Summary
The transcription factor SOX2 is widely known to play a critical role in the central nervous system; however, its role in peripheral neurogenesis remains poorly understood. We recently developed an hESC-based model in which migratory cells undergo epithelial to mesenchymal transition (EMT) to acquire properties of neural crest (NC) cells. In this model, we found that migratory NC progenitors downregulate SOX2, but then start re-expressing SOX2 as they differentiate to form neurogenic dorsal root ganglion (DRG)- like clusters. SOX2 downregulation was sufficient to induce EMT and resulted in massive apoptosis when neuronal differentiation was induced. In vivo, downregulation of SOX2 in chick and mouse NC cells significantly reduced the numbers of neurons within DRG. We found that SOX2 binds directly to NGN1 and MASH1 promoters and is required for their expression. Our data suggest that SOX2 plays a key role for NGN1-dependent acquisition of neuronal fates in sensory ganglia.
Introduction
The SRY (sex-determining region)-box 2 (SOX2) gene encodes a transcription factor required for maintenance of the pluripotent state of human embryonic stem cells (hESCs) (Adachi et al., 2010). Because a morphologically recognizable neural plate expresses SOX2, it is considered one of the earliest functional markers of neuroectodermal specification (Rex et al., 1997). The role of SOX2 in central nervous system (CNS) development and adult neurogenesis has been extensively investigated (Cavallaro et al., 2008; Ferri et al., 2004; Graham et al., 2003; Miyagi et al., 2008). However, its function in the peripheral nervous system (PNS) is less studied. At E12.5, prospective satellite glial cells and Schwann cell precursors express SOX2, where it blocks myelination and terminal differentiation; SOX2 is downregulated in both of the mature glial cell lineages (Le et al., 2005). NC delamination from dorsal neuroepithelium is a classic example of epithelial-mesenchymal transition (EMT) (Meulemans and Bronner-Fraser, 2004). SOX2 is downregulated in delaminating dorsal neuroepithelial cells (migratory NC), and its enforced expression in the avian prospective NC impedes EMT and the acquisition of NC fates (Wakamatsu et al., 2004; Wegner and Stolt, 2005). However, migratory NC cells transiently re-express SOX2 when they reach the dorsal root ganglia (DRG) (Wakamatsu et al., 2000). The DRG neurons are formed in three successive overlapping waves. Ngn2 initiates the first wave and Ngn1 initiates the second (Ma et al., 1999). The Ngn2-dependent wave mainly gives rise to the small clusters of TrkB+ and TrkC+ neurons (mechanoreceptors and proprioceptors), whereas Ngn1 dependent wave gives rise to the large clusters of TrkA+ neurons (peptidergic and nonpeptidergic neurons) as well as peripheral glia (Marmigère and Ernfors, 2007). The third wave of neurogenesis arises from the boundary cap cells and contributes to TrkA+ neurons and glia (Marmigère and Ernfors, 2007). We have previously described a rapid and efficient protocol for differentiation of hESC into NC lineages, including sensory and autonomic neurons, Schwann cells, smooth muscle cells, melanocytes, and cartilage (Curchoe et al., 2010). Here we used hESCs as an in vitro model of human NC cells. In agreement with previous studies, we observed that SOX2 is a strong inhibitor of EMT and human NC delamination. We showed that SOX2 downregulation is sufficient to induce EMT and acquisition of migratory phenotypes in culture. Surprisingly, re-expression of SOX2 in NC cells is essential for generation of peripheral neurons, likely through its direct as well as indirect modulation of proneural gene expression.
Results
Human ESC-Derived Model of Neural Crest
The neuroepithelial cells were derived from hESCs as previously described (Bajpai et al., 2009; Cimadamore et al., 2009) followed by the NC cultures (Curchoe et al., 2010). Analysis of neuroepithelial cells demonstrated the expression of NESTIN, MUSASHI1, PAX6, and SOX2 (Figures 1A-1C) as well as markers of dorsal neuroepithelium, such as PAX3 and SOX9 (Figures 1E and 1F). Adherent neuroepithelial clusters were also positive for the NC marker p75NTR (Figure 1D), which is expressed in the human neural tube (Thomas et al., 2008). Little or no expression of ventral neural tube markers NKX2.2 and HNF3b (Figures 1G and 1H) was seen. The addition of a Sonic Hedgehog (SHH) agonist (Purmorphamine) ventralized the cultures, decreased the expression of dorsal markers (GDF7, SOX9, and SLUG), and increased the expression of ventral markers (HNF3b, NKX2.2, and SHH) (Figure 1I). These data are consistent with the dorsal identity of hESC-derived neuroepithelium (dNE), which harbors premigratory neural crest cells. We propose to consider hESC-derived dNE clusters as a surrogate model for the EMT transition from dNE to migratory NC (Figure 1J). Indeed, when hES-derived neurospheres were plated on fibronectin (FN)—a permissive substrate for neural crest (NC) migration (Henderson and Copp, 1997)—a migratory cell population emerged (Figure 1K), which was positive for SOX10 (Figure 1L), a classic marker of migratory NC cells (Paratore et al., 2001). Compared with cells in neuroepithelial clusters, we found that migratory NC cells strongly downregulated the neural adhesion molecule N-CADHERIN (N-CAD) (Akitaya and Bronner-Fraser, 1992) (Figure 1M) and lost nuclear expression of SOX2 (Figure 1N), consistent with observations in chick embryos (Wakamatsu et al., 2004). Furthermore, while the spheres were positive for the CNS marker PAX6, we observed the loss of PAX6 in the migratory cells and an acquisition of the NC marker Integrin-a4 (Figure 1O). Integrin-a4 is expressed in migrating cells and in DRG (Kil et al., 1998) and is a classic cellular receptor for fibronectin (Mould et al., 1994). Thus the expression patterns of SOX2, SOX10, N-CAD, PAX6, and INT-a4 allowed us to delineate the dNE clusters from the migratory NC cells, suggesting that hESC-based cultures faithfully reproduce early events of NC delamination from dNE. Flow cytometry revealed _5% of cells positive for INT-a4 in the early cultures (Figure 1P) and up to 80% afterward (Figure 1Q). These results suggest that under the present culture conditions, the original CNS identity of primary dNE cells was rapidly lost in favor of the NC identity. In agreement with the strong upregulation of INT-a4, the CNS marker PAX6 was almost completely absent in NC cells from the first passage on (see Figure S1D available online). The INT-a4 expression remained approximately at the same level for at least six passages (Figures S1A and S1B) along with NESTIN expression (Figure S1C). We conclude that under current culture conditions, the dNE cells rapidly emigrate from clusters and acquire characteristics of NC cells.
Figure 1. Dorsal Identity of hESC-Derived Neuroepithelium.
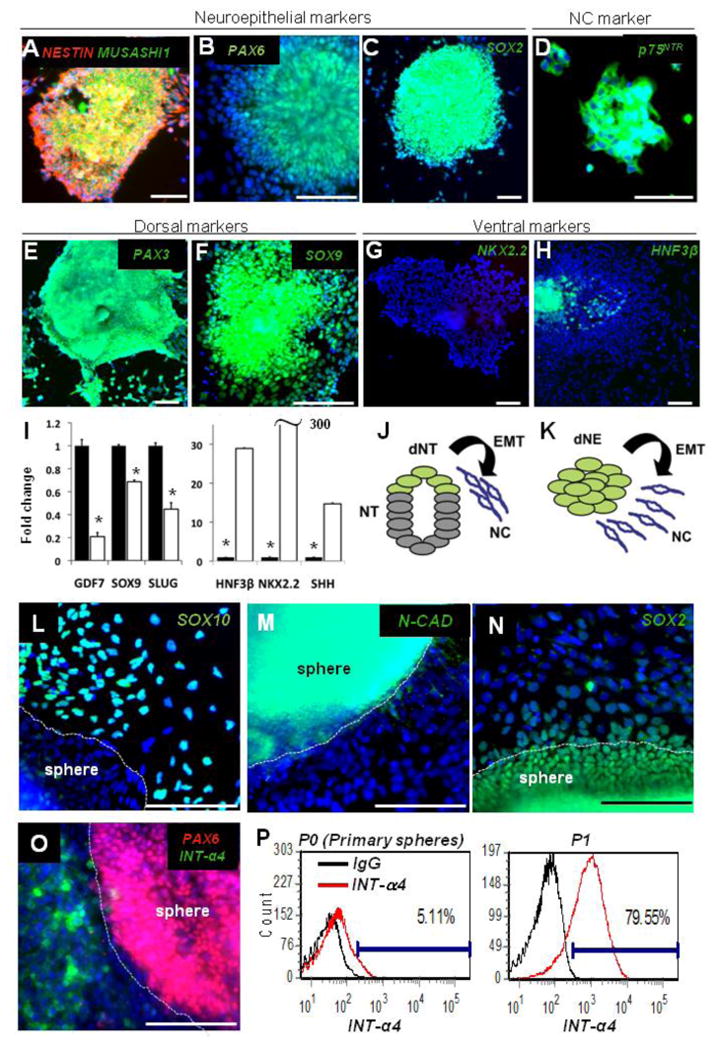
(A-H) Neural clusters at day 6 of neural induction express NESTIN, MUSASHI1 (A), PAX6 (B), SOX2 (C), p75 (D), and dorsal markers PAX3 (E) and SOX9 (F) and lack the ventral marker NKX2.2 (G) and the floor plate marker HNF3b (H).
