and controls during the Demonstration, it slowed more for the participant group. Beneficiaries assigned to PGPD participants were more expensive than those in the control group in every year except 2007. Finally, the figure shows that our multivariate regression model predicts unadjusted spending well, as our modeled values are very similar to the unadjusted values.
Fig. 1.
Annual Medicare Payments per Medicare Beneficiary with Prevalent Cancer. Notes: Annual per beneficiary spending is inflated to 2009 dollars using the gross domestic product deflator.
Source: Author analyses of Medicare claims files, 2001–2005 (20% sample), 2006–2009 (100%).
Our participant and local control groups are similar along demographic characteristics, including age, proportion female and proportion eligible for Medicaid (Table 1). A similar proportion have metastatic cancer across the two groups (14% in participants, 13% in controls), and the proportion with other chronic conditions is also similar in the pre-period. In both groups, the most prevalent cancers were prostate, breast, and colon.
Mean annual Medicare spending on patients with cancer was higher in PGPD participants than local controls in both periods, though the gap narrowed in the Demonstration period (Table 1). The unadjusted percentage change in spending between the preand post-period mean payments was 8% in the participant group and 10% in the control group. Over a third of annual spending was on acute care, and procedures made up the next largest share.
The annual mortality rate among the cancer cohort was 11.6% in patients assigned to PGPD participants and 11.3% in the local control group (Table 1). The mortality rate declined in the post-period in both groups, with a greater unadjusted drop in the participant group. The proportion dying in the hospital was lower in PGPD participants in both time periods and declined in both groups during the Demonstration. Hospice use was higher in PGPD participants in both periods; during the Demonstration hospice fell among participants while it rose in the control group. Similarly, days in hospice decreased in the participant group and increased in the control group during the study period. Number of intensive care unit (ICU) days remained constant in the participant group, while it increased in the control group. The acute care visit rate and the number of inpatient days declined in both participants and controls during the study period, but both measures declined more in the participant group on an unadjusted basis.
3.2. Regression results
We next used difference-in-difference models to examine performance of participants compared to controls. Our difference-in-difference models indicate that the Physician Group Practice Demonstration was associated with Medicare spending reductions of $721 annually on average in payments for cancer patients across the 10 participating sites (Table 2), compared to the previous finding of $114 annually for all beneficiaries.8 This equates to an annual 3.9% reduction in payments per cancer patient, compared to the previous finding of 1.4% for all beneficiaries. The magnitude of savings in cancer patients is similar to that observed among beneficiaries eligible for both Medicaid and Medicare, another high-cost, high-risk group ($532 annually per beneficiary, or 5%).8 The reductions in spending were not distributed evenly across the years of the Demonstration: analyses of savings by year show that savings for PGPD sites were greatest in 2007 and smallest in 2009.
Table 2.
Annual per-beneficiary change in payments associated with PGPDa.
| N | Total payments (95% CI) | Acute care payments (95% CI) | Cancer-specific payments (95% CI) | Imaging payments (95% CI) | Procedures payments (95% CI) | Hospice payments (95% CI) | |
|---|---|---|---|---|---|---|---|
| AH PGPD Participants | 988,781 | −$720.89 (−969, −473) | −$736.62 (−936, −538) | $144.76 (79, 210) | −$12.63 (−43, 18) | $23.22 (−12, 58) | −$83.88 (−138,−30) |
| Billings clinic | 17,579 | −612.75 (−718, −507) | −1401.45 (−1482, −1321) | 117.69 (90, 145) | −28.95 (−43, −15) | −234.67 (−260, −209) | −23.75 (−49, 1) |
