Abstract
About 43 million individuals in the US currently suffer from disabilities due to arthritis. Cartilage defects are the major source of pain in the affected joints. Current treatments, whilst alleviating some of the clinical symptoms, prove insufficient to cure the underlying irreversible cartilage loss. Stem cells represent a unique source for restoration of cartilage defects. Pre-clinical and clinical trials are currently pursued to investigate the potential of various types of stem cells and stem cell derived chondrocytes to repair arthritic joints. A major challenge with all stem cell-mediated tissue regeneration approaches is death of the transplanted cells with clearance by the immune system. Our current inability to diagnose successful or unsuccessful engraftment of transplanted cells non-invasively in vivo represents a major bottleneck for the development of successful stem cell therapies. A large variety of non-invasive Magnetic Resonance (MR) imaging techniques have been developed over the last decade, which enable sensitive in vivo detection of Matrix Associated Stem Cell Implants (MASI) and early diagnosis of related complications. While initially focused on successfully harvesting cellular MR imaging approaches with easily applicable SuperParamagnetic Iron Oxide Nanoparticles (SPIO), our team began to observe details that will facilitate clinical translation. We therefore started a broader effort to define a comprehensive set of novel, clinically applicable imaging approaches for stem cell transplants in patients. We established immediately clinically applicable nanoparticle labeling techniques for tracking stem cell transplants with MR imaging; we have evaluated the long term MR signal effects of iron oxide nanoparticle labeled MASI in vivo; and we have defined distinct signal characteristics of labeled viable and apoptotic MASI. This review article will provide an overview over these efforts and discuss important implications for clinical translation.
Keywords: SPIO, MR imaging techniques, MASI
Introduction
Arthritis is a major cause of disability, resulting in 992,100 hospitalizations and 44 million outpatient visits in the US each year and causing annual expenses on the order of $95 billion in direct costs (medical treatment) and $47 billion in indirect costs (lost earnings [1,2]). Articular cartilage defects are the key source of pain and functional disability [1-3]. The treatment of cartilage defects provides a major challenge due to the lack of self-regeneration of injured cartilage. New therapies based on transplants of autologous chondrocytes, stem cells, or stem cell derived chondrocytes provide a potentially curative therapeutic option [4]. Advantages of using stem cells for a cell transplant rather than autologous chondrocytes include: (1) one less knee surgery, (2) decreased donor-site morbidity and (3) higher cost effectiveness, while yielding equal or better long term outcomes [5,6]. However, a major barrier for long-term success of cell transplants in cartilage defects is our inability to recognize complications of the engraftment process in a timely manner. To date, a large proportion of transplanted stem cells and chondrocytes undergo apoptosis and/or are cleared from the transplantation site by macrophages [4,7,8]. An imaging method that could visualize and monitor stem cell transplants in cartilage defects directly, non-invasively, and longitudinally in vivo would greatly enhance our ability to develop successful cell transplantation techniques. MR imaging is currently the only non-invasive diagnostic test capable of providing high resolution, anatomical and functional information of cartilage defects in vivo [9,10]. Over the last 10 years, we have developed non-invasive MR imaging techniques for early detection of complications of the engraftment process of Matrix Associated Stem Cell Transplants (MASI). By exploiting novel, clinically applicable, cell tracking techniques as a new tool to monitor stem cell engraftment outcomes non-invasively in vivo, we anticipate significantly improving and accelerating the development of successful therapies for cartilage regeneration in patients, and ultimately, alleviating long term disabilities and related costs to society.
Cell Labeling with Iron Oxide Nanoparticles
The development of a non-invasive imaging technique for in vivo detection of stem cell transplants is crucial for monitoring the safety and efficacy of virtually any stem cell therapy. The ability to non-invasively track transplanted stem cells in vivo, in real time, allows for evaluations of correct stem cell deposition, immediate engraftment patterns, local proliferation, long-term retention at the target site and immune rejection processes (Figure 1). With regards to stem cell transplants in arthritic joints, MR imaging is the only directly clinically applicable imaging modality available for this purpose.
Figure 1.