(I) Quantitative PCR of dorsal (GDF7, SOX9, SLUG) and ventral (HNF3b, NKX2.2, SHH) markers in hESCs neuralized for 6 days without (black bars, set to 1) or with 10 mM of Purmorphamine (open bars). All values were normalized to 18S expression; *p < 0.005.
(J and K) Schematic representation of NC delamination from the dorsal neural tube (J) and a model of human NC delamination from hESC-derived dNE clusters (K). NT, neural tube; dNT, dorsal neural tube; dNE, dorsal neuroepithelium; EMT, epithelial to mesenchymal transition; NC, Neural Crest.
(L–O) Cells emigrating from human dNE clusters acquire SOX10 (L) and lose N-CAD (M) and SOX2 (N). Cytoplasmic staining in (N) is a nonspecific staining. Expression of NC marker Integrin-a4 (INT-a4) and the absence of the CNS marker PAX6 separate human NC and dNE clusters (O).
(P and Q) Flow cytometry analysis of INT-a4 showing the switch from CNS to NC identity when primary spheres (P) were cultured for one passage on FN-coated plates (Q). Normal mouse IgG was used as a negative control; scale bars, 100 mm; blue, Hoechst. Error bars ± SE.
SOX2 Downregulation Induces EMT
Forced expression of SOX2 blocks EMT and inhibits NC delamination and NC migration in chick and quail embryos (Wakamatsu et al., 2004), thus overriding the activity of Bone Morphogenetic Protein 4 (BMP4), a well-known NC inducer (Sela-Donenfeld and Kalcheim, 1999; Shoval et al., 2007). We investigated whether downregulation of SOX2 (loss of function) is sufficient to induce EMT using the ESC-based model of human NC. Human ESCs were stably transduced with lentivirus carrying the inducible SOX2 shRNA and the efficiency of SOX2 downregulation upon addition of dox was verified (75% reduction; Figures S2A and S2B). The specificity of knockdown was verified by assessing the transcript levels of other SOXB1 members (i.e., SOX1 and SOX3) (Figure S2C). Human ESCs stably transduced with inducible SOX2 shRNA-expressing lentivirus were neuralized for 5 days and cells were allowed to emigrate from dNE neurospheres for 4 days in the presence of dox. SOX2 downregulation significantly enhanced cell migration when compared to control cells carrying inducible scrambled shRNA (Figures 2A– 2C), suggesting that SOX2 downregulation in dNE cells in the absence of exogenous BMP4 is sufficient to induce migratory activity.
Figure 2. SOX2 Counteracts Epithelial Mesenchymal Transition.
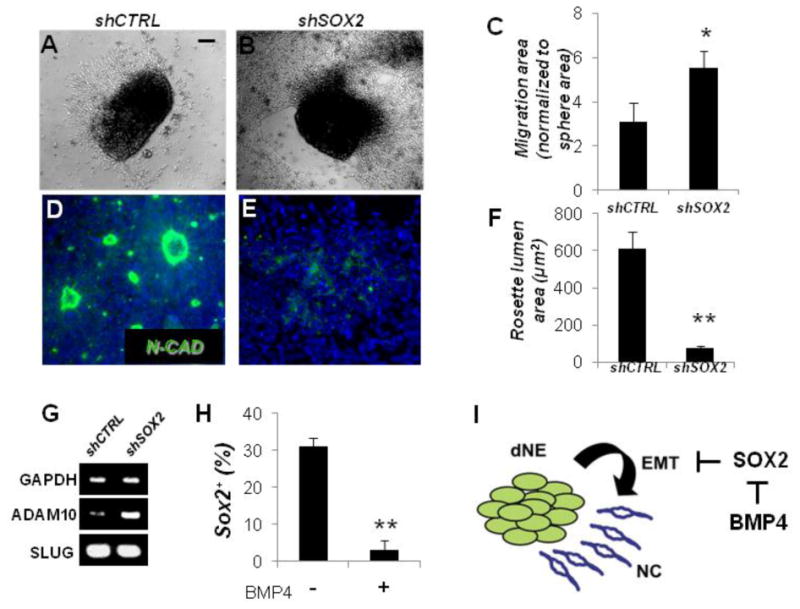
(A and B) Bright field images of neurospheres carrying dox-inducible control scrambled (shCTRL, [A]) or SOX2 shRNA (shSOX2, [B]). Spheres were plated on laminin/polyornithine and allowed to migrate for 4 days in the presence of dox (1 mg/ml).
(C) Quantification of migration in conditions shown in (A) and (B). Migration area for each sphere was normalized to the sphere size.
(D–F) N-CAD staining showing rosette-epithelial organization in the center of neurospheres flattened for 4 days on lamin/polyornithine. shCTRL (D) and shSOX2 (E) neurospheres plated in the presence of dox; quantified in (F).
(G) RT-PCR analysis of SLUG and ADAM10 in dNE cells expressing scrambled control and SOX2 shRNA.
(H) The percentage of SOX2+ cells after 4 days in the presence or the absence of 100 ng/ml BMP4.
(I) A model proposing the role of SOX2 in delamination of dorsal neuroepithelial cells. Scale bars, 100 mm; Hoechst nuclear dye (Blue) was used to stain cell nuclei; *p < 0.05; **p < 0.005. Error bars ± SE.
We observed that dNE cells within the clusters tend to organize into N-CAD+ neuroepithelial rosettes (Figure 2D). The neuroepithelial rosette cytoarchitecture was almost completely disrupted and N-CAD staining was largely eliminated following SOX2 knockdown (Figures 2D–2F). We also investigated whether SOX2 downregulation altered expression of SLUG and ADAM10, two well-established inducers of EMT in neural crest cells (Carl et al., 1999; Shoval et al., 2007). As shown in Figure 2G, RT-PCR analysis showed that SLUG expression remained unchanged and was abundant in cells treated with either SOX2 shRNA or scrambled control shRNA. SLUG expression in vivo is required but not sufficient for EMT in dNE cells, since not all SLUG-expressing dNE cells migrate (Linker et al., 2000). Thus, our results suggest that hES-derived dNE cells are competent for EMT and that SOX2 downregulation does not alter SLUG expression. The ADAM10 metalloprotease can enhance migration in dNE cells via a mechanism involving N-CAD cleavage and internalization that, in turn, results in loss of intercellular adhesion and enhanced Wnt signaling (Shoval et al., 2007). In contrast to SLUG, ADAM10 expression was upregulated upon SOX2 knockdown (Figure 2G), consistent with the role of ADAM10 in promoting NC delamination. We also investigated whether the NC inducer BMP4 alters the level of SOX2 expression in human dNE cells. To this end, we seeded dNE cells in the presence of BMP4 and determined the percentage of cells positive for nuclear SOX2 after 4 days of treatment (Figure 2H). BMP4 treatment efficiently downregulated SOX2 expression when compared to untreated cells, indicating that SOX2 is downstream of BMP signaling during early phases of NC specification (Figure 2I). Our results suggest that SOX2 downregulation is sufficient to initiate delamination from dNE clusters and that BMP4 may induce NC delamination, at least in part, through the downregulation of SOX2.