| Dartmouth-Hitchcock clinic | 84,085 | −384.61 (−539, −230) | −508.14 (−669, −347) | 148.40 (92, 205) | 78.26 (60, 97) | 128.07 (90,166) | 4.50 (−43, 52) |
| Everett clinic | 94,174 | −417.26 (−576, −258) | −216.36 (−338, −95) | 460.98 (408, 514) | −84.98 (−110, −60) | 179.71 (143, 217) | −430.66 (−490, −371) |
| Forsyth medical group | 58,527 | 1111.78 (836, 1388) | 882.69 (626, 1139) | −49.48 (−132, 33) | −4.43 (−43, 34) | 84.46 (10,159) | 77.27 (−10,165) |
| Geisinger clinic | 115,997 | 149.04 (0, 298) | −257.28 (−394, −121) | −69.50 (−113, −26) | 23.07 (5, 41) | −207.11 (−240, −174) | −24.70 (−63, 13) |
| Marshfield clinic | 43,212 | −612.66 (−883, −342) | −487.38 (−703, −271) | 314.78 (245, 385) | −196.55 (−229, −164) | −20.56 (−53,12) | −146.77 (−195, −98) |
| Middlesex health system | 146,635 | −32.15 (−179,115) | 43.01 (−62, 148) | −311.96 (−348, −276) | 50.63 (35, 67) | 170.10 (148, 192) | 13.91 (−18, 46) |
| Park Nicollet clinic | 74,500 | −447.96 (−690, −206) | −345.11 (−532, −158) | 210.36 (149, 272) | −0.37 (−31, 31) | 223.50 (202, 245) | 133.57 (84, 183) |
| St. John's clinic | 46,557 | −1289.91 (−1731, −0.849) | −640.68 (−0.965, −0.316) | 75.84 (−33,185) | −35.04 (−90, 20) | −108.98 (−141, −77) | −241.66 (−326, −157) |
| University of Michigan Faculty group practice | 307,515 | −3526.58 (−3709, −3344) | −3732.14 (−3891, −3573) | 535.31 (501, 570) | 99.18 (82, 116) | 204.42 (174, 235) | −196.17 (−229, −163) |
PGPD=Physician Group Practice Demonstration, CI=Confidence Interval INotes: Spending is inflated to 2009 US dollars using the gross domestic product deflator. Negative estimates represent savings. Estimates derived from a linear model adjusting for area-year indicators, age, black race, woman, Medicaid eligibility, disability, cancer type, and indicator for metastatic cancer. The model adjusts for zip code-level rates of poverty and high income. The model adjusts for the rate of low-variation conditions for each of the 10 local areas for each year separately for treatment and control groups. Low-variation condition rate is the number of individuals experiencing the conditions hip fracture, stroke, and acute myocardial infarction per thousand Medicare beneficiaries Source: Author analyses of Medicare claims files, 2001-2005 (20% sample), 2006-2009 (100%)
Results for cancer beneficiaries were quite heterogeneous by participating site, as was found in analysis of the Demonstration.8 Estimates of changes in payment for cancer patients associated with the Demonstration varied from reductions in payments of $3527 at the University of Michigan Faculty Group Practice to increases in spending during the Demonstration associated with the Demonstration of $1112 at Forsyth Medical Group. These cancer-specific spending results were not associated with results from the full demonstration; some sites that did not perform well in the full demonstration did well with cancer patients. For example, St. John's Clinic did not have savings associated with the Demonstration for the full cohort, but estimated savings among cancer patients were $1290 annually.
Estimated savings among beneficiaries with cancer were derived entirely from reductions in acute care payments (Table 2). On average, reductions in acute care payments for cancer patients associated with the Demonstration were $737 annually across the 10 sites and heterogeneity across the 10 sites displayed a similar pattern as the overall effects. Reductions in acute care payments could be due to either reductions in utilization or reductions in the “price” of an acute care stay – either from changes in policy affecting payments or from changes in diagnosis related groups (DRGs) used for charges. There was a significant decrease in the hospital discharge rate (Table 3) indicating that the reduction in acute care payments is due, at least in part, to a reduction in inpatient utilization. The reductions in acute care payments were offset by a small, significant increase in payments for cancer-related treatment. There were no significant changes overall in payments for imaging or procedures more broadly, though some sites exhibited small significant reductions in these spending categories.
Table 3.