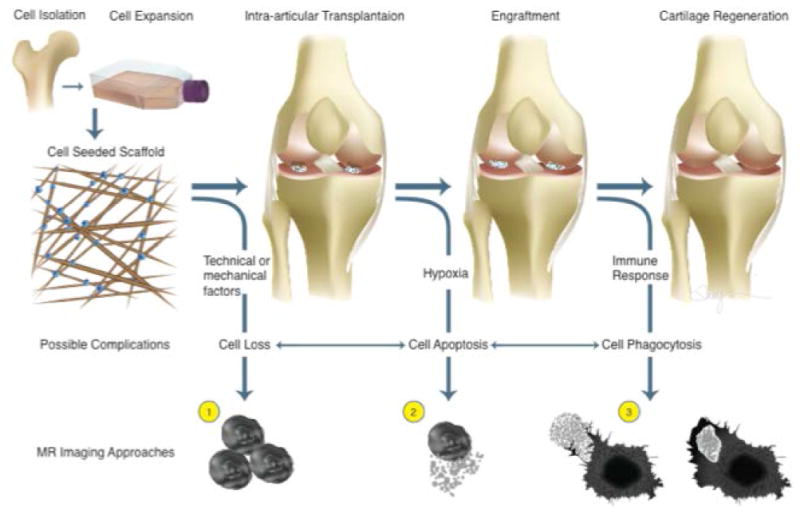
Concept of stem cell-mediated regeneration of osteochondral defects with possible complications and related imaging approaches.
Most cell tracking studies have been performed with iron oxide nanoparticles, because these are easier to introduce into stem cells and provide a higher sensitivity for stem cell detection compared to clinically applicable gadolinium chelates [11-19]. Nanoparticles for MR imaging are categorized based on their size: Superparamagnetic Iron Oxide Nanoparticles (SPIO) with diameters of more than 50 nm are phagocytosedby stem cells in high quantities and therefore, provide highly efficient cell labeling. Conversely, “UltraSmall Superparamagnetic Iron Oxide Nanoparticles” (USPIO) with diameters in the order of 20-50 nm are typically introduced into stem cells via endocytosis and generally provide weaker MR signal effects [20-23,15]. Cell labeling with SPIO is usually possible with simple incubation techniques while efficient cell labeling with USPIO requires transfection techniques [20,21]. Therefore, previous approaches for MR-based cell tracking have been almost exclusively performed with SPIO which allow for easier cell labeling and more sensitive cell detection, such as ferumoxides and ferucarbotran (Feridex™, FDA-approved; Endorem®and Resovist™, clinically approved in Europe) [14,15,24-26]. Unfortunately, recently, all clinically applicable SPIO have been taken of the market in the US and in Europe. Major contrast agent companies are developing USPIO as second generation nanoparticles, which offer a wider spectrum of applications and which may have fewer effects on stem cell physiology and differentiation.
A list of clinically applicable MR contrast agents, which have been used or could be used for clinical stem cell tracking applications are listed in Figure 2. Ferumoxytol (Feraheme™) is a USPIO, which has been recently FDA-approved for intravenous treatment of anemia in patients [27-31]. This agent exerts strong signal effects on MR images and can thus be applied “off label” for cell labeling and cell tracking purposes. Ferumoxytol is composed of an iron oxide core and a carboxydextran coat. The agent has a mean hydrodynamic diameter of 30 nm and a high r2 relaxivity of 83 L mmol-1 s-1 at 20 mHz [32]. We previously applied ferumoxytol as an intravenous contrast agent for MR imaging of arthritis [32] and we performed initial ferumoxytol-labeling experiments of hMSCs and other stem cells [23]. Ferumoxytol is currently the only USPIO, which would be immediately available for clinical stem cell tracking applications in the US via an “off label” use. Protamine sulfate can be used as a clinically applicable transfection agent, which can shuttle ferumoxytol into stem cells [33]. Using protamine-transfection, we could detect ferumoxytol-labeled stem cells by a significant negative (dark) signal effect on T2-weighted MR images, which lasted forseveral weeks ([33]; Figure 2). Thu et al. [34] reported a stem cell labeling technique based on ferumoxytol-heparinprotamine complexes, which was more efficient than ours. However, for our application of MASI, our clinicians requested a “heparin-free” labeling technique, in order to avoid potential secondary bleeds or heparin-induced cartilage damage [35]. With our protocol, this potential side effect of heparin, delivered with labeled cells, would be excluded.
Figure 2.