NC Cells Re-Express SOX2 within DRG-Like Secondary Clusters
Although SOX2 expression antagonizes EMT and NC delamination, it is re-expressed in nascent chicken DRG (Wakamatsu et al., 2000) along with N-CAD (Akitaya and Bronner-Fraser, 1992). We found that SOX2 and N-CAD are reacquired in nascent embryonic day 10 (E10) murine DRG (Figures 3A and 3B) along with SOX10 (Figure S3A). In mouse and rat DRG, SOX10 marks multipotent cells able to give rise to both glia and neurons (Kim et al., 2003; Montelius et al., 2007). These observations suggest that gangliogenesis involves upregulation of SOX2 and N-CAD by migratory NC cells coalescing into ganglia (Figure 3C). We asked whether hESC-NC cells could re-express SOX2 and N-CAD. We cultured single cell dNE clusters in the presence of BMP4 for 4 days and obtained near homogeneous SOX2- population (Figure 3D, left panel). Exposure to Leukemia Inhibitory Factor (LIF), shown to promote Mesenchymal to Epithelial Transition (MET) in the rat metanephros (Barasch et al., 1999) resulted in SOX2+ clusters (Figure 3D, right panel). We have also used a modified collagen invasion assay (Voroteliak et al., 2002). Single dNE cells seeded on top of 1% collagen gel were allowed to migrate through the gel and the cells adhered to the bottom of the well were assessed for SOX2/N-CAD expression (Figures 3E–3G). Many cells reacquired both SOX2 and N-CAD expression and formed tight clusters surrounded by N-CAD-, SOX2- cells (Figures 3E and 3F). hESC-NC cultured in the presence of EGF (see Experimental Procedures) routinely yielded a similar clear segregation between migratory cells and secondary clusters forming polarized neuroepithelial rosettes (Figure 3H) or tight clusters (Figure 3I) surrounded by the SOX2- cells. These clusters were positive for Integrin-a4 (Figure 1Q and data not shown) and SOX10 (Figure S3) and negative for the human CNS marker PAX6 (Figure 4F). Taken together, these results suggest that secondary clusters routinely observed in monolayer cultures of hESC-NC cells after single cell dissociation of primary dNE clusters resembles the in vivo ganglia (DRG) with respect to their expression of SOX2, N-CAD, Integrin-a4, SOX10, and PAX6.
Figure 3. hESC-Derived NC Cells Form DRG-Like Clusters.
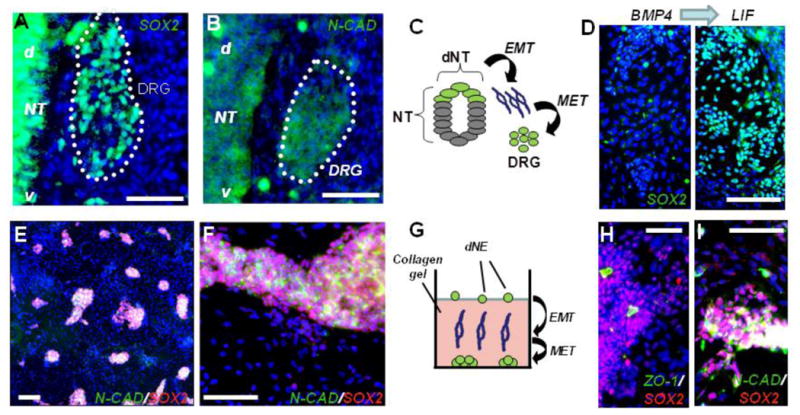
(A–C) Migratory NC cells reacquire both SOX2 (A) and N-CAD expression (B) after coalescing into embryonic day 10 (E10) dorsal root ganglia (DRG); (d) and (v) denote the dorsal-ventral orientation of the neural tube schematically illustrated (C).
(D) dNE cells reacquire SOX2 expression following LIF treatment. Left panel shows SOX2 staining in cells treated for 4 days with 100 ng/ml BMP4. 6.69 ± 1.25% of total cells were SOX2+. Four days of treatment with 100 ng/ml LIF (right panel) resulting in formation of numerous SOX2+ clusters with 34.15 ± 3.1% of total cells expressing SOX2.
(E and F) hES-derived migratory neural crest cells reform clusters after migration through the collagen gel; low (E) and high (F) magnifications.
(G) Schematic of human dNE migrating through collagen to form colonies at the bottom of the plate.
(H and I) Clusters of cultured dNE cells. SOX2+ cells are found in the polarized epithelial rosettes identified by the apical accumulation of clusters positive for the tight junction protein ZO-1 (H) and N-CAD (I). Scale bar, 100 mm; blue, Hoechst dye. Error bars ± SE.
Figure 4. SOX2 Expression is Associated with Peripheral Neuronal Differentiation.
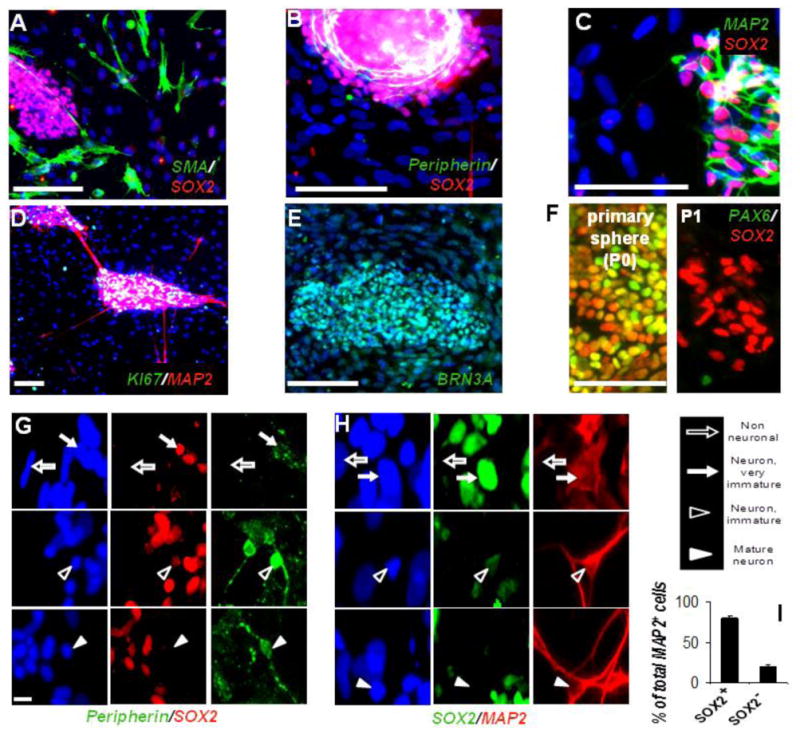
(A-C) Spontaneous differentiation in SMA+ smooth muscle cells was always seen outside of SOX2+ clusters (B and C). SOX2+ aggregates are associated with Peripherin and MAP2.
(D and E) Secondary neurogenic clusters immunostained for MAP2 and Ki67 (D) and sensory neuronal marker BRN3A (E).
(F) SOX2 and PAX6 coexpression in dNE cells (primary spheres [P0], left panel). NC cells in secondary neurogenic clusters are SOX2+ PAX6_ (passage 1 [P1], right panel).
(G and H) SOX2 is expressed in cells lacking neuronal morphology and weakly positive for Peripherin or MAP2 (solid arrows); neurons with mature morphology and strong MAP2, Peripherin staining are weak positive / negative for SOX2 (open and solid arrowheads, respectively).
(I) Quantification of MAP2/SOX2 colabeling from the experiment illustrated in (H). In all images blue, Hoechst dye. Scale bars: (A–F) 100 mm, and (G–H) 10 mm; **p < 0.005. Error bars ± SE.
SOX2 Expression is Associated with Neuronal Markers
Upon spontaneous differentiation of NC cultures, smooth muscle actin (SMA)+ cells were always found among the mesenchymal SOX2- cells (Figure 4A), whereas Peripherin or the pan-neuronal marker Microtubule Associated Protein-2 (MAP2) were strictly associated with the SOX2+ aggregates (Figures 4B and 4C). Reminiscent of proliferating neurogenic DRG in vivo (Kahane and Kalcheim, 1998; Wakamatsu et al., 2000) and in accordance with the well-known role of SOX2 in proliferation (Ferri et al., 2004), cells in secondary neurogenic clusters actively proliferated (Figure 4D), whereas surrounding mesenchymal cells were mostly postmitotic (Figure 4D). The vast majority of the cells were positive for BRN3A (Figure 4E), a marker for both early migrating neural crest and sensory neurons (Fedtsova and Turner, 1995). In sharp contrast to cells found in primary spheres, SOX2+ cells in secondary neurogenic clusters did not coexpress the CNS marker PAX6 (Figure 4F), therefore suggesting their peripheral identity. High-resolution imaging showed that weakly MAP2+ cells were strongly SOX2+, whereas some MAP2+ cells with mature neuronal morphology were often weakly positive for SOX2 (Figure 4G). Similar results were obtained for SOX2 and Peripherin coexpression (Figure 4H). Approximately 80% of MAP2+ cells were also positive (strongly or weakly) for SOX2 under this condition (Figure 4I). We investigated if SOX2 was coexpressed with neuronal markers in vivo. Confocal analysis of E11 DRG showed clearly detectable levels of Sox2 coexpressed with an early neuronal marker, TuJ1, in the same cells (Figure S4). We also found that in vivo, SOX2 tends to be coexpressed with SOX10 in E10 DRG neural progenitors (Figure S3A). Similarly, SOX2+ cells in secondary hESC-derived neurogenic clusters also showed SOX10 expression (Figure S3B). Taken together, these data suggest that SOX2 expression is associated with peripheral neurogenesis and provide evidence that secondary hESC-derived neurogenic clusters resemble embryonic DRG.