Annual per-beneficiary change in outcomes associated with PGPD. Source: Author analyses of Medicare claims files, 2001-2005 (20% sample), 2006-2009 (100%).
| Mortality (95% CI)a | Death in hospital (95% CI)a | Hospice use (95% CI)a | Hospice days (95% CI)a | ICU days (95% CI) | Days in hospital (95% CI) | Hospital discharges (95% CI) | |
|---|---|---|---|---|---|---|---|
| All PGPD participants | −0.65 (−1.00, −0.29) | 0.04 (−0.18, 0.26) | −0.73 (−1.11, −0.35) | −0.43 (−0.70, −0.17) | −0.08 (−0.12, −0.04) | −0.08 (−0.18, 0.02) | −0.03 (−0.05, −0.02) |
| Billings clinic | −0.93 (−1.16, −0.70) | −0.54 (−0.61, −0.47) | 0.29 (0.07, 0.51) | −0.15 (−0.28, −0.01) | 0.11 (0.09, 0.13) | −0.17 (−0.19, −0.14) | −0.05 (−0.06, −0.04) |
| Dartmouth-Hitchcock clinic | −0.70 (−1.14, −0.25) | −0.45 (−0.60, −0.31) | −0.07 (−0.45, 0.32) | 0.06 (−0.17, 0.30) | −0.11 (−0.15, −0.07) | −0.35 (−0.43, −0.27) | −0.05 (−0.07, −0.04) |
| Everett clinic | −1.56 (−1.97, −1.15) | 0.55 (0.31, 0.78) | −2.75 (−3.16, −2.33) | −2.19 (−2.52, −1.86) | −0.35 (−0.39, −0.30) | −0.19 (−0.29, −0.08) | 0.02 (0.01, 0.04) |
| Forsyth medical group | −0.18 (−0.72, 0.36) | −0.13 (−0.50, 0.25) | 0.89 (0.32, 1.45) | 0.13 (−0.34, 0.59) | 0.05 (−0.01, 0.11) | 0.20 (−0.01, 0.41) | 0.08 (0.05, 0.10) |
| Geisinger clinic | 0.24 (−0.01, 0.49) | 0.44 (0.26, 0.63) | −0.05 (−0.31, 0.21) | −0.33 (−0.55, −0.11) | −0.09 (−0.13, −0.06) | 0.40 (0.29, 0.51) | 0.02 (0.01, 0.03) |
| Marshfield clinic | −0.69 (−1.02, −0.37) | −0.01 (−0.22, 0.20) | −1.06 (−1.40, −0.71) | −1.00 (−1.24, −0.77) | −0.15 (−0.18, −0.11) | −0.18 (−0.29, −0.06) | −0.05 (−0.07, −0.04) |
| Middlesex health system | 0.80 (0.60, 1.00) | 0.64 (0.50, 0.78) | −0.17 (−0.38, 0.04) | 0.24 (0.09, 0.38) | −0.08 (−0.11, −0.06) | 0.36 (0.32, 0.41) | 0.05 (0.04, 0.06) |
| Park Nicollet clinic | −0.40 (−0.67, −0.14) | 0.15 (−0.05, 0.34) | 0.27 (−0.08, 0.61) | 0.63 (0.39, 0.86) | −0.16 (−0.20, −0.12) | −0.06 (−0.13, 0.01) | −0.01 (−0.03, 0.00) |
| St. John's clinic | −1.56 (−1.97, −1.14) | 0.09 (−0.27, 0.44) | −2.65 (−3.21, −2.09) | −1.35 (−1.74, −0.95) | 0.09 (0.03, 0.15) | −0.41 (−0.52, −0.31) | −0.09 (−0.11, −0.06) |
| University of Michigan Faculty group practic | −1.39 (−1.71, −1.07) | 0.04 (−0.05, 0.13) | −1.23 (−1.51, −0.95) | −0.23 (−0.40, −0.07) | −0.18 (−0.21, −0.15) | 0.00 (−0.07, 0.06) | −0.10 (−0.11, −0.09) |
PGPD=Physician Group Practice Demonstration, CI=Confidence Interval. Notes: Estimates derived from a linear model adjusting for area-year indicators, age, black race, woman, Medicaid eligibility, disability, cancer type, and indicator for metastatic cancer. The model adjusts for zip code-level rates of poverty and high income. The model adjusts for the rate of low-variation conditions for each of the 10 local areas for each year separately for treatment and control groups. Low-variation condition rate is the number of individuals experiencing the conditions hip fracture, stroke, and acute myocardial infarction per thousand Medicare beneficiaries.