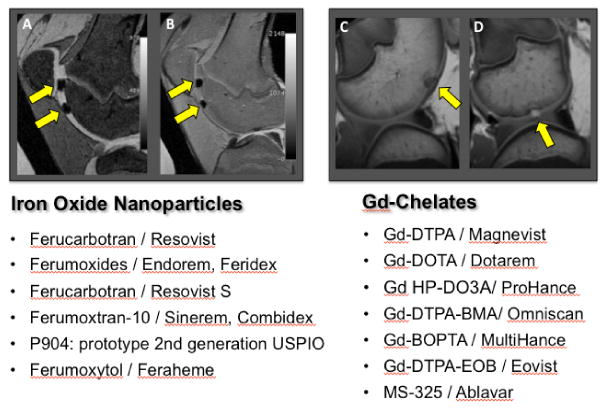
Overview over clinically applicable MR contrast agents which either have been used for clinical cell tracking studies in the past orwhich could, in principle, be applied “off label” for cell tracking studies in patients, because they are FDA approved for other indications. Sagittal T2-weighted (A) and proton-density-weighted (B) MR scans show example of two transplants of iron oxide nanoparticle labeled cells in cartilage defects (left, arrows) and sagittal T1-weighted MR scans (right) show a representative example of an unlabeled (C) or Gd-DTPA labeled (D) transplant (arrows).
Both SPIO-labeling and ferumoxytol-labeling results in lysosomal retention of iron oxide nanoparticles in the cells' cytoplasm, where they undergo slow iron metabolism over several weeks [20,24,36]. The labeled cells provide a strong, significant, negative (dark) signal effect on MR images, in vitro and in vivo [20,15,24,36,37]. We have shown that optimized protocols for nanoparticle labeling do not impair the viability or differentiation capacity of iron oxide labeled stem cells: While exposing stem cells to excessive amounts of iron oxides has impaired stem cell differentiation, particularly chondrogenesis, labeling stem cells with limited quantities of iron oxide nanoparticles had no apparent effect on stem cell viability, proliferation or differentiation [24,38]. Our team developed stem cell labeling protocols that provide a compromise between cellular iron load that allows MR detection (the higher the better) and cellular iron load that ensures preserved stem cell function and differentiation capacity (the lower the better). Optimized cell labeling protocols lead to an unimpaired chondrogenesis of hMSCs when compared to unlabeled controls [15,24]. In general, an iron load of less than 10 picogram per cell has not shown any impairment in chondrogenesis in our experience, although this would have to be confirmed for other cell types. Ex vivo cell labeling could be used to diagnose correct stem cell deposition or stem cell loss from the target site, and it could also help detecting in vivo tumor formation based on observations of expanding cell deposits and too fast dilution of the iron label (Figure 3). Depending on transplanted cell type, viable cell transplants metabolize the iron label within 2-4 weeks. Therefore, the iron oxide induced MR signal loss of stem cell transplants at 0-4 weeks after MASI does usually not interfere with more long term indicators of cartilage defect repair, defined by the MOCART score (magnetic resonance observation of cartilage repair tissue: “defect fill,” “cartilage interface,” “surface,” “adhesions,” “structure,” “signal intensity,” “subchondral lamina,” and “effusion”, [39,40]). Future studies have to show, if abnormal MR signal kinetics of iron labeled stem cells, such as early loss of iron signal at the transplant site, correlate with late findings of incomplete or failed cartilage repair, as defined by the MOCART score.
Figure 3.
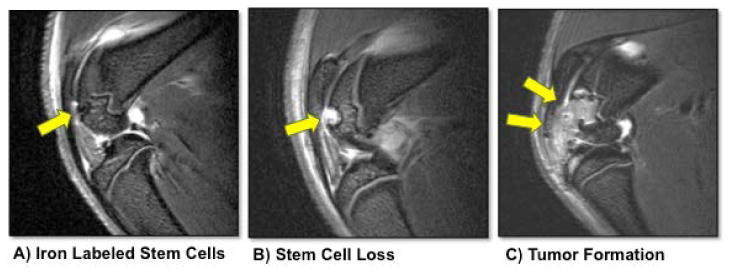
Sagittal T2-weighted MR images of rat knee joints with an osteochondral defect of the distal femur (arrow) and status post implantation of a ferumoxitol-labeled stem cell transplant (A), loss of labeled cells from the transplant site (B) and tumor formation (C).
In Vivo Labeling of Bone Marrow Derived Stem Cells
As of today, seven clinical trials have been reported, in which researchers tracked ex vivo labeled cells in patients [41]. However, due to stringent FDA-controlled regulatory issues, all of these studies have been performed outside the United States [41]. Furthermore, first generation iron oxide nanoparticles (Feridex orEndorem), which had been used for ex vivo stem cell labeling for these previously reported trials, have been discontinued by the pharmaceutical industry due to economical reasons. The limited biodistribution of SPIO lead to limited applications and limited revenues. As outlined above, our group and others used the ultrasmall SPIO (USPIO) ferumoxytol (Feraheme) as an alternative stem cell label. However, the combination of “off label” use of several drugs needed for ex vivo cell labeling together with cell manipulations (such as cell washing to remove excess label) represents a hurdle for FDA-approval and clinical translation in the United States. We solved this problem by labeling mesenchymal stem cells (MSC) in vivo, via a simple intravenous injection of ferumoxytol, which is endocytosed by MSC in bone marrow [42]. After their harvest from bone marrow and transplantation into arthritic joints, the iron labeled MSC could be tracked with clinical MR imaging tools. This new procedure does not require any alteration of stem cell harvest or transplantation procedures, does not involve any ex vivo stem cell manipulation, is immediately clinically translatable via “off label” use of the FDA-approved iron supplement ferumoxytol and could be widely utilized for numerous novel stem cell therapies currently entering clinical trials. The ability to directly track ferumoxytol labeled MSC in vivo could enable a wide variety of tissue regeneration approaches and stem cell imaging applications beyond arthritis research.