SOX2 is Required for Sensory Neurogenesis in Human ESC-Derived NC Cultures
Culturing of hESC-NC cells with LIF for 7 days enriches for MAP2+ and Peripherin+ cells and depletes SMA+ and GFAP+ cells from the cultures. Addition of BMP4 had the opposite effect (Figures 5A and 5B). These results suggest that the enrichment for neuronal outcomes correlates with the levels of SOX2 (Figure 3D), consistent with our hypothesis that SOX2 is required for neurogenesis, but not for glial or smooth muscle cell differentiation. Next we engineered hESC lines harboring SOX2-specific inducible shRNA and investigated the differentiation outcomes under neurogenic, myogenic, or gliogenic conditions upon downregulation of SOX2. The differentiation of NC cells under neurogenic conditions (see Experimental Procedures) results in a high proportion of cells expressing neuronal markers (Figures 5C and 5D, upper panels). The downregulation of SOX2 under these conditions resulted in dramatic loss of Peripherin+ and MAP2+ cells when compared to control scrambled shRNA (Figures 5C and 5D, lower panels). Immunodetection of the active form of Caspase3 (AC3) revealed extensive cell death upon SOX2 knockdown (Figure 5E). To determine the time when SOX2 function is required during neuronal differentiation, we knocked down SOX2 expression at various time points after the onset of neuronal differentiation, but before any mature Peripherin+ neurons can be detected (Figure S5). SOX2 downregulation at day 3 following neuronal induction dramatically impaired neuronal differentiation, whereas SOX2 downregulation at day 7 had no significant effect on the total number of Peripherin+ cells detected at day 14 (Figure S5). These data suggest that SOX2 plays a critical role during the early commitment to the peripheral neuronal fates. We identified gliogenic culture conditions yielding almost exclusively P0+/GFAP+ double-positive putative early Schwann cells (see Experimental Procedures). SOX2 knockdown under such conditions had no significant effect on the number of differentiated P0+/GFAP+ cells (Figure 5F and data not shown for GFAP). In sharp contrast to neurogenic culture conditions, we did not observe increased cell death upon downregulation of SOX2 under gliogenic conditions (data not shown). Next we investigated the effect of SOX2 knockdown under differentiation conditions yielding mixed myogenic/neurogenic or gliogenic/neurogenic cultures (see Experimental Procedures). SOX2 downregulation under mixed conditions significantly increased the proportion of SMA+ cells (Figure 5G) and P0+/GFAP+ cells (Figure 5H and data not shown for GFAP expression). In both cases, the observed increase in SMA+ and P0+ cells happened at the expense of neuronal differentiation (data not shown). We engineered dNE cells carrying the dox-inducible SOX2 shRNA (targeting the mRNA 30UTR) with lentivirus expressing a SOX2-GFP fusion protein (which lacks the 30UTR and therefore is not affected by SOX2 shRNA). Enforced expression of SOX2-GFP, but not GFP alone, rescued neuronal differentiation as assessed by staining for the early neuronal marker Tuj1 (Figure 5I). Expression of a more mature marker MAP2 was not observed, suggesting that SOX2 may promote early, but not late, phases of neuronal commitment. This is consistent with the previous reports in chick embryos, where the overexpression of SOX2 blocked differentiation of neural precursors into mature neurons (Graham et al., 2003). Taken together, these results suggest that in hESC-NC cells, SOX2 function is critical at the early stages of neuronal differentiation, but dispensable for the differentiation into SMA+ and P0+ cells.
Figure 5. SOX2 is Required for Peripheral Neuronal Differentiation.
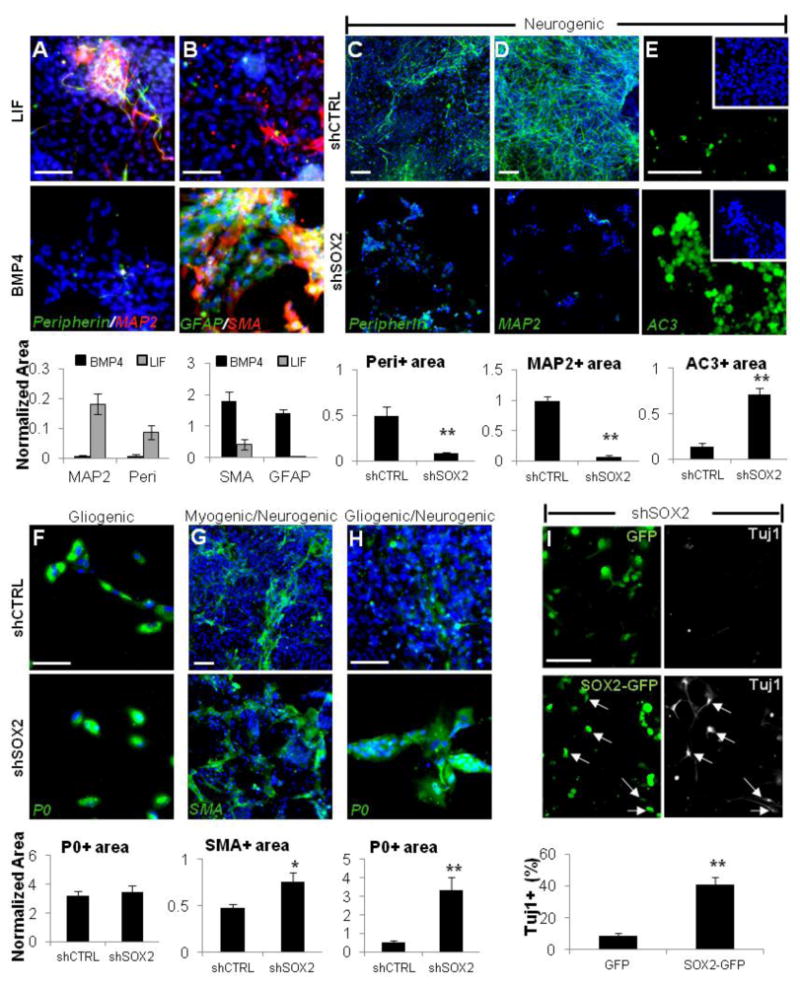
(A and B) hESC-NC cultured with LIF (upper row) are enriched for Peripherin and MAP2; the addition of BMP4 (lower row) enriches for the smooth muscle (SMA) and glial (GFAP) markers. (C and D) Under the neurogenic conditions, differentiation into Peripherin- (C) and MAP2- (D) positive neurons is abolished when SOX2 expression is downregulated using dox-inducible SOX2 shRNA, but not control scrambled shCTRL.
(E) Immunostaining for active Caspase3 (AC3) under neurogenic culture conditions in shCTRL (upper panel) and shSOX2 cells (lower panel) cultured in the presence of dox.
(F–H) Expression of SOX2 shRNA versus scrambled shCTRL under gliogenic conditions did not affect P0+ cells (F) and resulted in the increase in SMA+ cells (G) and P0+ cells (H) under the mixed conditions.
(I) SOX2 overexpression rescues neuronal differentiation. The dNE cells carrying dox-inducible SOX2 shRNA were transduced with lentivirus expressing GFP or a SOX2-GFP fusion protein (lacking the endogenous SOX2 3′UTR targeted by SOX2-specific shRNA) and cultured under neurogenic conditions in the presence of dox. After 14 days, neuronal differentiation was assayed by Tuj1 immunostaining. Arrows point to TuJ1+ neurons coexpressing exogenous SOX2-GFP. Blue, Hoechst. Area values for a given marker were normalized to total Hoechst area (see Experimental Procedures for details). Scale bars, 100 mm; *p < 0.05, **p < 0.005. Error bars ± SE.