Model is weighted using only county weights (not follow up time which incorporates deaths).
Table 3 displays regression results from non-spending outcome models, which again varied considerably across sites. The Physician Group Practice Demonstration was associated with a signifi-cant reduction in mortality of 0.65 percentage points among cancer patients, a 5.6% reduction. Reductions in mortality in three sites were large: Everett Clinic, St. John's Clinic, and the University of Michigan Faculty Group Practice. There was no significant change in in-hospital death across all sites. However, two sites significantly reduced death in hospital (Billings Clinic and Dartmouth-Hitchcock Clinic) while other sites had an increase in in-hospital death associated with the Demonstration. There was a reduction in hospice use associated with the Demonstration, mainly focused in Everett Clinic, St. John's Clinic, and the University of Michigan Faculty Group Practice. Across all sites, there was a significant reduction in ICU days of 0.08, but no significant reduction in days in the hospital.
A major limitation of our analysis is that we use a prevalent rather than an incident cancer cohort. While an incident cohort would allow for a more homogenous set of patients facing similar decisions, the sample size in participant organizations would be very small and we would only be able to capture initial treatment decisions, rather than the broad spectrum of cancer care (which, importantly, includes end-of-life care). The reliance on a prevalent cohort means that in our overall spending outcomes, we are capturing a large amount of non-cancer care in addition to active cancer treatment. We completed two sensitivity analyses to test for changes in care for those actively being treated for cancer. We first examined results in a cohort of just those beneficiaries with cancer-specific spending in a given year. In this group, annual perbeneficiary savings associated with the PGP were $844 (standard error: $409), concentrated entirely in acute care. In this cohort, the Demonstration was associated with a negative, non-significant effect on mortality. Second, we examined results in a cohort restricted to those with an indication of metastatic cancer in a given year. In this group, annual per-beneficiary savings associated with the PGP were $2009 (standard error: $442), with acute care savings of $1647 annually (standard error: $286). In the metastatic cohort, the Demonstration was associated with a negative, nonsignificant effect on mortality.
In addition, we wanted to ensure that the prevalent cohorts were similar in the participants and controls. For example, if the participants were diagnosing cancer earlier, this would result in healthier cohorts of cancer patients that may have lower spending or mortality outcomes. We find that the proportion of our prevalent cohort with payments for cancer-specific care in a given year is 28% in cases and 27% in controls, indicating that coding is similar across areas for cancer that is not actively being treated.
4. Conclusions
Our study finds that the Physician Group Practice Demonstration, the best evidence to date on the possible effect of ACO payment reforms, was associated with a significant reduction in Medicare spending on cancer patients of $721 annually per bene-ficiary. The spending reduction was concentrated in acute care payments, and this reduction in acute care spending mirrors findings from another high-risk, high-cost population, those dually eligible for Medicare and Medicaid.8 Significant decreases in hospital discharges indicate there were changes made in utilization patterns. The Demonstration did not reduce the number of deaths occurring in the acute care hospital, did not increase referral to hospice, and did not increase the number of days in hospice care. The Demonstration was associated with a small decrease in the number of ICU days and a reduction in the hospitalization rate. Additionally, there was an improvement in mortality.
Despite the overall spending reduction and reduction in acute care payments, the Demonstration was also associated with small increases in cancer-specific spending (procedures and chemotherapy). Interestingly, the University of Michigan Faculty Group Practice, which had the largest reductions in overall acute care payments associated with the Demonstration, had the largest increases in cancer-related treatment payments. As in the effect of the Demonstration on spending across all Medicare benefici-aries, the effect of the Demonstration on Medicare payments for cancer beneficiaries varied greatly across the 10 participating sites.