Differentiating Viable and Dead Stem Cell Transplants
Another complication of a stem-cell transplant is stem cell loss due to cell death. This often arises when cells are moved from a nurturing cell culture environment into hostile in vivo environments of osteochondral injuries. While the in vitro environment provides stable temperature, oxygen levels, pH, and nutrition conditions, an osteochondral defect is characterised by a hypoperfused, hypoxic environment [43,44], with high levels of inflammatory mediators [45]. As a result, a large portion of implanted cells undergo apoptosis [46] and are cleared from the transplantation site by macrophages, [4,7,8], which leads to incomplete repair [47]. If we were able to determine cell viability and cell death with an imaging test, then we could better develop MASI approaches that lead to successful cartilage regeneration outcomes.
Our studies have shown that viable and apoptotic MASI demonstrate distinct signal characteristics on MR images, when the cells are labeled with iron oxide nanoparticles [15,36,37,48]. Iron oxide labeled viable MASI demonstrated an increasing area of T2-signal loss in the early post-transplant period (1-2 weeks post MASI), which correlated to stem cell expansion over a larger areaat the transplantation site. The cells persisted in the defect for about 1-2 weeks, as proven by MRI and histopathology [15]. By contrast, transplants of iron oxides labeled apoptotic stem cells did not expand and demonstrated a central loss of T2-signal on follow up MR imaging studies due to loss of stem cells from the transplantation site and lack of repair of the defect [15]. Histopathology confirmed persistence of viable labelled and unlabeled hMSCs in the defects for up to 2 weeks and repair of the underlying defect, while transplantation of apoptotic hMSCs lead to faster cell elimination from the transplant site via macrophage phagocytosis and resulted inpersistent cartilage defects [15].
Imaging Technique to Track Interactions of Host Macrophages with MASI
Death of transplanted stem cells could be also detected indirectly, based on host immune responses [4,49]. Proteins released from apoptotic stem cells serve as a chemotactic factors for bone marrow macrophages, which migrate to and home in MASI, where they phagocytose the dead cells [15]. Converseley, Arinzeh et al. [29] showed that successful bone regeneration was not associated with significant immune cell migration into the transplant. An imaging technique that could visualize macrophage migrations into MASI could help to detect stem cell death and monitor the effect of supportive factors, such as scaffolds, growth factors and immune modifiers on stem cell engraftment outcomes. We have shown in preclinical and clinical investigations, that bone marrow macrophages can be labeled in vivo with USPIO and that migration of ferumoxytol-labeled macrophages into MASI can be detected with MR imaging [50-52]. The approach relies on pre-loading bone marrow macrophages via intravenous ferumoxytol administration, followed by transplantation of unlabeled stem cells. Iron oxide labeling of bone marrow macrophages allows for detection of macrophage migration into unlabeled MASI. Our data have shown that bone marrow macrophages migrate in larger quantities into apoptotic MASI as opposed to viable MASI [42]. Since this approach relies on “off label” use of an FDA-approved drug, without changing route or dose of administration, this new procedure could likely be more easily translated to the clinic than ex vivo labeling procedures described above, and thereby directly benefit stem cell therapies currently entering clinical trials.