SOX2 is Required for the Generation of a Subset of DRG Neurons In Vivo
To test the role of Sox2 in vivo, we performed a targeted knockdown in chick embryos with shRNA under the control of an enhancer that mediates expression of the neural crest marker FoxD3 (Fishwick et al., unpublished data). The chicken Sox2- specific shRNA or control shRNA were electroporated into the trunk neural tube of HH10 (10 somite stage; Hamburger, 1988) chick embryos, efficiently targeting a large percentage of the premigratory NC. Embryos were fixed 2 days later, by which time neural crest cells had completed migration and condensed to form DRG. Consistent with the fact that neuronal differentiation is ongoing, we observed that, on average, 53% of neural crest-derived cells transfected with control constructs expressed the definitive neuronal marker HUC/D within the DRG of control electroporated embryos (Figure 6A). In contrast, only an average of 26% of neural crest-derived cells transfected with Sox2 shRNA stained positive for HUC/D. These results suggest that Sox2 downregulation in the migratory NC in chick embryos inhibits neuronal differentiation with DRG. Next, we used conditional knockout of Sox2 in the mouse NC lineage using classic Cre recombinase under the control of Wnt1, which is expressed in NC cells at the time of their initial emigration (Hollyday et al., 1995). Mice carrying homozygous Sox2LoxP alleles (Favaro et al., 2009), Wnt1:Cre (Danielian et al., 1998), and Z/EG reporter (Novak et al., 2000) (which activates GFP upon Cre recombination) revealed a homogeneous GFP expression in E11 DRG cells (Figure 6B). Indeed, the Wnt1-controlled Cre activation resulted in efficient elimination of Sox2 in E11 ganglionic cells of Wnt1:Cre/Sox2LoxP/LoxP mice (Figure 6C). Note that Sox2 is expressed in E10 DRG predominantly in the dorsal part of the ganglion. Neuronal differentiation was assessed by immunostaining for the neuronal markers Tuj1 and HuC/D in the DRG of wild-type (WT) and Sox2 conditional knockout (cKO) mice at E11 and E14.5. Quantification of serial sections starting from the trunk region revealed a 25% and 29% reduction respectively in Tuj1+ and HuC/D+ neurons at E11 (Figures 6D and 6E) in DRG of Sox2 cKO mice. At E14.5, serial sections aligned at the level of the forelimbs were stained for Tuj1 and quantified. Compared to WT, Sox2 cKO mice displayed a 45% reduction in Tuj1+ ganglionic neurons (Figure 6F). Together, changes in Sox2 expression in NC decreased the numbers of DRG neurons by half both in chick and in mouse embryos and supported the critical role of SOX2 in sensory neurogenesis.
Figure 6. SOX2 Downregulation In Vivo Reduces the Number of DRG Neurons.
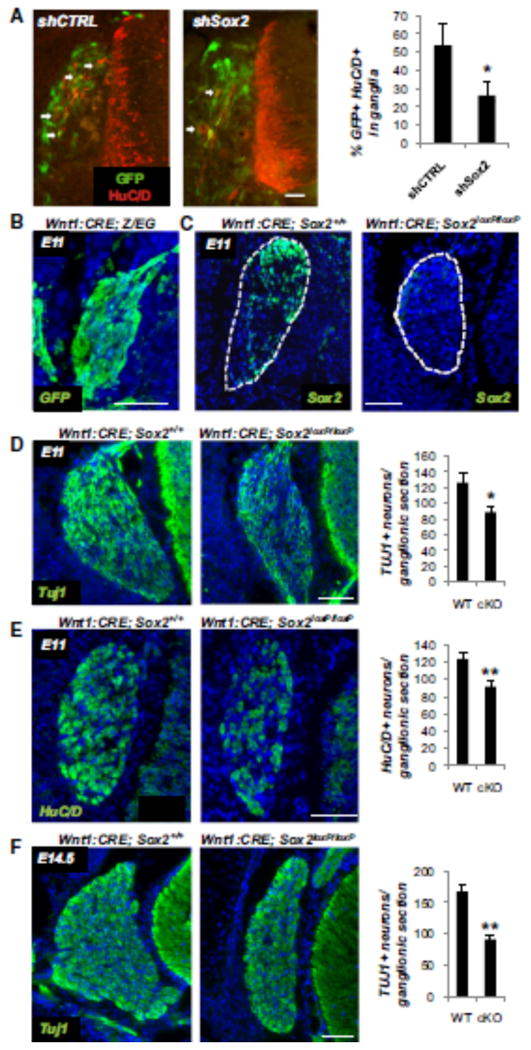
(A) Immunohistochemistry for the neuronal marker HuC/D and GFP (labeling shRNA expressing cells) in electroporated chick embryos; arrows point to cells coexpressing HuC/D and GFP within DRG. Quantification of HuC/ D-GFP coexpression (graph) revealed a reduction of HuCD+ cells in the embryos electroporated with Sox2-specific shRNA compared to control shRNA (shCTRL). *p < 0.01 Students t test Scale bar, 30 mm. Error bars ± SD.
(B) Cre activation in DRG was probed in Wnt1:Cre x Z/EG mice by GFP staining. At E11, ganglia were homogeneously positive for GFP, demonstrating Cre activation.
(C) Sox2 expression is efficiently eliminated in murine E11 ganglia of Wnt1:CRE x Sox2LoxP/LoxP mice (right panel, compare with Sox2 wild-type mice, left panel).
(D) Tuj1 expression in E11 ganglia of the wild-type (left panel) and Sox2 conditional knockout mice (right panel); quantification of Tuj1+ neurons in ganglionic sections. n = 23.
(E) HuC/D expression in E11 ganglia of WT (left panel) and Sox2 cKO mice (rightpanel); quantification of HuC/D+ neurons in ganglionic sections. n = 32.
(F) Tuj1 expression in E14.5 ganglia of the wild-type (left panel) and Sox2 conditional knockout mice (rightpanel); note the smaller size of the ganglia in Sox2 mutants at this time point. Quantification of Tuj1+ neurons in ganglionic sections. n = 50. (WT, wild-type; cKO, conditional knock-out). Scale bars: (B–F), 100 mm; *p < 0.05, **p < 0.005. Error bars ± SE.
SOX2 Regulates Proneural bHLH Genes
Reminiscent of the effects of SOX2 downregulation in hESC-NC cultures, the absence of proneural factors can trigger apoptosis during early phases of neurogenesis in vivo (Guillemot et al., 1993; Ma et al., 1999). We therefore asked whether SOX2 loss altered expression of bHLH factors such as NGN1 and MASH1, critical for the development of sensory and autonomic (sympathetic, parasympathetic, enteric) neurons (Guillemot et al., 1993; Ma et al., 1999). First, we determined the kinetics of cell death upon downregulation of SOX2 under neurogenic conditions using the activation of Caspase3 as readout No substantial cell death was observed for the first 3 days, but massive apoptosis was observed thereafter (Figures 7A and 7B). We then isolated total RNA from day 3 cultures, prior to the onset of apoptosis, and compared the expression of NGN1 and MASH1 in cells treated with SOX2 shRNA or control scrambled shRNA. Both RT-PCR and quantitative PCR (qPCR) analyses revealed that SOX2 downregulation dramatically reduced the expression of both of the bHLH transcription factors analyzed (Figures 7C and 7D). These findings are consistent with the massive neuronal death upon downregulation of SOX2. Next, we determined whether SOX2 protein is actually bound to the promoters of proneural bHLH transcription factors in human ESC-derived NC cells. We identified three putative SOX2 binding sites on both the NGN1 and MASH1 promoters (Figure 7E). Using chromatin immunoprecipitation (ChIP) coupled to qPCR, we found statistically significant evidence that SOX2 is bound to NGN1 and MASH1 promoters at least at two out of three putative SOX binding sites (Figures 7F and 7G). These results suggest that at the onset of neuronal differentiation, SOX2 functions to promote cell survival by facilitating the expression of proneural bHLH factors, likely via direct interaction with their promoter regions. On the other hand, the NOTCH pathway has long been implicated in the repression of proneural genes (Cau et al., 2000; Chen et al., 1997; Ishibashi et al., 1994). Upon binding of NOTCH transmembrane receptors to JAGGED and DELTA ligands, the NOTCH intracellular domain (NOTCH-ICD) cleaves, translocates to the nucleus, and forms a complex with RBPJ transcriptional modulator. NOTCH-ICD/RBPJ complex triggers the expression of bHLH proteins HES1 and HES5, known to repress the expression of proneural genes such as MASH1 and NGN1 (Cau et al., 2000; Chen et al., 1997) and block neuronal differentiation (Ishibashi et al., 1994). We therefore investigated the effect of SOX2 knockdown on several components of the NOTCH pathway. The qPCR analysis revealed that SOX2 downregulation in dNE cells cultured for 3 days under neurogenic conditions resulted in _2 fold upregulation of NOTCH2, JAGGED1, and RBPJ (Figure 7H). The expression of HES1 and HES5 was upregulated 3- and 2-fold, respectively (Figure 7H). The NOTCH1 and DELTA1 (DLL1) transcripts were slightly (_30%) downregulated, compared to control shRNA. These data suggest that, at the population level, SOX2 knockdown results in activation of the NOTCH pathway, including HES1 and HES5 genes, providing an alternative mechanism for the observed downregulation of proneural genes and the lack of neuronal differentiation.
Figure 7. SOX2 Regulates Proneural bHLH Genes at the Onset of Neurogenesis.