This study is the first to examine how a broad Medicare payment reform might specifically impact cancer patients. The study also has a few limitations worth noting. First, the Physician Group Practice Demonstration only included 10 participating sites, and these sites were selected in a competitive process. Thus, it is unclear how these sites may differ from others on characteristics not observed in this study. However, the sites that participated in the Physician Group Practice Demonstration are likely similar to the sites applying for and being selected for the new Medicare ACO programs. Thus, our results are likely relevant for understanding how Medicare ACO programs may impact cancer care. In addition, results were highly variable across sites, possibly because of relatively small sample sizes; as a result, site-specific conclusions should be interpreted with caution. Finally, we have very limited insight into the quality of care. We offer mortality and claims-based utilization measures for use of the hospital and hospice, but we do not have clinical or process outcome measures, such as guideline adherence. Our mortality models are limited by the information available in administrative claims: we are only able to control for metastatic stage, and changes in coding or detection of cancer under the Demonstration may have changed the composition of the cohort relative to the controls.
Improving value in the health care system, and in particular in cancer care, is a major goal of payment reform. Earlier work showed that the PGPD modestly reduced spending for Medicare beneficiaries, but significantly reduced spending in higher-cost groups.8 This study mirrors those findings. Our results suggest that while the PGPD changed inpatient admission policies for cancer patients, it was not associated with reductions in spending on cancer-specific treatments. This could be viewed optimistically: payment reform was not associated with stinting on cancer-specific treatment. Alternatively, it may be disappointing that the PGPD did not induce changes in discretionary services (such as imaging), which are both expensive and common in cancer treatment. Admission policies play an important role in current cancer costs, constituting about a third of spending; however, as more expensive chemotherapy agents and new procedures are introduced to the market, payments for inpatient care may be dwarfed by spending on cancer treatments. Indeed, in the latter years of the Demonstration many new chemotherapy agents were introduced, and this may be the reason why average spending in PGPD participants again rose above the control group starting in 2008.
ACOs have the potential to align incentives that could support a variety of value-based approaches to cancer care. These approaches might include encouraging physicians to consider patient preferences and value when weighing possible treatments, implementing evidence-based treatments centered on guidelines, and discouraging overuse of imaging or expensive chemotherapy agents with suitable substitutes (see for example Memorial Sloan-Kettering's decision to rule out use of Zaltrap).15 In addition, ACO incentives align with other efforts that encourage physicians and patients to carefully consider if test and treatment options are appropriate in each individual case, such as the Choosing Wisely recommendations.16,17 Finally, moving away from fee-for-service may allow physicians to take more time to communicate with patients about the benefits and risks of treatment options, since under fee-for-service this time is often not reimbursed.
While it is difficult or impossible to measure changes in these approaches to cancer care using administrative claims, our results on cancer-specific spending suggest that changes were not made in treatment decisions for cancer care. Changes in cancer treatment are difficult to implement: Smith and Hillner recognize that the few changes they suggest to cancer care will cause “discomfort and adjustments” and that “changing practice will not be easy.”16 Value-based approaches to cancer care take time to implement on a broad scale. In addition, cancer care is almost entirely in the domain of specialists. The Demonstration was short and perhaps not long enough for organizations to make structural changes in their approach to the highly specialized field of cancer care. If improving value in cancer care is a process that takes time, it is possible the reduction in inpatient admissions we observe is a first step on this path.
These findings have implications for how policymakers and insurers design ACO programs and for how ACO provider organizations respond to payment reform. A focus on high-cost, high-risk groups such as cancer patients is important for success of the reforms. Oncologists and others providing cancer care will play an important role in decisions that affect spending. Under Medicare's ACO programs, oncologists may be part of or partner with ACOs to provide high quality, specialized cancer care. The commercial health insurance sector has been active in pursuing innovative payment methodologies for oncology services. For example, cancer-specific ACO provider groups have formed partnerships with payers18 and bundled payment programs for cancer care are also being piloted.19 These disease-specific ACOs and programs may not be necessary if a more holistic ACO approach can adequately care for specialized patients' needs and decrease spending growth. This study shows that in the PGPD, providers were able to reduce spending on acute care for cancer patients, but did not reduce treatment-related spending. Costs of cancer care make up nearly 10% of Medicare spending, so focusing on care for cancer patients could represent a large potential pool of savings for Medicare ACOs if providers are able to implement value-based approaches to care.