Safety and Practical Considerations for Clinical Translation
We have applied various iron oxide nanoparticle compounds as MR contrast agents in clinical trials [50,52-58]. These agents are generally well tolerated and show excellent safety profiles [28,31,52,56,57]. The delivered iron dose for potential ferumoxytol cell tracking studies in patients would be less than 1 mg Fe (Note: these are coated iron oxide nanoparticles, not free iron), which is considerably less than the currently administered dose for anemia treatment (5 mg/kg) or the iron dose administered with one blood transfusion. Iron oxide nanoparticles, which have been released from apoptotic cells, are either phagocytosed and metabolized by resident macrophages or they are absorbed by the synovium, enter the blood and are slowly metabolized by macrophages in liver and spleen [15,26,32,59]. Ferumoxytol is not excreted via the kidneys and not associated with any risk of nephrogenic sclerosis (a potential adverse event with Gd-chelates) [30,31,60]. In fact, ferumoxytol has been FDA-approved for the treatment of anemia in patients with renal insufficiency and has been safely applied in more than 150,000 patients to date. Anaphylaxis or anaphylactoid reactions with ferumoxytol were reported in 0.1-0.2% of exposed subjects, which is comparable to other MR contrast agents [30,31,60]. Our group uses ferumoxytol « off label » via an IND with the FDA as an intravenous contrast agent for patients with bone tumors. Results from these clinical trials will provide valuable information about contrast agent signal characteristics and safety in patients. We plan to use this experience towards clinical translation of our cell tracking techniques.
Most studies on stem cell-mediated cartilage repair have been performed with either chondrocytes or MSC. Alternative cell types comprise Adipose Derived Fat Cells (ADSC), synovial and periosteal derived stem cells. The ideal cell source for MASI should allow easy and inexpensive cell harvesting, expansion and maintenance, involve minimal donor morbidity, contribute directly to chondrogenic matrix regeneration and have a low risk of immune responses or transmission of other diseases. MSC represent very heterogenous cell populations and it is highly debated, wether these cells contribute directly to chondrogenic matrix regeneration orif they rather cause a supportive microenvironment for cartilage defect repair [61]. While using autologous stem cells for cartilage repair would be desirable to minimize host immune responses, the yield of MSC which could be retrieved from bone marrow aspirates and which are capable of forming cartilage would likely be too low for most « one-stop-shop » approaches and require ex vivo enrichment and expansion of cartilage forming phenotypes (positive for CD105, CD73, CD90 and negative for CD45, CD34, CD11b, CD19, HLA-DR; according to the International Society for Cell Therapy; ISCT). Using conventional separation technologies, 5 ml of human unprocessed bone marrow produces approximately 250 million mononuclear cells ±90 million. One in 100,000=2500 of these cells are cartilage-forming MSC as defined above. In an outpatient procedure, up to about 180 mlof bone marrow aspirate or 90,000 MSC could be retrieved. In an inpatient procedure, 10-15 ml/kg could be harvested, which would translate into 10 cc/kg=350,000 MSC for a 70 kg patient. These numbers are too low for most cartilage regeneration needs. Since cell culture expansion could lead to changes in cell phenotypes and cell de-differentiation, « off the shelf » cell products are preferred by many investigators because of their immediate and potentially unlimited availability and better characterization. However, these products will require close observations of potential host immune responses that could lead to rejection of the transplant.
Another barrier for clinical translation is the limited availability of immediately clinically applicable scaffolds. Hydrogel-based bioengineered scaffolds have shown very promising results in preclinical studies. However these products are not FDA-approved and thus, not clinically available within the foreseeable future [61]. Table 1 provides a list of FDA-approved scaffolds which would be potentially more immediately clinically applicable via an « off label » use. Scaffold features that affect stem cell engraftment outcomes and which have to be evaluated for every new scaffold product include the following [62-64]: (1) Biocompatibility; Ability of the scaffold material to integrate into living tissue. (2) Mechanical property: Resistance to local mechanical forces. (3) Pore size: Influences cell aggregation and differentiation. (4) Structure and geometry: Influences proliferation and differentiation of the transplanted cells. (5) Biodegradation property: Fast or slow elimination from the body. (6) Biochemical integration: Long term integration and availability of growth factors or cytokines. Clinic-to-bench-and-back research is urgently needed to evaluate the effect of various stem-cell scaffold compositions on bone and cartilage regeneration outcomes.
Table 1.
Overview over clinically applicable scaffolds.