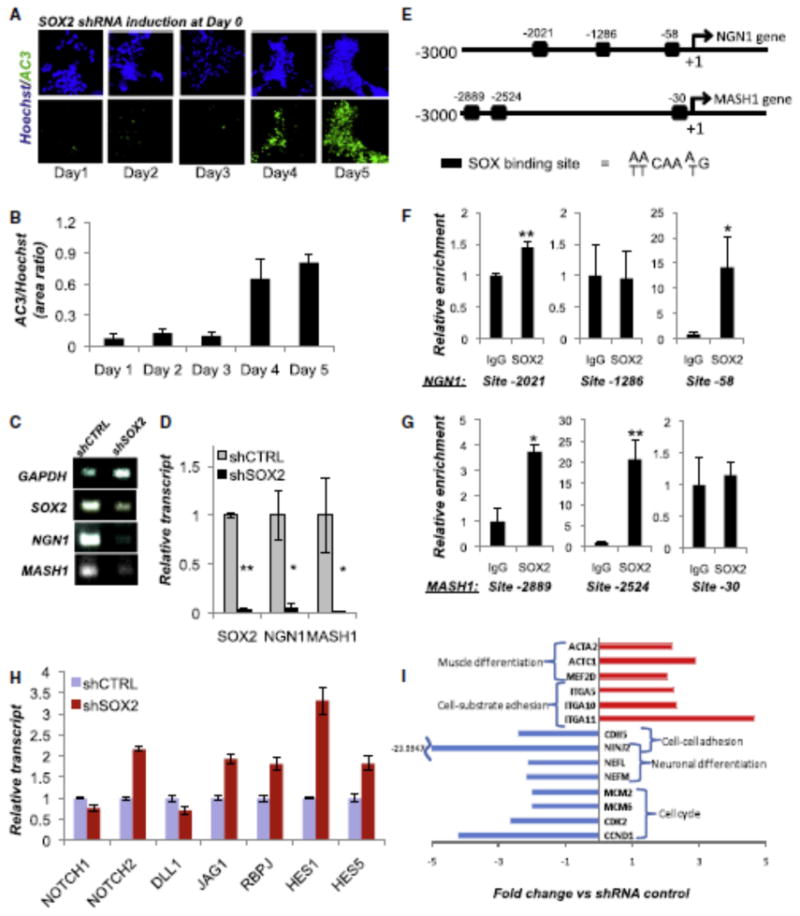
(A and B) Representative images and (B) quantification of active Caspase3 (AC3) staining in NC cells carrying the DOX-inducible SOX2 shRNA at various time points during neuronal differentiation. SOX2 was knocked down by dox administration on day 0. Day 3 was the last time point tested before a detectable increase in cell death. All SOX2 knockdown experiments (C and D and H and I) were performed with the cells harvested at day 3 after dox administration.
(C) RT-PCR gene expression analysis for the bHLH proneuronal genes NGN1 and MASH1 in scrambled control (shCTRL) and SOX2 (shSOX2) shRNA-transduced NC cells (day 3).
(D) qPCR confirmation of the results in (C); normalized to 18S transcripts.
(E) Position of the putative SOX binding sites (black boxes) on the human NGN1 and MASH1 promoters. Transcription initiation sites are shown as +1.
(F and G) Chromatin immunoprecipitation using SOX2-specific antibody demonstrated significant enrichment over the isotype controls, suggesting a direct binding of SOX2 at two sites of the NGN1 (F) and MASH1 (G) promoters; *p < 0.05, **p < 0.005.
(H) qPCR analysis of the NOTCH pathway in shSOX2 and shCTRL cells 3 days after dox administration (the onset of neuronal differentiation). Values are normalized to 18S. Error bars ± SE.
(I) Representative examples from the microarray analysis of gene expression upon downregulation of SOX2 by shRNA (day 3).
Identification of SOX2-Interacting Proteins in hESC-NC
To identify potential cofactors of SOX2 during peripheral neurogenesis, we performed immunoprecipitation (IP) of the endogenous SOX2-containing complexes from hESC-NC cultures. As demonstrated in these cultures, SOX2 is only expressed in the DRG-like clusters (Figures 3 and 4; Figures S1 and S3). Tryptic peptides from the IP material were subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an LTQ Orbitrap Velos mass spectrometer equipped with electron transfer dissociation (Thermo Fisher Scientific). SOX2 protein was identified in both biological duplicate IP samples that used SOX2 specific antibodies and was absent from both biological replicate IP samples with isotype-matched IgG controls. Meta-analysis using the NextBio search engine (www.nextbio.com) identified biogroups and pathways potentially regulated by the SOX2 interacting proteins (Tables S1–S3). We detected SIX4 and CREB nuclear factors, known to be important in early neuronal survival during development Six4 is expressed in cranial sensory placodes (Kobayashi et al., 2000) and olfactory placode (Chen et al., 2009); however, Six4 is not expressed in the neural tube at any time point in development, and Pax3, a marker of dorsal neuroepithelium, is expressed normally in the dorsal neural tube of Six4 null embryos, suggesting that premigratory NC field is not perturbed (Grifone et al., 2005). The loss of Six4 results in massive loss of the sensory neurons in developing trigeminal ganglia (Konishi et al., 2006) and contributes to the block of neurogenesis in olfactory placode (Chen et al., 2009). Curiously, this is accompanied by the loss of proneurogenic transcription factors Ngn1 and Mash1 (Chen et al., 2009). Therefore, it appears that Sox2-Six4 complex is unique to peripheral ganglia and, taken together with previously published data, our results suggest that Sox2-Six4 complex plays a critical role in sensory/olfactory neurogenesis. In mouse CNS, Creb1 deletion leads to neurodegeneration only in a Crem_/_background (Mantamadiotis et al., 2002). However, the peripheral nervous system is more sensitive to Creb1 loss since extensive apoptosis and peripheral neuron loss was seen during early gangliogenesis in Creb1-deficient mice even in the presence of the wild-type Crem (Lonze et al., 2002). These data hint at the existence of critical cofactors of SOX2, with exclusive or contextual role in DRG compared to CNS tissue.
Meta-Analysis of Gene Expression upon SOX2 Knockdown
To reveal transcriptional changes associated with SOX2 knockdown in hESC-NC cells, we performed global gene expression analysis (Illumina microarrays, 24K human referenced genes). Volcano algorithm (GeneSpring software, Agilent Technologies), identified ∼200 transcripts altered over 2-fold upon SOX2 knockdown. NextBio meta-analysis (www.nextbio.com) identified biogroups and pathways altered by SOX2 downregulation (Figure S6). Downregulated biogroups included cell cycle progression genes, neuronal differentiation and maturation factors, and cell-cell adhesion genes. Upregulated biogroups included cell-extracellular matrix adhesion, muscle differentiation pathways, and genes regulated by Serum Response Factor (SRF). Representative genes of each biogroup are shown in Figure 7I. Well-known G1/S regulators (CyclinD1, CCND1; Cyclin-dependent kinase 2, CDK2; and DNA replication regulators, MCM2 and MCM6) were among downregulated genes in the cell cycle-related biogroups. Several integrins—such as ITGA5, ITGA10, and ITGA11, which are important for cell adhesion to the fibronectin and collagen matrices—were upregulated, whereas transcripts encoding cell-cell adhesion molecules—such as CDH5 and NINJURIN2 (NINJ2), constitutively expressed in peripheral sensory and enteric neurons (Araki and Milbrandt, 2000)—were downregulated upon SOX2 knockdown. Because NC migration is dependent upon the increased affinity for the extracellular matrix and decreased cell-cell adhesion (Perris and Perissinotto, 2000), these results are consistent with the increased delamination and migration of human dNE cells following SOX2 knockdown (Figure 2). SOX2 knockdown triggered the downregulation of neurofilament light and medium polypeptides NEFL and NEFM. Mutations in the NEFL gene are associated with the peripheral neuropathies Charcot-Marie-Tooth types 2E and 1F (Jordanova et al., 2003; Mersiyanova et al., 2000). In summary, SOX2 knockdown under neurogenic conditions triggers the downregulation of molecular signatures indicative of cell proliferation, cell-cell adhesion and neuronal differentiation, whereas the genes promoting muscle differentiation and cell-matrix adhesion were upregulated.
Discussion
Early stages of human gestation are virtually inaccessible for experimental research, making hESC cultures a unique model from which to study the development of human NC lineages.