Acknowledgements
Supported by National Institutes on Aging Grant Nos. P01AG19783 and R21 AG044251, and a Norris Cotton Cancer Center Prouty Grant.
Footnotes
For the control group, all analyses are weighted to reflect the population with cancer from each contributing county in the participant group. Beneficiaries who die or age into Medicare during the year are weighted according to the person-months they contribute for analyses of spending and utilization.
References
- 1.Potetz L, DeWilde LF. Cancer and medicare: a chartbook. 2009 Feb; 〈 http://www.allhealth.org/briefingmaterials/CancerandMedicareChartbookFinalfulldocumentMarch11-1412.pdf 〉 .
- 2.Balogh E, Patlak M, Nass SJ, Institute of Medicine . Delivering affordable cancer care in the 21st century. The National Academies Press; 2013. Workshop Summary. [PubMed] [Google Scholar]
- 3.McGinnis T, Small DM. Accountable care organizations in medicaid: emerging practices to guide program design. Center for Health Care Strategies, Inc.; Hamilton, NJ: 2012. [Google Scholar]
- 4.Miller RH, Luft HS. HMO plan performance update: an analysis of the literature, 1997–2001. Health Affairs. 2002;21:63–86. doi: 10.1377/hlthaff.21.4.63. [DOI] [PubMed] [Google Scholar]
- 5.Ubl S. Affairs H, editor. ACOs: improved care or roadblocks to innovation? 〈 http://healthaffairs.org/blog/2011/04/25/acos-improved-care-or-roadblocks-to-innovation/〉; 2011 Accessed 25.04.11.
- 6.Berenson RA, Burton RA. Accountable care organizations in medicare and the private sector: a status update. Urban Institute; Nov, 2011. 〈 http://www.healthreformgps.org/wp-content/uploads/ACO-in-Medicare-RWJC-Urban.pdf 〉 . [Google Scholar]
- 7.Department of Health and Human Services, Centers for Medicare & Medicaid Services Medicare Program; Medicare shared savings program: accountable care organizations, final rule. 2011. Accessed 02.11.11.
- 8.Colla CH, Wennberg D, Meara ER, et al. Spending differences associated with the Medicare Physician Group Practice Demonstration. JAMA: Journal of the American Medical Association. 2012;308:1015–1023. doi: 10.1001/2012.jama.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services Medicare Physician Group Practice Demonstration Fact Sheet. 2011 Jul; 〈 http://www.cms.gov/DemoProjectsEvalRpts/downloads/PGP_Fact_Sheet.pdf 〉 .
- 10.Kautter J, Pope GC, Trisolini M, Grund S. Medicare Physician Group Practice Demonstration design: quality and efficiency pay-for-performance. Health Care Financing Review. 2007;29:15–29. [PMC free article] [PubMed] [Google Scholar]
- 11.United States Census . U.S. Census Bureau; Washington, DC: 2000. [Google Scholar]
- 12.Huskamp HA, Newhouse JP. Is health spending slowing down? Health Affairs. 1994;13:32–38. doi: 10.1377/hlthaff.13.5.32. [DOI] [PubMed] [Google Scholar]
- 13.The World Bank World development indicators, GDP deflator. 2011 〈 http://data.worldbank.org/indicator/NY.GDP.DEFL.KD.ZG 〉 .
- 14.Cameron AC, Gelbach JB, Miller DL. Robust inference with multiway clustering. Journal of Business and Economic Statistics. 2011;29:238–249. [Google Scholar]
- 15.Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times; New York, NY: 2012. [Google Scholar]
- 16.Smith TJ, Hillner BE. Bending the cost curve in cancer care. The New England Journal of Medicine. 2011;364:2060–2065. doi: 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Society of Clinical Oncology Choosing wisely. 2012 〈 http://www.choosingwisely.org/doctor-patient-lists/american-society-of-clinical-oncology/ 〉 .
- 18.Kluding P. Florida Blue And Moffitt Cancer Center create cancer-specific accountable care arrangement. Dec 20, 2012.
- 19.Burns J. UnitedHealthcare's bold effort to deal with cancer drug costs. Managed Care. 2011 Jan; [PubMed] [Google Scholar]