| Product Name | Company | Primary Indication | Composition |
|---|---|---|---|
| INTEGRA™ FlowableWound Matrix | Integra | Wound healing | Granulated cross-linked bovme tendon collagen and glycosaminoglycan |
| lnf orce®Reinforcement Matrix | Integra | Tendon repair | Cross-linked porcine derived collagen I |
| SurgiMend | TEl Biosciences | Soft tissue repair | Acellular dermal Collagen matrix derived from fetal and neonatal bovine dermis |
| AlloPatch HD™ | MTF Sports Medicine | Tendon augmentation | Acellular Human Dermis |
| FlexHo® | Ethicon | Hernia repair breast reconstruction | Acellular dermal matrix derived from donated human allograft skin |
| BioFiber'™ | Tornier | Soft tissue repair | Absorbable biologic polymer |
| TRUFITTM CB Plug | Smith & Nephew | Cartilage repair | Polymers, ceramics and fibers |
| ChondroMimetic™ | TiGenix | Cartilage repair | Readily absorbed, non-synthetic, collagen, GAG, calcium phosphate |
| OseoFit | Kensev Nash | Cartilage repair | Collagen formulation1 synthetic polymer and ceramic |
| Matrix Collagen Particles | Collagen Matrix | Wound healing | Type I collagen |
| Collagen Sponge | Collagen Matrix | Wound healing | Type I collagen |
| Collagen Film | Collagen Matrix | Wound healing | Type I collagen |
| Collagen Film/Collieva | lnnocoll | Wound healing | Purified type 1 collagen |
| Pelvicol®Acellular Collagen Matrix | C.R. Bard | Soft tissue repair | Cross-linked Porcine dermal collagen |
| ARTISS | Baxter Healthcare Corporation | Wound healing | .Human fibrinogen and a synthetic fibrinolysis inhibitor / low-concentration human thrombin solution In a calcium chloride solution |
| TISSEEL | Baxter Healthcare | Hemostasis / Sealine | Human fibrinogen and a synthetic fibrinolysis inhibitor, aprotinin |
| EVICEL®Fibrin Sealont | Ethicon360 | Hemostasis | The only all human, aprotinin free, fibrin sealant |
Summary
Clinically applicable cell tracking techniques will enable us to overcome the bottle neck of diagnosing stem cell transplant failures, avoid long term and invasive follow up studies of lost or dead transplants and help to assign patients with transplant failure to early interventions or alternative treatment options. Clinical translation of the described imaging techniques could help to direct patients with apoptotic and/or lost MASI, as diagnosed by cellular MR imaging, to repeated or alternative treatments. On the other hand, patients with successful transplants could be spared from invasive follow up studies. Cellular imaging tools could be also utilized to study the effect of new types of cell transplants (e.g. chondrocytes or chondrogenic precursors derived from induced pluripotent stem cells or embryonic stem cells [65]), scaffolds, growth factors and immune response modifiers on MASI engraftment outcomes, which could in turn inform the development of more successful MASI approaches. Since clinical trials of new combination therapies are expensive and take years to complete, the impact of our imaging technique could be immense. Our cellular imaging techniques would be in principle readily clinically applicable, could help to improve and tailor individualized therapeutic approaches, and ultimately, improve successful joint regeneration and long term outcomes.
Acknowledgments
This work was supported by NIH grant R01AR054458 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. We thank Malcolm Debaun and Jason Dragoo for valuable input regarding clinical translation of our cell tracking approaches. We would also like to acknowledge Amy Thomas who created Figure 1 for this manuscript.
References
- 1.Brooks PM. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. CurrOpinRheumatol. 2002;14:573–577. doi: 10.1097/00002281-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford) 2005;44:1531–1537. doi: 10.1093/rheumatology/kei049. [DOI] [PubMed] [Google Scholar]
- 3.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. CurrOpinBiotechnol. 2004;15:406–410. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Nejadnik H, Hui JH, FengChoong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 6.Trattnig S, Domayer S, Welsch GW, Mosher T, Eckstein F. MR imaging of cartilage and its repair in the knee--a review. EurRadiol. 2009;19:1582–1594. doi: 10.1007/s00330-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 7.Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports TraumatolArthrosc. 2009;17:1289–1297. doi: 10.1007/s00167-009-0782-4. [DOI] [PubMed] [Google Scholar]
- 8.Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, et al. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding C, Cicuttini F, Jones G. How important is MRI for detecting early osteoarthritis? Nat ClinPractRheumatol. 2008;4:4–5. doi: 10.1038/ncprheum0676. [DOI] [PubMed] [Google Scholar]
- 10.Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. EurRadiol. 2007;17:1135–1146. doi: 10.1007/s00330-006-0453-5. [DOI] [PubMed] [Google Scholar]