Here we used hESC-NC to model NC delamination and differentiation into neurons, glia, and smooth muscle. We demonstrated that downregulation of SOX2 in dNE clusters is sufficient to induce EMT/migratory NC. These loss-of-function data are consistent with the previously published gain-of-function data in which overexpression of SOX2 in chick dNE (premigratory NC cells) blocks NC delamination (Wakamatsu et al., 2004). Our results suggest that SOX2 may function downstream of BMP4, a classical inducer of EMT (Sela-Donenfeld and Kalcheim, 1999), but upstream of ADAM10, a protease known to cleave the extracellular portion of N-CAD (Shoval et al., 2007). We found that SOX2 function is required during the onset of neuronal differentiation, such that downregulation of SOX2 in hESC-NC cells interfered with their ability to acquire neuronal, but not glial or mesenchymal, fates. Chromatin IP suggested that under the culture conditions optimized for neuronal differentiation, SOX2 is bound to the promoter of proneural genes NGN1 and MASH1 in the progenitors of peripheral neurons. The knockdown of SOX2 under these conditions resulted in dramatic downregulation of proneural bHLH transcription factors and massive apoptotic cell death. This is reminiscent of the situation documented during CNS development where proneural genes are critical for both the acquisition of neuronal (but not glial) fates and the survival of young neuroblasts (Cai et al., 2000; Olson et al., 2001). Moreover, in the periphery, the lack of Ngn1 resulted in massive neuronal death in the cranial sensory ganglia (Ma et al., 1999). In chick, specific downregulation of Sox2 in the migratory NC cells reduced the numbers of neurons in DRG by ∼50%.
Because Sox2 was downregulated using shRNA, it is possible that partial loss of neurons is due to the incomplete elimination of Sox2 protein. Alternatively, Sox2 might be required for the generation of a subset of peripheral neurons in DRG. The mouse conditional knockout data is consistent with the latter scenario. Conditional ablation of Sox2 using NC specific expression of Cre (Wnt1-Cre) resulted in reduction of neurons in E14.5 DRG by 45%, and the ganglia were also smaller in Sox2 mutant animals at this time point. The requirement of SOX2 for NGN1 expression (as found in human ESC-derived NC cultures, Figures 7C and 7D) could explain the loss of the Ngn1-dependent subset of DRG neurons, mimicking the Ngn1 knockout phenotype in the ganglia (Ma et al., 1999). Indeed, at earlier time points (E11), when primarily the Ngn2-dependent neurons are formed, only a 25% reduction of TuJ1+ cells was seen in the Sox2 ablated embryos. It will be informative to find out if the trkA+ DRG neurons are predominantly lost in Sox2 ablated embryos compared to trkB+/C+ cells. The lack of NGN1 expression in human ESC-derived NC would explain a stronger in vitro phenotype upon SOX2 knockdown, compared to conditional ablation of Sox2 in mice. It remains possible that in the human embryo, similarly to that documented in mice, NGN1-expressing NC cells are able to generate peripheral neurons in the absence of SOX2. At E10, Sox2 staining was mainly detected within the dorsal portion of the DRG, consistent with its role in Ngn1 induction and initiation of the second wave of neurogenesis in the ganglia (Montelius et al., 2007). Because the satellite cells, the glial cell type associated with neuronal cell bodies in the DRG, are thought to develop during a later period from E10.5–E13.5 (Farinas et al., 2002), it is unlikely that numerous Sox2+ cells observed at E10 are committed glial cells. At E11, ∼11% of all Sox2+ cells in ganglia expressed various levels of the early neuronal marker TuJ1. Comprehensive lineage tracing experiments will be required to determine if a transient upregulation of Sox2 initiates the Ngn1 expression in the nascent sensory neurons. In hESC-NC, SOX2 knockdown results in upregulation of NOTCH2, JAG1, HES1, and HES5—known inhibitors of proneural genes. NOTCH signaling in this case seems to be mediated by NOTCH2 and JAG1, which have been implicated in the cardiac myogenic program of neural crest cells (Varadkar et al., 2008), and in the CNS, Sox2 was shown to directly bind and repress GFAP expression and glial fate (Cavallaro et al., 2008). This mechanism could also contribute to the glial differentiation in human NC cultures upon knockdown of SOX2. In developing CNS, both SOX2 overexpression (Graham et al., 2003) and downregulation (Cavallaro et al., 2008; Miyagi et al., 2008) negatively affect CNS neurogenesis. In chick embryos, the overexpression of SOX2 DNA binding domain fused to the Engrailed activator domain (SOX2ER) inhibits the onset of neurogenesis in the developing CNS and prevents delamination from the dorsal neural tube (Graham et al., 2003). These discrepancies may reflect differences in model organism and experimental approaches employed. For instance, the overexpression of SOX2ER likely blocks the function of all SOXB1 members (Episkopou, 2005), while the induction of SOX2 shRNA did not significantly inhibit the level of SOX1, and the expression of SOX3 was upregulated (Figure S2). In addition, SOX2 is likely to function in a dose-dependent and context-dependent fashion (Pevny and Nicolis, 2010). High levels of SOX2 expression may reinforce the neural progenitor characteristics, whereas low levels of SOX2 under neurogenic conditions may promote the neuronal fates, while suppressing nonneuronal fates. In our hands, the overexpression of SOX2-GFP resulted in rescuing the early steps (TuJ1+) but not later steps (Peripherin+, MAP2+) of peripheral neuronal differentiation (Figure 5I). A proneuronal role for SOX2 has been reported in the mammalian CNS. Weak Sox2 expression was observed in Map2+ cells in cultures and neurospheres generated from Sox2 mouse hypomorphs in which Sox2 expression was reduced by ∼30%, and these cells showed normal gliogenic potential in vitro but severely reduced neuronal differentiation (Cavallaro et al., 2008). These authors also found that Sox2 directly binds the GFAP promoter and suppresses the expression of the GFAP gene (Cavallaro et al., 2008). In vivo, such mutant mice show a 40%–60% decrease in GABAergic neurons. Furthermore, reduced neuronal but not glial differentiation is also seen using neurospheres derived from mice with conditional ablation of Sox2 in the CNS (Miyagi et al., 2008). Our findings imply that disrupted SOX2 function might be linked to NC-related pathologies, or neurocristopathies. Although most SOX2 mutations are likely to lead to early embryonic lethality (Avilion et al., 2003), some neurocristopathies might be linked to nonlethal SOX2 loss-of-function mutations, or mutations in genes encoding SOX2-interacting factors or downstream effectors. Indeed, nonlethal mutations in human SOX2 (a major cause of anophthalmia/microphthalmia; Fantes et al., 2003) and the NC-related CHARGE syndrome (Sanlaville and Verloes, 2007) are often marked by common defects, such as sensorineural deafness (Hagstrom et al., 2005), which might be linked to defective peripheral neurogenesis. In such cases, modulating SOX2 expression and function might help develop therapeutic applications.
Experimental Procedures
Derivation, Maintenance, and Differentiation of Human dNE Cells dNE was generated from hEC as described (Cimadamore et al., 2009). For propagation, cells were seeded onto Matrigel-coated plates (BD Biosciences, final concentration = 1:30, 2h coating at room temperature) using base medium (1:1 ratio of DMEM/F12 Glutamax-neurobasal medium [GIBCO], 2% B27 supplement without vitamin A [GIBCO], 10% BIT 9500 [StemCell Technologies], and 1 mM glutamine [GIBCO]) supplemented with 20 ng/ml EGF (Chemicon), 20 ng/ml bFGF, 5 mg/ml insulin (Sigma) and 5 mM nicotinamide (Sigma). For segregation between epithelial and mesenchymal cells, accutase- dissociated dNE cells were seeded onto FN-coated plates (1 mg/ml, overnight coating) at a density of 45,000 cells/cm2 in the presence of EGF 100 ng/ ml for 12 days. For differentiation into smooth muscle cells, dNE cells were seeded onto FN-coated plates (1 mg/ml, overnight coating) at a density of 45,000 cells/cm2 in base medium supplemented with 40 ng/ml EGF. Cells were allowed to differentiate for 7 days. Gliogenic conditions were obtained by seeding dNE cells at 20,000 cells/cm2 in FN-coated plates in base medium supplemented with 1% horse serum. Cells were allowed to differentiate for 12 days. For neuronal differentiation (neurogenic conditions), dNE cells were seeded onto FN-coated plates at 45,000 cells/cm2 in base medium supplemented with 40 ng/ml bFGF and 40 ng/ml BDNF and allowed to differentiate for 14 days. Other conditions were as described in the text For neurosphere-based migration assays, hES-derived neurospheres were plated ondifferent substrates as described and cells allowed to migrate for a minimum of 1 day to a maximum of 4 days on laminin/polyornithine-coated plates in base media supplemented with 20 ng/ml EGF, 20 ng/ml bFGF, 5 mg/ml insulin, and 5 mM nicotinamide.