- 11.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229:838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 12.Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 13.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 14.Daldrup-Link HE, Rudelius M, Piontek G, Metz S, Bräuer R, et al. Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology. 2005;234:197–205. doi: 10.1148/radiol.2341031236. [DOI] [PubMed] [Google Scholar]
- 15.Henning TD, Gawande R, Khurana A, Tavri S, Mandrussow L, et al. Magnetic resonance imaging of ferumoxide-labeled mesenchymal stem cells in cartilage defects: in vitro and in vivo investigations. Mol Imaging. 2012;11:197–209. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. ProcNatlAcadSci U S A. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nejadnik H, Henning TD, Castaneda RT, Boddington S, Taubert S, et al. Somatic differentiation and MR imaging of magnetically labeled human embryonic stem cells. Cell Transplant. 2012;21:2555–2567. doi: 10.3727/096368912X653156. [DOI] [PubMed] [Google Scholar]
- 18.Syková E, Jendelová P. Magnetic resonance tracking of transplanted stem cells in rat brain and spinal cord. Neurodegener Dis. 2006;3:62–67. doi: 10.1159/000092095. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 20.Daldrup-Link HE, Rudelius M, Oostendorp RA, Settles M, Piontek G, et al. Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology. 2003;228:760–767. doi: 10.1148/radiol.2283020322. [DOI] [PubMed] [Google Scholar]
- 21.Metz S, Bonaterra G, Rudelius M, Settles M, Rummeny EJ, et al. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. EurRadiol. 2004;14:1851–1858. doi: 10.1007/s00330-004-2405-2. [DOI] [PubMed] [Google Scholar]
- 22.Golovko DM, Henning T, Bauer JS, Settles M, Frenzel T, et al. Accelerated stem cell labeling with ferucarbotran and protamine. EurRadiol. 2010;20:640–648. doi: 10.1007/s00330-009-1585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaneda RT, Khurana A, Khan R, Daldrup-Link HE. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp. 2011:e3482. doi: 10.3791/3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henning TD, Sutton EJ, Kim A, Golovko D, Horvai A, et al. The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast Media Mol Imaging. 2009;4:165–173. doi: 10.1002/cmmi.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha P, Golovko D, Bains S, Hostetter D, Meier R, et al. Monitoring of natural killer cell immunotherapy using noninvasive imaging modalities. Cancer Res. 2010;70:6109–6113. doi: 10.1158/0008-5472.CAN-09-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton EJ, Henning TD, Boddington S, Demos S, Krug C, et al. In vivo magnetic resonance imaging and optical imaging comparison of viable and nonviable mesenchymal stem cells with a bifunctional label. Mol Imaging. 2010;9:278–290. [PMC free article] [PubMed] [Google Scholar]
- 27.Landry R, Jacobs PM, Davis R, Shenouda M, Bolton WK. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am J Nephrol. 2005;25:400–410. doi: 10.1159/000087212. [DOI] [PubMed] [Google Scholar]
- 28.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am SocNephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balakrishnan VS, Rao M, Kausz AT, Brenner L, Pereira BJ, et al. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest. 2009;39:489–496. doi: 10.1111/j.1365-2362.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 30.Coyne DW. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert OpinPharmacother. 2009;10:2563–2568. doi: 10.1517/14656560903224998. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85:315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 32.Simon GH, von Vopelius-Feldt J, Fu Y, Schlegel J, Pinotek G, et al. Ultrasmallsupraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Invest Radiol. 2006;41:45–51. doi: 10.1097/01.rli.0000191367.61306.83. [DOI] [PubMed] [Google Scholar]
- 33.Khurana A, Nejadnik H, Chapelin F, Lenkov O, Gawande R, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 2013;8:1969–1983. doi: 10.2217/nnm.12.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18:463–467. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roosendaal G, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Cartilage damage as a result of hemarthrosis in a human in vitro model. J Rheumatol. 1997;24:1350–1354. [PubMed] [Google Scholar]
- 36.Henning TD, Wendland MF, Golovko D, Sutton EJ, Sennino B, et al. Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5T and 3T: discrimination of viable from lysed cells. MagnReson Med. 2009;62:325–332. doi: 10.1002/mrm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedopil A, Klenk C, Kim C, Liu S, Wendland M, et al. MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis. Invest Radiol. 2010;45:634–640. doi: 10.1097/RLI.0b013e3181ed566c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 39.Welsch GH, Zak L, Mamisch TC, Resinger C, Marlovits S, et al. Three-dimensional magnetic resonance observation of cartilage repair tissue (MOCART) score assessed with an isotropic three-dimensional true fast imaging with steady-state precession sequence at 3.0 Tesla. Invest Radiol. 2009;44:603–612. doi: 10.1097/RLI.0b013e3181b5333c. [DOI] [PubMed] [Google Scholar]
- 40.Welsch GH, Zak L, Mamisch TC, Paul D, Lauer L, et al. Advanced morphological 3D magnetic resonance observation of cartilage repair tissue (MOCART) scoring using a new isotropic 3D proton-density, turbo spin echo sequence with variable flip angle distribution (PD-SPACE) compared to an isotropic 3D steady-state free precession sequence (True-FISP) and standard 2D sequences. J MagnReson Imaging. 2011;33:180–188. doi: 10.1002/jmri.22399. [DOI] [PubMed] [Google Scholar]
- 41.Bulte JW. Science to practice: can stem cells be labeled inside the body instead of outside? Radiology. 2013;269:1–3. doi: 10.1148/radiol.13131753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khurana A, Nejadnik H, Gawande R, Lin G, Lee S, et al. Intravenous ferumoxytol allows noninvasive MR imaging monitoring of macrophage migration into stem cell transplants. Radiology. 2012;264:803–811. doi: 10.1148/radiol.12112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Human gene therapy. 2008;19:807–819. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 45.Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: an overview. PlastReconstrSurg. 2006;117:1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 48.Nedopil AJ, Mandrussow LG, Daldrup-Link HE. Implantation of ferumoxides labeled human mesenchymal stem cells in cartilage defects. J Vis Exp. 2010 doi: 10.3791/1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. ExpHematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Daldrup-Link HE, Rummeny EJ, Ihssen B, Kienast J, Link TM. Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin's lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. EurRadiol. 2002;12:1557–1566. doi: 10.1007/s00330-001-1270-5. [DOI] [PubMed] [Google Scholar]
- 51.Simon GH, Raatschen HJ, Wendland MF, vonVopelius-Feldt J, Fu Y, et al. Ultrasmall superparamagnetic iron-oxide-enhanced MR imaging of normal bone marrow in rodents: original research original research. Academic radiology. 2005;12:1190–1197. doi: 10.1016/j.acra.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Metz S, Lohr S, Settles M, Beer A, Woertler K, et al. Ferumoxtran-10-enhanced MR imaging of the bone marrow before and after conditioning therapy in patients with non-Hodgkin lymphomas. EurRadiol. 2006;16:598–607. doi: 10.1007/s00330-005-0045-9. [DOI] [PubMed] [Google Scholar]
- 53.Daldrup-Link HE, Kaiser A, Link TM, Settles M, Helbich T, et al. Comparison between gadopentetate and feruglose (Clariscan)-enhanced MR-mammography: preliminary clinical experience. AcadRadiol. 2002;9:S343–347. doi: 10.1016/s1076-6332(03)80225-8. [DOI] [PubMed] [Google Scholar]
- 54.Daldrup-Link HE, Brasch RC. Macromolecular contrast agents for MR mammography: current status. EurRadiol. 2003;13:354–365. doi: 10.1007/s00330-002-1719-1. [DOI] [PubMed] [Google Scholar]
- 55.Daldrup-Link HE, Kaiser A, Helbich T, Werner M, Bjørnerud A, et al. Macromolecular contrast medium (feruglose) versus small molecular contrast medium (gadopentetate) enhanced magnetic resonance imaging: differentiation of benign and malignant breast lesions. AcadRadiol. 2003;10:1237–1246. doi: 10.1016/s1076-6332(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 56.Daldrup-Link HE, Rydland J, Helbich TH, Bjørnerud A, Turetschek K, et al. Quantification of breast tumor microvascular permeability with feruglose-enhanced MR imaging: initial phase II multicenter trial. Radiology. 2003;229:885–892. doi: 10.1148/radiol.2293021045. [DOI] [PubMed] [Google Scholar]
- 57.Simon G, Link TM, Wörtler K, Doebereiner F, Schulte-Frohlinde E, et al. Detection of hepatocellular carcinoma: comparison of Gd-DTPA- and ferumoxides-enhanced MR imaging. EurRadiol. 2005;15:895–903. doi: 10.1007/s00330-005-2669-1. [DOI] [PubMed] [Google Scholar]
- 58.Daldrup-Link HE, Mohanty A, Cuenod C, Pichler B, Link T. New perspectives on bone marrow contrast agents and molecular imaging. SeminMusculoskeletRadiol. 2009;13:145–156. doi: 10.1055/s-0029-1220885. [DOI] [PubMed] [Google Scholar]
- 59.Corot C, Robert P, Idée JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Schwenk MH. Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy. 2010;30:70–79. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 61.Hyun JS, Tran MC, Wong VW, Chung MT, Lo DD, et al. Enhancing stem cell survival in vivo for tissue repair. BiotechnolAdv. 2013;31:736–743. doi: 10.1016/j.biotechadv.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 64.Uebersax L, Hagenmüller H, Hofmann S, Gruenblatt E, Müller R, et al. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006;12:3417–3429. doi: 10.1089/ten.2006.12.3417. [DOI] [PubMed] [Google Scholar]
- 65.Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]