ChIP and qPCR
ChIP was performed using the Ez-ChIP kit (Millipore) according to the manufacturer'srecommendations with the following modifications: 2 3 106 cells were used for each immunoprecipitation, cells were sonicated in order to yield chromatin fragment of 200–500 bp, and 5 mg of immunoprecipitating antibodies were employed in each ChIP. Antibodies were rabbit anti-SOX2 (Millipore AB5603) and normal rabbit IgG (Millipore PP64) as a nonspecific control. qPCR amplification was performed with site-specific primers designed to flank the putative SOX binding sites (Supplemental Experimental Procedures). qPCR values were analyzed with the D(DCT) method and normalized to the values obtained with the nonspecific antibody.
Sox2 Manipulation in Chick Embryos
Fertilized chicken embryos (Gallus gallus domesticus) were obtained from McIntyre Farms, Lakeside, CA, and incubated in a 38_C humidified incubator until HH10 according to the staging of Hamburger (Hamburger, 1988). miR30 plasmid (4 mg/ml concentration) was injected by air pressure into the neural tube of the embryo in ovo using a pulled glass needle. Platinum electrodes were placed across the neural tube and a current of 3 3 21 V of 50 ms in 100 ms intervals was used to electroporate the cells on one half of the neural tube. Embryos were resealed and reincubated a further 24 hr.
Transgenic Mice
Mice carrying Sox2LoxP alleles (Favaro et al., 2009) were crossed with the mice expressing Cre recombinase under the control Wnt1 promoter (wnt1:Cre). A total of 23 sections were used for Tuj1 quantification at E11, 32 sections for Hu/CD quantification E11, and 50 sections for Tuj1 quantification at E14.5. Wnt1:Cre/Sox2LoxP/LoxP mice were crossed to Z/EG mice (Jackson Laboratories), which activates GFP upon Cre recombination to monitor Wnt1:Cre activity in DRG. Additional details are available in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank C.-T. Huang and K. Liu for their help with cloning, lentiviral production, and microarray analysis. We thank Dr. S. Albini, Dr. S. Forcales, and Professor L. Puri for sharing their expertise in chromatin immunoprecipitation techniques. Sox2LoxP mice were kindly provided by Dr. Nicolis, and Wnt1- CRE mice were kindly provided by Dr. Y. Yamaguchi. We thank Dr. J. Hou for helping with IP-MS data analysis. This work has been supported by CIRM postdoctoral fellowship to F.C., CIRM grant RS1004661 to A.T., and transient research support to A.V. Terskikh from the Sanford-Burnham Medical Research Institute and an NIH Blueprint core grant (PI, S.A. Lipton).
References
- Adachi K, Suemori H, Yasuda SY, Nakatsuji N, Kawase E. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. 2010;15:455–470. doi: 10.1111/j.1365-2443.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- Araki T, Milbrandt J. Ninjurin2, a novel homophilic adhesion molecule, is expressed in mature sensory and enteric neurons and promotes neurite outgrowth. J Neurosci. 2000;20:187–195. doi: 10.1523/JNEUROSCI.20-01-00187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R, Coppola G, Kaul M, Talantova M, Cimadamore F, Nilbratt M, Geschwind DH, Lipton SA, Terskikh AV. Molecular stages of rapid and uniform neuralization of human embryonic stem cells. Cell Death Differ. 2009;16:807–825. doi: 10.1038/cdd.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, et al. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999;99:377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- Cai L, Morrow EM, Cepko CL. Misexpression of basic helixloop- helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development. 2000;127:3021–3030. doi: 10.1242/dev.127.14.3021. [DOI] [PubMed] [Google Scholar]
- Carl TF, Dufton C, Hanken J, Klymkowsky MW. Inhibition of neural crest migration in Xenopus using antisense slug RNA. Dev Biol. 1999;213:101–115. doi: 10.1006/dbio.1999.9320. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci USA. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadamore F, Curchoe CL, Alderson N, Scott F, Salvesen G, Terskikh AV. Nicotinamide rescues human embryonic stem cellderived neuroectoderm from parthanatic cell death. Stem Cells. 2009;27:1772–1781. doi: 10.1002/stem.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curchoe CL, Maurer J, McKeown SJ, Cattarossi G, Cimadamore F, Nilbratt M, Snyder EY, Bronner-Fraser M, Terskikh AV. Early acquisition of neural crest competence during hESCs neuralization. PLoS ONE 5. 2010:e13890. doi: 10.1371/journal.pone.0013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifeninducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, et al. Mutations in SOX2 cause anophthalmia. Nat Genet. 2003;33:461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- Farinas I, Cano-Jaimez M, Bellmunt E, Soriano M. Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia. Brain Res Bull. 2002;57:809–816. doi: 10.1016/s0361-9230(01)00767-5. [DOI] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Fedtsova NG, Turner EE. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 Functions to Maintain Neural Progenitor Identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development. 2005;132:2235–2249. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Hagstrom SA, Pauer GJ, Reid J, Simpson E, Crowe S, Maumenee IH, Traboulsi EI. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am J Med Genet A. 2005;138A:95–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Ontogeny of neuroembryology. J Neurosci. 1988;8:3535–3540. doi: 10.1523/JNEUROSCI.08-10-03535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DJ, Copp AJ. Role of the extracellular matrix in neural crest cell migration. J Anat. 1997;191:507–515. doi: 10.1046/j.1469-7580.1997.19140507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES-1 prevents mammalian neural differentiation in the central nervous system. EMBO J. 1994;13:1799–1805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanova A, De Jonghe P, Boerkoel CF, Takashima H, De Vriendt E, Ceuterick C, Martin JJ, Butler IJ, Mancias P, Papasozomenos S, et al. Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain. 2003;126:590–597. doi: 10.1093/brain/awg059. [DOI] [PubMed] [Google Scholar]
- Kahane N, Kalcheim C. Identification of early postmitotic cells in distinct embryonic sites and their possible roles in morphogenesis. Cell Tissue Res. 1998;294:297–307. doi: 10.1007/s004410051180. [DOI] [PubMed] [Google Scholar]
- Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev Biol. 1998;202:29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Osanai H, Kawakami K, Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech Dev. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Ikeda K, Iwakura Y, Kawakami K. Six1 and Six4 promote survival of sensory neurons during early trigeminal gangliogenesis. Brain Res. 2006;1116:93–102. doi: 10.1016/j.brainres.2006.07.103. [DOI] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C, Bronner-Fraser M, Mayor R. Relationship between gene expression domains of Xsnail, Xslug, and Xtwist and cell movement in the prospective neural crest of Xenopus. Dev Biol. 2000;224:215–225. doi: 10.1006/dbio.2000.9723. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, et al. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- Marmigère F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nature reviews Neuroscience. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Mersiyanova IV, Perepelov AV, Polyakov AV, Sitnikov VF, Dadali EL, Oparin RB, Petrin AN, Evgrafov OV. A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet. 2000;67:37–46. doi: 10.1086/302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development Dev. Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Miyagi S, Masui S, Niwa H, Saito T, Shimazaki T, Okano H, Nishimoto M, Muramatsu M, Iwama A, Okuda A. Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 2008;582:2811–2815. doi: 10.1016/j.febslet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Montelius A, Marmigère F, Baudet C, Aquino JB, Enerback S, Ernfors P. Emergence of the sensory nervous system as defined by Foxs1 expression. Differentiation. 2007;75:404–417. doi: 10.1111/j.1432-0436.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Mould AP, Askari JA, Craig SE, Garratt AN, Clements J, Humphries MJ. Integrin alpha 4 beta 1-mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J Biol Chem. 1994;269:27224–27230. [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Olson JM, Asakura A, Snider L, Hawkes R, Strand A, Stoeck J, Hallahan A, Pritchard J, Tapscott SJ. NeuroD2 is necessary for development and survival of central nervous system neurons. Dev Biol. 2001;234:174–187. doi: 10.1006/dbio.2001.0245. [DOI] [PubMed] [Google Scholar]
- Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- Perris R, Perissinotto D. Role of the extracellular matrix during neural crest cell migration. Mech Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet. 2007;15:389–399. doi: 10.1038/sj.ejhg.5201778. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126:4749–4762. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, Munnich A, Lyonnet S, Vekemans M, Etchevers HC. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17:3411–3425. doi: 10.1093/hmg/ddn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev Dyn. 2008;237:1144–1152. doi: 10.1002/dvdy.21502. [DOI] [PubMed] [Google Scholar]
- Voroteliak EA, Shikhverdieva ASh, Vasil'ev AV, Terskikh VV. Simulation of the migration process of human epidermal keratinocytes over three-dimensional collagen gel. Izv Akad Nauk Ser Biol. 2002;1:30–37. [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Weston JA. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Endo Y, Osumi N, Weston JA. Multiple roles of Sox2, an HMG-box transcription factor in avian neural crest development. Dev Dyn. 2004;229:74–86. doi: 10.1002/dvdy.10498. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development Trends. Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


